Back to Journals » Clinical Interventions in Aging » Volume 15
Plasma Interleukin-37 is Elevated in Acute Ischemic Stroke Patients and Probably Associated With 3-month Functional Prognosis
Authors Zhang F, Zhu T, Li H, He Y, Zhang Y, Huang N, Zhang G , Li Y, Chang D, Li X
Received 7 September 2019
Accepted for publication 6 May 2020
Published 4 August 2020 Volume 2020:15 Pages 1285—1294
DOI https://doi.org/10.2147/CIA.S230186
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zhi-Ying Wu
Feng Zhang,1 Tianrui Zhu,1 Heng Li,1 Yi He,2 Yuanyuan Zhang,1 Nana Huang,1 Guitao Zhang,3 Yanshuang Li,1 Dujuan Chang,1 Xiaohong Li1
1Department of Neurology, Jinan Central Hospital, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, People’s Republic of China; 2Department of Neurology, The Second Affiliated Hospital of Xi’an Medical University, Xi’an, Shanxi, People’s Republic of China; 3Department of Neurology, Capital Medical University Affiliated Beijing Tiantan Hospital, Beijing, People’s Republic of China
Correspondence: Xiaohong Li Email [email protected]
Background: Interleukin-37 is a novel cytokine emerging as a natural suppressor of inflammatory responses. Inflammation and the immune response play important roles in acute ischemic stroke. This study aimed at evaluating the plasma levels and the association with 3-month outcomes of interleukin-37 in acute ischemic stroke patients.
Patients and Methods: In total, 152 consecutive patients with acute ischemic stroke and 45 healthy controls were included. Plasma interleukin-37 levels were determined in the first morning after admission using an enzyme-linked immunesorbent assay. The primary outcome was the 3-month functional outcome (modified Rankin Scale score > 2). Logistic regression was used to evaluate the risk and 3-month outcome of stroke according to plasma interleukin-37 level.
Results: Plasma interleukin-37 levels were significantly higher in the patients with acute ischaemic stroke than in the healthy controls (182.26 versus 97.89 pg/mL, p< 0.001). Patients with large-artery atherosclerosis had significantly higher IL-37 levels than those with small-artery occlusion (202.12± 35.82 versus 175.67± 33.71pg/mL, p< 0.001). Plasma interleukin-37 levels were positively correlated with National Institutes of Health Stroke Scale scores (r=0.521, p< 0.0001) and lesion volume (r=0.442, p< 0.0001). Ninety-four and 58 patients had favourable and unfavourable 3-month outcomes, respectively. Elevated plasma interleukin-37 levels were independently associated with unfavourable 3-month outcomes (adjusted odds ratio=1.033, p=0.001, 95% confidence interval: 1.015– 1.056).
Conclusion: Admission plasma interleukin-37 levels were significantly increased after acute ischemic stroke. Elevated interleukin-37 levels were independently associated with unfavourable 3-month prognoses in acute ischemic stroke patients. Further studies with other populations are needed.
Keywords: interleukin-37, acute ischemic stroke, functional outcome, prognosis
Introduction
Stroke is the second leading cause of death in the world and the leading cause of death and disability in China.1–3 There are 2.4 million new stroke cases each year, approximately 70% of who have experienced various types of ischemic stroke in China.1 Meanwhile, in American, about 795,000 people experience a new or recurrent stroke each year and 87% are ischemic.4 Deaths from stroke increased from 5.29 million (5.22–5.40) to 6.17 million (6.04–6.33) between 2007 and 2017 globally.5 Rapidly effective measurable biomarkers to indicate disease development and prognosis are crucial for the optimization of care and the allocation of healthcare resources.
It is well accepted that immunity and inflammation play critical roles in the pathogenesis of stroke and are implicated in the primary and secondary progression of ischemic lesions, as well as in the recovery, and overall outcome after a stroke.6–10 The brain responds to ischemic injury with an acute and prolonged inflammation process triggered by both resident and peripheral innate immunity,10 and characterized by rapid activation of resident cells, production of pro-inflammatory mediators and infiltration of different types of inflammatory cells in the ischemic area of the brain. New biomarkers in immune reaction might be of great value in predicting the prognosis of acute ischemic stroke (AIS).
Interleukin (IL)-37 is a newly identified member of the IL-1 family. It has emerged as a natural suppressor of innate inflammation and adaptive immune responses11,12 and is expressed at low concentrations in peripheral blood mononuclear cells, mainly monocytes, and dendritic cells. IL-37 is up regulated in the inflammatory context in an inducible manner13–15 and inhibits the secretion of pro-inflammatory cytokines such as IL-1β, IL-6, and tumor necrosis factor-α (TNF-α) in peripheral blood monocytes, macrophages, dendritic cells, and epithelial cells.13
Accumulating evidence from animal and clinical studies has confirmed that IL-37 participates in atherosclerotic disease. IL-37 has been shown to ameliorate myocardial ischemia/reperfusion (I/R) in mouse models.16 IL-37 has also been shown to reduce the inflammatory response after cerebral ischemia and reperfusion injury by down-regulating pro-inflammatory microglia-macrophages and cytokines and up-regulating expression of anti-inflammatory cytokines in mice.17,18 Furthermore, Zhang SR and Zafar A found that IL-37 increases in patients after ischemic stroke respectively17,19 and the plasma levels of IL-37 were found to be significantly elevated in patients with acute coronary syndrome (ACS),20 and the increased IL-37 concentration was associated with a worse clinical in-hospital outcome in ST-segment elevation myocardial infarction (STEMI) patients undergoing primary percutaneous coronary interventions (PPCI).21
The expression and function of IL-37 in patients with AIS has few been well demonstrated. The goal of this study was to estimate the plasma levels of IL-37 in AIS patients and investigate the association between IL-37 levels and 3-month functional outcomes of AIS.
Patients and Methods
Study Population
From November 2017 to August 2018, a total of 223 consecutive patients with first-ever AIS were identified in the Department of Neurology, Jinan Central Hospital affiliated to Shandong University. All the patients were first admitted to our Stroke Center within 24 hours from symptoms onset, followed by confirmation with magnetic resonance imaging (MRI). The inclusion criteria were based on the clinical history, neurological symptoms and MRI according to the criteria of the World Health Organization. The exclusion criteria consisted of transient ischemic stroke, intracranial hemorrhage, aneurysmal subarachnoid hemorrhage, venous sinus thrombosis, a recent history of surgery or myocardial infarction during the past 3 months, vascular malformation, malignant tumor, severe liver or kidney failure, and systemic inflammatory and autoimmune diseases. Among these patients, 62 patients were excluded, and 161patients were finally included in the present study. Meanwhile, 136 healthy subjects from the Physical Examination Centre in our hospital were collected, and after matching for age and sex, 45 were included as healthy controls. All participants or their relatives (patients unable to communicate) were informed of the study protocol and signed the consent form in accordance with the Helsinki declaration. The local ethics committee of the Jinan Central Hospital affiliated to Shandong University approved this study.
Demographic Characteristics and Neuroimaging
The participants’ demographic characteristics (including age, sex and body mass index (BMI)) and history of risk factors were obtained at admission. The common risk factors for AIS include hypertension, diabetes mellitus, coronary heart disease, atrial fibrillation, cigarette smoking, alcohol consumption, a family history of AIS, high systolic blood pressure (SBP) (≥140mmHg) and diastolic blood pressure (DBP)(≥90mmHg). Clinical severity was assessed at admission using the National Institutes of Health Stroke Scale (NIHSS) score.22 The patients with AIS were aetiologically classified into 5 subtypes: large-artery atherosclerosis (LAA), small-vessel occlusion (SVO), cardio-embolism (CE), other cause and stroke of undetermined aetiology (U) according to the Trial of Org 10,172 in the Acute Stroke Treatment (TOAST) system.23 The clinical stroke syndrome was categorized using the classification criteria of the Oxfordshire Community Stroke Project (OCSP) as follows: total anterior circulation infarct (TACI), partial anterior circulation infarct (PACI), posterior circulation infarct (POCI), lacunar infarct (LACI), and uncertain.24 The infarct volume was calculated using the formula: 0.5×a×b×c.25 In detail, “a” represents the largest cross-sectional diameter, “b” represents a second diameter drawn at right angles to the first, and “c” represents the height of the ellipsoid (5-mm slices containing the infarct were use in the present study). The clinical syndromes and lesion volumes were confirmed by Prof. Yan-xin Zhao, who was blinded to the clinical and laboratory results.
End-Point and Follow-Up
The primary end-point was the functional outcome at 3 months from the baseline, which was assessed by the modified Rankin Scale (mRS).26 A favourable functional outcome was defined as a mRS score of 0 to 2 points, whereas an unfavourable outcome was defined as a mRS score of >2 points.27 Outcome assessment was performed by one trained medical staff member who was blinded to the laboratory results during the outpatient review.
Blood Collection and Quantification
For all patients, fasting blood samples were drawn on the first morning after admission, namely 48 hours from stroke onset. The levels of laboratory indicators, including total cholesterol (TC), total triglycerides (TG), low-density lipoprotein (LDL), homo-cysteine (HCY), fasting blood glucose (FBG), glycosylated haemoglobin (HbA1c), blood urea nitrogen (BUN), and creatinine (Cr), were determined according to standard methods in the clinical laboratory of our hospital. The acute-stage plasma IL-37 levels were assessed within 48 hours of stroke onset. A total of 5 mL of citrate anti-coagulated venous blood was obtained from each patient. After centrifugation at 4000 g for 10 min, the plasma of the samples was immediately stored at −80 °C before the assay. IL-37 levels were measured in the central laboratory of our hospital by investigators blinded to the clinical outcome and neuroimaging findings using a human IL-37 ELISA reagent kit (ElabScience, Hongshan district, Wuhan, China), and the results are expressed as picograms per millilitre (pg/mL).
Statistical Analysis
The results are expressed as percentages for categorical variables and as the means (standard deviation (SD)) and medians (inter- quartile ranges (IQRs)) for continuous variables, depending on whether the distribution of the data was normal or non-normal. Shapiro–Wilk tests were used to determine the normality of data distribution. Proportions were compared using the chi-squared (χ2) test. A two-group comparison of non-normally distributed data was performed using the Mann–Whitney U-test, and a two-tailed Student’s unpaired t-test was used for normally distributed continuous variables. Spearman’s rank correlation was used for bivariate correlations. The relationship of IL-37 with the end point was investigated with the use of logistic regression models. We used crude models and multivariable models adjusted for all outcome predictors significant on univariate analysis, and the results are reported as the odds ratios (ORs). All statistical analyses and graphs were performed using R software (Version 3.5.3, http://www.R-project.org). Statistical significance was defined as P<0.05.
Results
Baseline Characteristics of the Study Population
From 223 screened patients, AIS was diagnosed in 161 patients (11 with transient ischemic attack (TIA), 16 with onset of symptoms >24 h, 7 with haemorrhagic stroke, 12 who did not provide informed consent, 4 with epileptic seizures, 7 with systemic infections, and 5 with malignant tumours were not analysed) and 152 completed the 3-month follow-up (6 were lost to follow-up and 3 withdrew). During the same period, a total of 136 healthy subjects from the Physical Examination Centre in our hospital were collected, and after matching for age and sex, 45 participants were included as healthy controls.
The descriptive characteristics of the AIS patients and healthy controls are shown in Table 1. The sex and age distributions of the AIS patients and healthy controls were well matched. The prevalence rates of hypertension, diabetes, ischaemic stroke history, coronary heart disease, and cigarette smoking were significantly higher in the AIS group than in the control group. In addition, the levels of BMI, FBG, HbA1c, LDL-c, SBP and DBP, were significantly higher in the AIS group than in the control group (P<0.05, respectively). At admission, the median NIHSS score was 5 (IQR, 3–8), the median lesion volume was 4.11 mL (IQR, 1.22–7.14), and the median IL-37 concentration was 182.26 pg/mL (IQR, 159.62–199.64). Of the AIS patients, 67, 72 and 13 exhibited LAA stroke, SAO stroke and other stroke types, respectively, according to the TOAST criteria and 80TACS, 20PACS, 45POCS, and 7LACS subtypes according to the OCSP criteria. Twenty (13.16%) of the 152 AIS patients included in this study underwent thrombolysis therapy, and 3 (1.97%) of the AIS patients underwent mechanical thrombectomy. Overall, an unfavourable outcome after 3 months was observed in 58 patients (38.16%).
 |
Table 1 Baseline Clinical Characteristics and Laboratory Data of the Study Population |
Plasma IL-37 Levels in Stroke Patients
The median plasma IL-37 levels in the AIS patients and the normal controls were 182.26 and 97.89 pg/mL, respectively (Table 1), demonstrating that the plasma IL-37 levels of the AIS patients were significantly higher than those of the normal controls (p < 0.001, Figure 1). The plasma IL-37 levels in the patients with different stroke subtypes are listed in Table 2. We compared the plasma IL-37 levels of the patients with LAA stroke, SAO stroke and other stroke types with those of the normal controls. The plasma IL-37 level of patients with each stroke subtype was higher than that of the normal controls (p < 0.001, Figure 2). In addition, we found that the level of IL-37 was significantly higher in patients with LAA stroke than in those with SAO stroke (202.12±35.82 versus 175.67±33.72 pg/mL, p<0.001, Figure 2).
 |
Table 2 Plasma IL37 Levels in Different Stroke Subtypes |
Correlations of Plasma IL-37 Levels with Infarct Volumes and NIHSS Scores
The median infarct volume of the AIS patients was 4.11 mL (Table 1). Plasma IL-37 levels were positively associated with infarct volume (r = 0.442, p < 0.0001, Figure 3); plasma IL-37 levels were higher in AIS patients with larger infarct volumes. We also investigated the correlation between plasma IL-37 level and NIHSS score; the plasma IL-37 level increased with increasing stroke severity, as defined by the NIHSS score (r = 0.521, p < 0.0001, Figure 4).
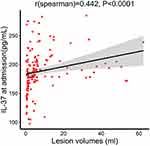 |
Figure 3 Correlation between plasma IL-37 level and lesion volumes. P values were determined using Spearman correlation analysis. |
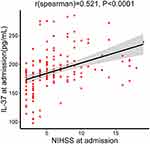 |
Figure 4 Correlation between plasma IL-37 level and NIHSS score. P values were determined using Spearman correlation analysis. |
Plasma IL-37 Levels and Different Long-Term Outcome Predictions
At 3 months, 94 patients exhibited favourable outcomes, while 58 patients exhibited unfavourable outcomes. The plasma IL-37 levels in patients with unfavourable outcomes were significantly higher than those in patients with favourable outcomes (204.50 versus 178.03 pg/mL, p < 0.0001, Figure 5). The logistic regression indicated that increased IL-37 levels were strongly associated with unfavourable outcomes, with an unadjusted OR of 1.044 (p < 0.001, 95% CI: 1.029–1.063, Table 3). After adjusting for other outcome predictors significant in the univariate logistic analysis, including SBP, atrial fibrillation, TC, LDL-c, NIHSS score and infarct volume, the plasma level of IL-37 was found to be an independent predictor of the 3-month outcome, with an adjusted OR of 1.033 (p = 0.001, 95% CI: 1.015–1.056, Table 3) by multivariable logistic analysis.
 |
Table 3 Logistic Regression Analysis for Three Months Outcome |
Discussion
In the present study, our results showed that the plasma IL-37 levels were markedly higher in AIS patients than in healthy controls (182.26 pg/mL versus 97.89 pg/mL, p<0.001). A correlation analysis showed that the levels of IL-37 were positively correlated with lesion volume and neurological deficit (as assessed by the NIHSS). Furthermore, our main finding was firstly showed a positive correlation between the plasma IL-37 level and an unfavourable 3-month prognosis in patients with AIS.
These results were consistent with Kun Liu’s research results, which showed that the level of IL-37 was elevated in patients with acute coronary syndrome and was associated with a worse clinical outcome after ST-segment elevation acute myocardial infarction.21 Xi-ling Shou found that plasma IL-37 levels were elevated in patients with chronic heart failure and predicted major adverse cardiac events.28
IL-37, a novel homologue of the IL-1 cytokine family mainly synthesized by activated macrophages and dendritic cells, is a natural suppressor of innate and adaptive inflammatory and immune responses11,12 and an active participant in cardio-cerebral vascular disease.21,28 Many studies have indicated that the production of IL-37 occurs at low levels in a normal physiological state and can be effectively induced in an inflammatory environment. IL-37 is not constitutively expressed by blood monocytes in healthy subjects but is rather induced by IL-1β, Toll-like receptors (TLR) agonists or transforming growth factor β (TGF-β).11 However, we remain uncertain about by what, when and how rapidly IL-37 is released into the circulation. Compared with the baseline IL-37 levels in the normal controls and based on previous observational studies,21,28,29 we speculate that significantly increased IL-37 levels are secondary to post-ischaemic inflammation and are induced by pro-inflammatory factors in the early phase of AIS as a feedback loop to maintain the dynamic balance between the pro- and anti-inflammatory responses and to suppress an acute and over-reactive inflammatory process.21,30
Inflammation is involved in atherosclerotic plaque formation, plaque destabilization and rupture31 and the occurrence of AIS.6 Ischemic stroke results in an abrupt deprivation of nutrient supplies that quickly leads to irreversible damage in the core of the affected area. Subsequently, excitotoxicity, oxidative stress, and mitochondrial disturbances spread cellular damage into the partly preserved penumbra. Ischemia-reperfusion injury also induces a robust inflammatory response.32 Within hours, stroke induces systemic immune changes that last for weeks and can affect clinical outcomes.33,34 All of these processes stimulate inflammatory responses and the production and activation of a large number of inflammatory mediators, such as cytokines (IL-1β, TNF, and IL-6), chemokines, nitric oxide, and reactive oxygen species (ROS). These inflammatory mediators then exacerbate cell death and eventually lead to dysfunction of the blood–brain barrier.35 Following this process, infiltrating leucocytes, neurons, astrocytes, and microglia participate in ischaemic brain injury through the release of pro-inflammatory mediators36–38 from several hours to days after AIS occurs. Peripheral blood monocytes migrate into the ischaemic brain tissues where they mature into different types of microglia and macrophage-like cells or dendritic cells.39 IL-37 may be produced by these cells, especially M2 macrophages, which exhibit anti-inflammatory properties together with other anti-inflammatory cytokines, such as IL-10 and TGF-β.7 Because few studies have demonstrated the secretion of IL-37 and the inducing factor for IL-37, our current work may be useful.
The concentration of IL-37 in each stroke subgroup was compared with that in the control group. Despite differences in the aetiology and pathophysiology of each subtype, the plasma IL-37 level was increased in all stroke subtypes. These findings suggest that the increase of IL-37 is probably caused by cerebral ischemia itself to a certain extent, as supported by the increase in pro-inflammatory cytokines caused by focal ischaemic lesions and the positive correlation observed between the lesion volume and the plasma IL-37 level. We further compared IL-37 levels between patients with LAA and SAO subtypes and found that IL-37 levels in patients with the LAA subtype were significantly higher than those in patients with the SAO subtype. Inflammation plays an important role in atheromatous plaque vulnerability and disruption.40 In addition, histopathological detection indicated that IL-37 is highly expressed in human atherosclerotic plaques.41 We hypothesized that when the atherosclerotic plaque ruptured in patients with the LAA subtype, the IL-37 level was probably increased due to both a systemic inflammatory response and the release from the plaque into the circulation.
We report that elevated plasma IL-37 levels are associated with unfavourable outcomes at 3 months after AIS, which is consistent with the observation that increased IL-37 levels correlate with a poor prognosis of ischaemic myocardial infarction and heart failure.21,28 Our present study revealed that NIHSS scores, lesion volumes, atrial fibrillation, SBP, TC, and LDL-c were all associated with the 3-month functional outcome. These results are in accordance with those of previous studies that have demonstrated that lifestyle factors and lipid metabolism are risk factors for stroke.42 Furthermore, we demonstrated that IL-37 remained a relevant factor for the 3-month functional outcome when these factors were all included in the multivariable logistic analysis. Our data suggest that the plasma IL-37 level might be a novel potential factor of poor prognosis in AIS patients and could help to screen for high-risk patients who need aggressive monitoring and treatment. These results may seem to contradict the biological functions of IL-37, such as anti-inflammation, immune regulation and a reduction in atherosclerosis. Nevertheless, these results were very similar to those previously reported for natriuretic peptide43 and hepatic growth factor (HGF),44 which are both known to be protective in AIS patients, even though elevated levels are strong predictors of poor outcomes. The underlying mechanisms linking the increased IL-37 level at admission and an unfavourable functional outcome have not been clearly demonstrated. However, several possible aspects should be considered. First, we found that the level of IL-37 was positively correlated with infarct volume and NIHSS score at the baseline. Second, many studies have suggested that inflammation exacerbates secondary brain injury in the acute stage36–38,42,45 Because the increased IL-37 level was hypothesized to be a compensation response to suppress the inflammatory response and maintain a dynamic balance between the pro-inflammatory and anti-inflammatory responses, it may actually act as an index of the anti-inflammatory response in AIS. This may indicate the degree of inflammatory response, which exacerbates secondary brain injury and thereby indicates the severity of the clinical prognosis. However, the exact mechanism underlying the function of IL-37 in the pathogenesis of AIS should be investigated in future studies.
To the best of our knowledge, this is the first study to determine IL-37 expression and its prognostic value for 3-month outcomes in Chinese AIS patients. The results showed a significantly higher plasma IL-37 concentration in AIS patients than in the healthy control group. Furthermore, IL-37 is found to be positively correlated with an unfavourable 3-month prognosis in patients with AIS.
However, several limitations should be taken into account in the present study. First, plasma IL-37 concentrations were only tested once at admission. We cannot provide information regarding when the circulating IL-37 level was increased or how long the increase in IL-37 level persisted in the progression of AIS. A previous study showed that when stimulated by Toll-like receptor (TLR) agonists, IL-37 expression in peripheral blood mononuclear cell (PBMCs) reached peak levels at 6–8 h and returned to baseline levels at 72 h.15 Relevant data in this population are not yet available. It could be interesting to evaluate changes in the plasma IL-37 levels in stroke patients at different time points in the acute phase. The rate of IL-37 level change may be a further indicator of outcome. Second, we detected the IL-37 levels in the peripheral plasma rather than cerebral spinal fluid (CSF), from which we would be able to infer local tissue or central nervous system (CNS) levels. Whether the plasma IL-37 levels reflect similar changes in the CNS remains unclear. However, it is not realistic to obtain cerebral spinal fluid under the emergency condition of acute stroke. Third, to fully evaluate the role of IL-37 in AIS, future studies are needed to examine the levels of relevant inflammatory factors, such as IL-6, IL-10, TNF-α, nuclear factor kappa-B (NF-kB) and their relationships with IL-37. Fourth, the number of subjects was small. When performing the prognostic analysis, the sample population of 152 subjects was too small, and the 3-month follow-up was too short. Although statistical significance was found, we did not find a notably high OR (OR = 1.033, 95% CI = 1.015–1.056, p = 0.001) for IL-37 concentration in the logistic analysis. If there were to be a larger number of subjects, the result might be more persuasive. Therefore, large samples of patients from multiple centres might be helpful in future studies.
Conclusions
In summary, our present study demonstrated for the first time a significantly higher plasma IL-37 concentration in Chinese AIS patients than in the healthy control group. Elevated IL-37 levels are probably independently associated with a poor 3-month prognosis in Chinese patients with AIS. Further prospective studies in patients from multiple large centres are needed to test and validate the diagnostic value of IL-37 in predicting the outcome of AIS.
Ethical Statement
The study was approved by the local ethics committee of the Jinan Central Hospital affiliated to Shandong University.
Acknowledgment
We greatly appreciate the technical assistance with the statistical analysis provided by Prof. Tao Zhang (Department of Biostatistics, School of Public Health, Shandong University). We also express our gratitude to all the participants in the study who made this work possible.
This work was supported by grants from the National Natural Science Foundation of China (81373635) and the Key Research and Development Plan of Shandong Province (2016GSF121044).
Disclosure
The authors declare that they have no competing interests.
References
1. Wu S, Wu B, Liu M, et al. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019;18(4):394–405. doi:10.1016/S1474-4422(18)30500-3
2. Zhou M, Wang H, Zhu J, et al. Cause-specific mortality for 240 causes in China during 1990–2013: a systematic subnational analysis for the global burden of disease study 2013. Lancet. 2016;387(10015):251–272. doi:10.1016/S0140-6736(15)00551-6
3. Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global and regional burden of stroke during 1990–2010: findings from the global burden of disease study 2010. Lancet. 2014;383(9913):245–255. doi:10.1016/S0140-6736(13)61953-4
4. Virani SS, Alonso A, Benjamin EJ, et al. Statistical update heart disease and stroke statistics-2020 update a report from the American heart association. Circulation. 2020;141. doi:10.1161/CIR.0000000000000757
5. Roth GA, Abate D, Abate KH, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392:1736–1788.
6. Esenwa CC, Elkind MS. Inflammatory risk factors, biomarkers and associated therapy in ischaemic stroke. Nat Rev Neurol. 2016;12(10):594–604. doi:10.1038/nrneurol.2016.125
7. Konsman JP, Drukarch B, Van Dam AM. (Peri)vascular production and action of pro-inflammatory cytokines in brain pathology. Clin Sci (Lond). 2007;112:1–25.
8. Jin R, Liu L, Zhang S, et al. Role of inflammation and its mediators in acute ischemic stroke. J Cardiovasc Transl Res. 2013;6(5):834–851. doi:10.1007/s12265-013-9508-6
9. Vidale S, Consoli A, Arnaboldi M, et al. Postischemic inflammation in acute stroke. J Clin Neurol. 2017;13(1):1–9. doi:10.3988/jcn.2017.13.1.1
10. Tsuyama J, Nakamura A, Ooboshi H, et al. Pivotal role of innate myeloid cells in cerebral post-ischemic sterile inflammation. Semin Immunopathol. 2018;40(6):523–538. doi:10.1007/s00281-018-0707-8
11. Nold MF, Nold-Petry CA, Zepp JA, et al. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol. 2010;11(11):1014–1022. doi:10.1038/ni.1944
12. Ding VA, Zhu Z, Mantz AA, et al. The role of IL-37 in non-cancerous diseases. Pathol Oncol Res. 2017;23(3):463–470. doi:10.1007/s12253-016-0137-7
13. Boraschi D, Lucchesi D, Hainzl S, et al. IL-37: a new anti-inflammatory cytokine of the IL-1 family. Eur Cytokine Netw. 2011;22(3):127–147. doi:10.1684/ecn.2011.0288
14. Li S, Amo-Aparicio J, Neff CP, et al. Role for nuclear IL-37 in the suppression of innate immunity. Proc Natl Acad Sci USA. 2019;16(10):4456–61.
15. Cavalli G, Dinarello CA. Suppression of inflammation and acquired immunity by IL-37. Immunol Rev. 2018;281(1):179–190. doi:10.1111/imr.12605
16. Wu B, Meng K, Ji Q, et al. IL-37 ameliorates myocardial ischaemia/reperfusion injury in mice. Clin Exp Immunol. 2014;176(3):438–451. doi:10.1111/cei.12284
17. Zhang SR, Nold MF, Tang S-C, et al. IL-37 increases in patients after ischemic stroke and protects from inflammatory brain injury, motor impairment and lung infection in mice. Sci Rep. 2019;9(1):6922. doi:10.1038/s41598-019-43364-7
18. Cavalli G, Justice JN, Boyle KE, et al. IL 37 reverses the metabolic cost of inflammation, increases oxidative respiration, and improves exercise tolerance. Proc Natl Acad Sci U S A. 2017;114(9):2313–2318. doi:10.1073/pnas.1619011114
19. Zafar A, Ikram A, Jillella DV, et al. Measurement of elevated IL-37 levels in acute ischemic brain injury: a cross-sectional pilot study. Cureus. 2017;9(10):e1767. doi:10.7759/cureus.1767
20. Ji Q, Zeng Q, Huang Y, et al. Elevated plasma IL-37, IL-18, and IL-18BP concentrations in patients with acute coronary syndrome. Mediators Inflamm. 2014;2014:165742. doi:10.1155/2014/165742
21. Liu K, Tang Q, Zhu X, et al. IL-37 increased in patients with acute coronary syndrome and associated with a worse clinical outcome after ST-segment elevation acute myocardial infarction. Clin Chim Acta. 2017;468:140–144. doi:10.1016/j.cca.2017.02.017
22. Brott T, Adams HP
23. Adams HP
24. Iłzecka J, Stelmasiak Z. Practical significance of ischemic stroke OCSP (Oxfordshire community stroke project) classification. Neurol Neurochir Pol. 2000;34:11–22.
25. Sims JR, Gharai LR, Schaefer PW, et al. ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology. 2009;72(24):2104–2110. doi:10.1212/WNL.0b013e3181aa5329
26. Bonita R, Beaglehole R. Recovery of motor function after stroke. Stroke. 1988;19(12):1497–1500. doi:10.1161/01.STR.19.12.1497
27. Katan M, Fluri F, Morgenthaler NG, et al. Copeptin: a novel, inde- pendent prognostic marker in patients with ischemic stroke. Ann Neurol. 2009;66(6):799–808. doi:10.1002/ana.21783
28. Shou X, Lin J, Xie C, et al. Plasma IL-37 elevated in patients with chronic heart failure and predicted major adverse cardiac events: a 1-year follow-up study. Dis Markers. 2017;2017:9134079. doi:10.1155/2017/9134079
29. Wang X, Cai X, Chen L, et al. The evaluation of plasma and leukocytic IL-37 expression in early inflammation in patients with acute ST-segment elevation myocardial infarction after PCI. Mediators Inflamm. 2015;2015:626934. doi:10.1155/2015/626934
30. Banchereau J, Pascual V, O’Garra A. From IL-2 to IL-37: the expanding spectrum of anti-inflammatory cytokines. Nat Immunol. 2012;13(10):925–931. doi:10.1038/ni.2406
31. Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–1143. doi:10.1161/hc0902.104353
32. Endres M, Engelhardt B, Koistinaho J, et al. Improving outcome after stroke: overcoming the translational roadblock. Cerebrovasc Dis. 2008;25(3):268–278. doi:10.1159/000118039
33. Macrez R, Ali C, Toutirais O, et al. Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurol. 2011;10(5):471–480. doi:10.1016/S1474-4422(11)70066-7
34. Prass K, Meisel C, Hoflich C, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. 2003;198(5):725–736. doi:10.1084/jem.20021098
35. Amantea D, Nappi G, Bernardi G, et al. Post-ischemic brain damage: pathophysiology and role of inflammatory mediators. FEBS J. 2009;276(1):13–26. doi:10.1111/j.1742-4658.2008.06766.x
36. Allan SM, Tyrrell PJ, Rothwell NJ. IL-1 and neuronal injury. Nat Rev Immunol. 2005;5(8):629–640. doi:10.1038/nri1664
37. McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008;5(1):45. doi:10.1186/1742-2094-5-45
38. Hayakawa K, Mishima K, Nozako M, et al. Delayed treatment with minocycline ameliorates neurologic impairment through activated microglia expressing a high-mobility group box1-inhibiting mechanism. Stroke. 2008;39(3):951–958. doi:10.1161/STROKEAHA.107.495820
39. Felger JC, Abe T, Kaunzner UW, et al. Brain dendritic cells in ischemic stroke: time course, activation state, and origin. Brain Behav Immun. 2010;24(5):724–737. doi:10.1016/j.bbi.2009.11.002
40. Avanzas P, Arroyo-Espliguero R, Cosin-Sales J, et al. Markers of inflammation and multiple complex stenoses (pancoronary plaque vulnerability) in patients with non-ST segment elevation acute coronary syndromes. Heart. 2004;90(8):847–852. doi:10.1136/hrt.2003.015826
41. Wang X, Keye X, Chen S, et al. Role of IL-37 in inflammatory and autoimmune diseases. Iran J Immunol. 2018;15(3):165–174. doi:10.22034/IJI.2018.39386
42. Fassbender K, Rossol S, Kammer T, et al. Pro-inflammatory cytokines in serum of patients with acute cerebral ischemia: kinetics of secretion and relation to the extent of brain damage and outcome of disease. J Neurol Sci. 1994;122(2):135–139. doi:10.1016/0022-510X(94)90289-5
43. Duello KM, Nagel JP, Blackshear JL, et al. B-type natriuretic peptides and mortality after stroke: a systematic review and meta-analysis. Neurology. 2014;83(3):292–293. doi:10.1212/WNL.0000000000000634
44. Zhu Z, Xu T, Guo D, et al. Serum hepatocyte growth factor is probably associated with 3-month prognosis of acute ischemic stroke. Stroke. 2018;49(2):377–383. doi:10.1161/STROKEAHA.117.019476
45. Hughes PM, Allegrini PR, Markus R, et al. Monocyte chemoattractant protein-1 deficiency is protective in a murine stroke model. J Cereb Blood Flow Metab. 2002;22(3):308–317. doi:10.1097/00004647-200203000-00008
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



