Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Plasma Exosomes from Children with Atopic Dermatitis May Promote Apoptosis of Keratinocytes and Secretion of Inflammatory Factors in vitro
Authors Zhu T , Sun J, Ma L, Tian J
Received 27 June 2022
Accepted for publication 3 September 2022
Published 14 September 2022 Volume 2022:15 Pages 1909—1917
DOI https://doi.org/10.2147/CCID.S380205
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Teng Zhu,* Jing Sun,* Lin Ma, Jing Tian
Department of Dermatology, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lin Ma, Department of Dermatology, Beijing Children’s hospital, Capital Medical University, National Center for Children’s Health, Beijing, 100045, People’s Republic of China, Tel/Fax +8601059616161, Email [email protected]
Purpose: Exosomes are important regulators of keratinocytes (KCs) that have been implicated in a variety of skin disorders. The effect of circulatory exosomes on KCs in pediatric atopic dermatitis (AD) has not been well studied. This study aims to explore the effect of plasma exosomes on KC activation, apoptosis and inflammation in pediatric AD patients.
Patients and Methods: Exosomes were extracted from plasma collected from 20 pediatric AD patients and 20 age-matched healthy controls. AD-exosomes were added with KCs at concentrations of 0 g/L, 10 g/L, 20 g/L and 30 g/L. Proliferation of KCs in each group was measured using Ki67 staining flow cytometry. Apoptosis was measured using Annexin V-FITC/PI double staining flow cytometry. KCs were divided into three groups according to the source of the exosomes they were cultured with: patients with AD, healthy controls and blank controls. Q-PCR was used to detect the activation (K6) and differentiation (K10) of cells, as well as inflammatory indicators (thymic stromal lymphopoietin (TSLP) and IL-33).
Results: The proliferation rate of KCs treated with 20 g/L exosomes from AD patients was significantly lower than that of other groups, while the apoptosis rate was significantly increased. Additionally, expression levels of K6, K10, TSLP and IL-33 were all up-regulated compared to keratinocytes treated with exosomes from healthy controls.
Conclusion: Exosomes from the peripheral blood of pediatric AD patients can regulate the activation, apoptosis and inflammatory cytokine secretion of KCs in vivo, which may participate in the pathogenesis of AD.
Keywords: atopic dermatitis, children, plasma, exosome, keratinocyte
Introduction
Atopic dermatitis (AD) is a chronic and recurrent inflammatory skin disease, characterized by pruritis.1 It usually first presents in childhood, affecting the physical and mental health of pediatric patients. Recent studies have broadened our knowledge of the immune mechanisms involved in AD, leading to the current understanding that the pathophysiology of AD stems from crosstalk between keratinocytes (KCs) and the immune system.2 KCs are the main functional and structural component of the epidermis, composing the most external layer of the skin that is essential as the first line of defense against external agents, as well as being important for the retention of body fluids and maintaining osmotic pressure in cells.3 Basal KCs can become either differentiated or activated, and specific keratin genes are often used as markers for these two alternative pathways. K5 and K14 are produced in basal KCs, while K6 and K16 are characteristically produced in activated KCs. That’s the reason for choosing K6 as our marker for KCs activation. Further the expression of K10 distinct the differentiated KCs from the keratins of the healthy epidermis.4 In AD patients, KCs have decreased filaggrin, loricrin and involucrin gene expression,5 even in patients without those genetic mutations, contributing to a defective skin barrier. Aberrant KC differentiation and altered epidermal barrier are crucial to the pathogenesis of AD.6
Exosomes are nanovesicles released by almost all cells and found in all body fluids.7 Exosome-delivered cargos include proteins, RNA, microRNA, and DNA, and their transfer between cells is essential to intercellular communication.8 Depending on the specific cargo packaged into exosomes, they can be involved in various cellular behaviors such as modulation of cell proliferation, migration, cytokine secretion and apoptosis.9 Some studies have demonstrated that exosomes can regulate the activation of KCs in certain skin diseases.10 However, there have been few reports of exosomes on pediatric AD patients, and the regulatory role of exosomes on KCs is still unclear. To address this gap, we explore the effect of plasma exosomes on KC activation and inflammation in AD patients, providing new insight on the crosstalk between circulatory exosomes and KCs, and expanding our understanding of the pathogenesis of AD.
Materials and Methods
Subject Recruitment and Data Collection
The study consisted of 20 patients with AD who were admitted to department of dermatology at the Beijing Children’s Hospital between 2018 and 2020 and 20 age-matched healthy controls. All patients met the Williams diagnostic criteria for AD. The patients enrolled in our study had no other known autoimmune or systemic diseases. This study was approved by the Ethics Committee of Beijing Children’s Hospital and was conducted according to the principles of the Helsinki Declaration. All individuals and their guardians signed informed consent forms. The average age of AD group was 4.3 and gender radio was 9/11 (female/male).
Exosome Isolation
Exosomes were isolated from the plasma by ultracentrifugation. 4 mL of peripheral blood was collected from each enrolled participant in a tube containing anti-coagulant EDTA. The blood samples were centrifuged for 15 min at 1500 xg at room temperature. After rewarming, the previously collected plasma sample was filtered and ultracentrifuged at 10,000 xg for 30 min at 4℃. The supernatant was collected and equalized in mass with cold 1X PBS, which were then spined in the preparative ultracentrifuge for 2 hours at 10,000 xg, 4℃. Resuspend the pellet with PBS and centrifuge for another 2 hours at 10,000 xg at 4℃ to wash the pellet containing exosome. Discard the supernatant and resuspend the pellet in 150μml PBS, store them at −80℃ until use.
Exosome Characterization
Exosomes were analyzed by nanoparticle tracking analysis (NTA) using a NanoSight NS300 (Malvern Panalytical, Amesbury, UK). Their movements were then analyzed by the NTA 3.2 software. A 3 μL aliquot of exosome preparation with a concentration of approximately 1000 particles/mL was placed on a formvar/carbon-coated grid and allowed to settle for 2 min. The samples were negatively stained with 2% phosphotungstic acid for 1 min. The grids were imaged with a transmission electron microscope(TEM)(HT-7700, Hitachi, Japan) operating at 120 kV.
Western blot was used to verify exosomal markers including CD9 and CD81. Plasma-derived exosomes were systematically lysed in RIPA buffer (Thermo Fisher Scientific Inc., USA) and sonicated in a 4℃ water bath (10 s, 2 times). Protein concentration was then quantified by a bicinchoninic acid (BCA) assay using the Pierce™ BCA Protein Assay kit (Thermo Fisher Scientific Inc., USA) according to manufacturer’s instructions. The protein concentration was adjusted to 10 μg/20 μL. Following electrophoresis on a 12% polyacrylamide gel, the proteins were transferred to a polyvinylidene fluoride membrane (PVDF, Millipore, USA) using a Trans-Blot Turbo Transfer System (Bio–Rad, USA). The membrane was blocked with 5% nonfat dry milk in TBST for 30 min at room temperature and incubated with primary antibodies (rabbit monoclonal anti-human CD9 antibody and rabbit monoclonal anti-human CD81 antibody, Abcam, UK) overnight at 4℃. After incubation with secondary antibody (horseradish peroxidase-labeled goat anti-rabbit antibody, Abcam, UK), images were captured on an Amersham Imager 600 (GE Healthcare Life Sciences, USA).
Cell Culture and Establishment of an Exosome Treatment Dose Curve
HaCaT cells (Morey biosciences, China) were cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum and 1% penicillin-streptomycin antibiotic in a humidified incubator at 37℃ under 5% CO2. The protein concentration of the peripheral blood exosomes extracted from AD patients were adjusted and exosomes added to culture dishes containing HaCaT cells in their growth phases at concentrations of 0g/L, 10 g/l,20 g/L, and 30g/L.
Detection of Cell Proliferation by Flow Cytometry Ki67 Staining
After culturing the HaCaT cells with exosomes for 24 h, the media was aspirated and the cells were digested with 0.25% trypsin (Thermo Fisher Scientific Inc., USA). Serum was added to terminate the digestion, then centrifugation at 300 x g for 5 min, then the supernatant was discarded. The cells were washed with ice cold PBS and centrifuged at 300 x g for 5 min. 3 mL of pre-cooled 70% ethanol was added dropwise to the cell solution, and incubate at −20 ℃ for 1 hour for streaming on-board analysis.
Detection of Apoptosis by Annexin V-FITC/PI Double Staining
HaCaT cells were cultured with exosomes for 48h, then the media was removed, the cells were digested with 0.25% trypsin, then serum terminated, and cells were collected by centrifugation at 300 x g for 5 min. Cells were then washed with ice cold PBS and centrifuged at 300 x g for 5 min and 200μL Binding Buffer (1X) was used to suspend cells; 5μL Annexin V-FITC (20μg/mL, BD, USA) were added and incubated at room temperature for 15 min in the dark; 5μL PI (50μg/mL, BD, USA) was added for staining for 5 min before the flow operation.
Detection of RNA Expression in HaCaT Cells by Quantitative PCR (q-PCR)
Total RNA was extracted using the RNeasy Mini kit (QIAGEN, Germany.) according to manufacturer’s instructions. The RNA concentration and OD value were detected by spectrophotometer and store at −80℃ for later use. Primer 5.0 was used to design primers, and the total RNA extracted was reverse-transcribed into cDNA using the miScriptReverse Transcription Kit (QIAGEN, Germany). PCR amplification was carried out on the cDNA using SYBR® Green PCR Kit (QIAGEN, Germany) in a 20μL reaction. The reaction conditions were as follows: 95 ℃ for 15 min, 1 cycle; 95 ℃ for 15s, 55 ℃ for 30s, 60 ℃ for 30s, 40 cycles. GAPDH was used as the internal reference gene in the experiment, and the relative expression was expressed in the form of 2-ΔΔCt, where ΔCT = CT (target gene)-CT(GAPDH).
Statistical Analysis
Statistical analysis was performed using SPSS version 12.0 statistical software (Chicago, IL, USA), and the data were visualized using Cytoscape 3.6.1 and GraphPad Prism 5.0 software. The quantitative data are displayed as the mean±standard deviation (SD) and were compared by Kruskal–Wallis test. P values < 0.05 indicated a statistically significant difference.
Results
Validation of Exosomes
The size of exosomes was measured by NTA and ranged from 50–150 nm, with an average particle size of approximately 84.31 nm (Figure 1A). To further characterize the exosomes, TEM was used to identify the morphology and structure of exosomes. TEM confirmed the spherical shape of exosomes and revealed the presence of clear lipid bilayer structures (Figure 1B). Western blotting provided the exosomal markers including CD9 and CD81 were indeed enriched in our isolated exosomes, as well as the negative marker calnexin was down regulated (Figure 1C). The particle size, morphology and structure of exosomes were similar for both AD and controls’ exosomes, as well as the Western blotting (WB) result.
The Effect of AD-Exosomes on the Proliferation of HaCaT Cells
AD-exosomes at concentrations of 0 g/L, 10 g/L, 20 g/L and 30 g/L were added to HaCaT cells. The expression level of Ki67 in HaCaT cells was detected by flow cytometry after a 24h treatment with AD-exosomes. The cell proliferation level of all concentrations of AD-exosome groups was significantly decreased (P<0.05) with the 20g/L exosome group being the most decreased (Figure 2).
The Effect of AD-Exosomes on the Apoptosis of HaCaT Cells
HaCaT cells were cultured with AD-exosomes at concentrations of 0 g/L, 10 g/L, 20 g/L and 30 g/L for 48 h, and the apoptosis of KCs was measured by the Annexin V-FITC/PI double staining method. The apoptotic rates of cells in the 0 g/L, 10 g/L, 20 g/L and 30 g/L AD-exosome groups were 5.34±0.3, 9.26±0.1, 12.44± 0.5, 10.37±0.1, respectively. AD-exosome treatment significantly increased apoptosis in KCs in a dose dependent manner (P<0.05), with the effect of the 20 g/L exosome group being particularly significant (Figure 3).
The Effect of AD-Exosomes on the Inflammation of HaCaT Cells
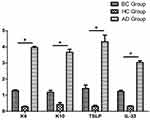
HaCaT cells were divided into three groups according to their treatment: the 20 g/L AD-exosome group, the healthy control exosome group and the blank group. q-PCR was used to detect the activation (K6) and differentiation (K10) of cells and their secretion of inflammatory markers (thymic stromal lymphopoietin (TSLP), IL-33). The results showed that compared to the control groups, K6, K10, TSLP, IL-25 and IL-33 were all significant upregulated in AD-exosome group (P<0.05) (Figure 4).
Discussion
AD is one of the most common chronic inflammatory skin diseases, with a prevalence of up to 7% in adults and up to 25% among children.11,12 At present, the etiology of AD has been shown to stem from genetic and environmental factors leading to insufficiencies in the epidermal barrier.13 Keratinocytes (KCs) are the main functional and structural components of epidermal barrier, highlighting its importance in AD.14 Recently, studies have demonstrated the participation of KCs in the onset, development as well as the chronicity of AD.15 It is thought that due to skin barrier defects, penetrating antigens interacting with KCs generate exaggerated responses characterized by an excess of cytokines and chemokines that promote local inflammatory processes.16 However, this theory cannot fully explain why children with AD present with multiple skin lesions all over the body. Exosomes function as essential messengers in the human body and may play an important role in the systemic pathogenesis of pediatric AD.
Exosomes are small membrane vesicles (30–150 nm size) released from cells into the extracellular space.17 They contain various biological components, such as nucleic acids, proteins, metabolites and more.18 Due to their small size and largely lipid compositions, these vesicles can easily squeeze between cells without damage and enter the circulation.19 Once in the circulation, exosomes can transport their cargo over long distances.20 Exosomes have been emerging as important players in allergies and autoimmune diseases due to their ability to modulate both the innate and adaptive immunity responses, and may have potential as diagnostic and therapeutic tools.21,22 A number of recent studies have revealed multiple regulatory effects of exosomes on KCs. Zhang et al demonstrated that exosomes in the plasma of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis are internalized by human primary keratinocytes. The exosomes promoted intrinsic apoptosis of these keratinocytes via down-regulation of the X-linked inhibitor of apoptosis protein (XIAP), a key apoptotic regulator in these cells.23 Neutrophil-derived exosomes from patients with generalized pustular psoriasis (GPP) can be rapidly internalized by keratinocytes, increasing the expression of inflammatory molecules through activation of NF-kB and MAPK signaling pathways. Proteomic profiling of neutrophil exosomes led to the identification of olfactomedin 4 as a critical differentially expressed protein mediating the autoimmune inflammatory responses characteristic of GPP. These results demonstrate that neutrophil exosomes have an immune-regulatory effect on keratinocytes, which modulates immune cell migration and inflammation in GPP.24 However, there has been no exploration thus far of the effect of peripheral blood exosomes on keratinocytes in pediatric AD patients. In this study, we aimed to explore whether exosomes in peripheral blood of pediatric AD patients can affect activation, apoptosis and inflammatory cytokine secretion in KCs.
Considering that exosomes in the peripheral blood of pediatric AD patients can be transported through blood to dermis in multiple regions of the body and further to epidermis by exocytosis, we hypothesized that they may play a role in the occurrence and development of disease. Keratinocyte apoptosis acts as a mechanism of eczema and spongiosis formation, which is mostly seen in acute and subacute AD lesions. Upregulation of Fas by IFN-g sensitizes keratinocytes to Fas-induced apoptosis by invading T cells with FasL expression. However, whether keratinocytes apoptosis in pediatric AD is promoted by plasma exosomes remains unknown. We carried out experiments to evaluate the effect of AD-exosomes on the proliferation and apoptosis of HaCaT cells. Our results revealed after the addition of different concentrations of AD-exosomes, cell proliferation was significantly decreased compared to blank control, while apoptosis was significantly increased. These changes were strongest after the addition of 20 g/L of AD-exosomes, so we conducted the follow-up experimental studies at this concentration. HaCaT cells were treated with either AD-exosomes, healthy control exosome or blank controls, and the activation (K6), differentiation (K10) and inflammation (TSLP, IL-33) indexes of the three groups were measured. The expressions of K6, K10, TSLP, and IL-33 in the AD-exosome group were significantly increased compared to controls. K10 is the earliest known protein marker for keratinocyte terminal differentiation in the normal epidermis, and remains present until cells are shed at the cornified layer.4 Activated keratinocytes also express K6 and produce paracrine signals to alert fibroblasts, endothelial cells, melanocytes, and lymphocytes, as well as signals targeted at neighboring keratinocytes.25 Our results confirmed that HaCaT cells become activated and differentiate under the action of AD-exosomes, resulting in significant production of K6 and K10. This may promote reciprocal immune responses in AD. Activated KCs cause itching of the skin by releasing additional pruritogens, including the alarmin TSLP, to directly activate pruritoceptive neurons.26 In addition to its direct role as a pruritogen, TSLP from keratinocytes promotes atopic skin inflammation and pruritus pathways indirectly by activating immune cells. TSLP binds to TSLPR on Th2 cells and type 2 innate lymphoid cells, leading to production of pruritogenic type 2 cytokines.27 Our experiments reveal that AD-exosome-treated HaCat cells secrete large amounts of TSLP, which may be the cause of syndromic itching in pediatric AD patients. IL-33 may be involved in the pathogenesis of AD through a variety of mechanisms, with the IL-33-ILC2s axis representing a potential central mediator.28 IL-33 is secreted extracellularly by keratinocytes, and activates ILC2 to produce type 2 cytokines which result in the formation of AD lesions. It reduces skin barrier function, and can also cause itching of the skin.29 Full-length biologically-active IL-33 is constitutively expressed in keratinocytes, and is normally stored in the cell nucleus.30 Upon cellular damage or stress, IL-33 is released rapidly as an alarmin to activate the innate immune system.31 The HaCat cells in the AD-exosome group in our experiment produced significant IL-33. Considering the important roles of IL-33 and TSLP in AD, exosomes circulating in peripheral blood represent a prime candidate as carriers of biological information to distant areas, activating keratinocytes in other parts of the body to produce AD-related and important cytokines, such as IL-33 and TSLP. Although we managed to prove that plasma exosome from pediatric AD patient may promote apoptosis of keratinocytes and secretion of inflammatory factors through vitro studies, there were still some limitations. It was difficult to remove plasma cytokines completely during exosome isolation in order to rule out the possibilities for their functions in AD pathogenesis. Since exosomes not only carry cytokines, but are transporters of important biological components such as protein, mRNA and microRNA, it is difficult to identify the specific component of exosome that plays the most important role in AD pathogenesis at the current stage of our study. Moreover, further vivo researches are needed for strengthening the evidence of relationships between plasma exosome and AD unset.
Conclusion
Altogether, our article confirms that peripheral blood exosomes of AD pediatric patients regulate the proliferation, apoptosis, activation and inflammation of KCs and participate in the occurrence and development of AD. Further research is warranted to obtain a deeper understanding of the role that specific components in circulatory exosomes play in this process.
Acknowledgments
This research was funded by Beijing Natural Science Foundation (7224331).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Yew YW, Thyssen JP, Silverberg JI. A systematic review and meta-analysis of the regional and age-related differences in atopic dermatitis clinical characteristics. J Am Acad Dermatol. 2019;80:390–401. doi:10.1016/j.jaad.2018.09.035
2. Möbus L, Rodriguez E, Harder I, et al. Atopic dermatitis displays stable and dynamic skin transcriptome signatures. J Allergy Clin Immunol. 2021;147:213–223. doi:10.1016/j.jaci.2020.06.012
3. Karsch S, Büchau F, Magin TM, Janshoff A. An intact keratin network is crucial for mechanical integrity and barrier function in keratinocyte cell sheets. Cell Mol Life Sci. 2020;77:4397–4411. doi:10.1007/s00018-019-03424-7
4. Freedberg IM, Tomic-Canic M, Komine M, Blumenberg M. Keratins and the keratinocyte activation cycle. J Invest Dermtol. 2001;116(5):633–640. doi:10.1046/j.1523-1747.2001.01327.x
5. Yamada K, Matsushita K, Wang J, Kanekura T. Topical glucose induces claudin-1 and filaggrin expression in a mouse model of atopic dermatitis and in keratinocyte culture, exerting anti-inflammatory effects by repairing skin barrier function. Acta Derm Venereol. 2018;98:19–25. doi:10.2340/00015555-2807
6. Boguniewicz M, Leung DYM. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev. 2011;242:233–246. doi:10.1111/j.1600-065X.2011.01027.x
7. Zhou X, Brown BA, Siegel AP, et al. Exosome-mediated crosstalk between keratinocytes and macrophages in cutaneous wound healing. ACS Nano. 2020;14:12732–12748. doi:10.1021/acsnano.0c03064
8. Estébanez B, Jiménez-Pavón D, Huang C-J, Cuevas MJ, González-Gallego J. Effects of exercise on exosome release and cargo in in vivo and ex vivo models: a systematic review. J Cell Physiol. 2021;236:3336–3353. doi:10.1002/jcp.30094
9. Noonin C, Thongboonkerd V. Exosome-inflammasome crosstalk and their roles in inflammatory responses. Theranostics. 2021;11:4436–4451. doi:10.7150/thno.54004
10. Shi A, Li J, Qiu X, et al. TGF-β loaded exosome enhances ischemic wound healing in vitro and in vivo. Theranostics. 2021;11:6616–6631. doi:10.7150/thno.57701
11. Bieber T. Atopic dermatitis. Ann Dermatol. 2010;22:125–137. doi:10.5021/ad.2010.22.2.125
12. Silverberg JI, Simpson EL. Associations of childhood eczema severity: a US population-based study. Dermatitis. 2014;25:107–114. doi:10.1097/DER.0000000000000034
13. Tsakok T, Woolf R, Smith CH, Weidinger S, Flohr C. Atopic dermatitis: the skin barrier and beyond. Br J Dermatol. 2019;180:464–474. doi:10.1111/bjd.16934
14. De Benedetto A, Rafaels NM, McGirt LY, et al. Tight junction defects in patients with atopic dermatitis. J Allergy Clin Immunol. 2011;127:
15. Patrick GJ, Archer NK, Miller LS. Which way do we go? complex interactions in atopic dermatitis pathogenesis. J Invest Dermatol. 2021;141:274–284. doi:10.1016/j.jid.2020.07.006
16. Leonard A, Wang J, Yu L, et al. Atopic dermatitis endotypes based on allergen sensitization, reactivity to staphylococcus aureus antigens, and underlying systemic inflammation. J Allergy Clin Immunol Pract. 2020;8:236–247.e3. doi:10.1016/j.jaip.2019.08.013
17. Gao X, Ran N, Dong X, et al. Anchor peptide captures, targets, and loads exosomes of diverse origins for diagnostics and therapy. Sci Transl Med. 2018;10:eaat0195. doi:10.1126/scitranslmed.aat0195
18. Wang L, Yu Z, Wan S, et al. Exosomes derived from dendritic cells treated with schistosoma japonicum soluble egg antigen attenuate DSS-induced colitis. Front Pharmacol. 2017;8:651. doi:10.3389/fphar.2017.00651
19. Su H, Rustam YH, Masters CL, et al. Characterization of brain-derived extracellular vesicle lipids in Alzheimer’s disease. J Extracell Vesicles. 2021;10:e12089. doi:10.1002/jev2.12089
20. Wozniak AL, Adams A, King KE, et al. The RNA binding protein FMR1 controls selective exosomal miRNA cargo loading during inflammation. J Cell Biol. 2020;219:e201912074. doi:10.1083/jcb.201912074
21. Pei W, Li X, Bi R, et al. Exosome membrane-modified M2 macrophages targeted nanomedicine: treatment for allergic asthma. J Control Release. 2021;338:253–267. doi:10.1016/j.jconrel.2021.08.024
22. Riazifar M, Mohammadi MR, Pone EJ, et al. Stem cell-derived exosomes as nanotherapeutics for autoimmune and neurodegenerative disorders. ACS Nano. 2019;13:6670–6688. doi:10.1021/acsnano.9b01004
23. Zhang C, Zhu Z, Gao J, et al. Plasma exosomal miR-375-3p regulates mitochondria-dependent keratinocyte apoptosis by targeting XIAP in severe drug-induced skin reactions. Sci Transl Med. 2020;12:eaaw6142. doi:10.1126/scitranslmed.aaw6142
24. Shao S, Fang H, Zhang J, et al. Neutrophil exosomes enhance the skin autoinflammation in generalized pustular psoriasis via activating keratinocytes. FASEB j. 2019;33:6813–6828. doi:10.1096/fj.201802090RR
25. Chieosilapatham P, Kiatsurayanon C, Umehara Y, et al. Keratinocytes: innate immune cells in atopic dermatitis. Clin Exp Immunol. 2021;204:296–309. doi:10.1111/cei.13575
26. Yosipovitch G, Berger T, Fassett MS. Neuroimmune interactions in chronic itch of atopic dermatitis. J Eur Acad Dermatol Venereol. 2020;34:239–250. doi:10.1111/jdv.15973
27. Wilson SR, Thé L, Batia LM, et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell. 2013;155:285–295. doi:10.1016/j.cell.2013.08.057
28. Imai Y, Yasuda K, Nagai M, et al. IL-33-induced atopic dermatitis-like inflammation in mice is mediated by group 2 innate lymphoid cells in concert with basophils. J Invest Dermatol. 2019;139:2185–2194.e3. doi:10.1016/j.jid.2019.04.016
29. Zheng J, Yao L, Zhou Y, et al. A novel function of NLRP3 independent of inflammasome as a key transcription factor of IL-33 in epithelial cells of atopic dermatitis. Cell Death Dis. 2021;12:871. doi:10.1038/s41419-021-04159-9
30. Salimi M, Barlow JL, Saunders SP, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013;210:2939–2950. doi:10.1084/jem.20130351
31. Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005;17:359–365. doi:10.1016/j.coi.2005.06.002
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.