Back to Journals » ClinicoEconomics and Outcomes Research » Volume 14
Planning and Budgeting of Medical Devices Among Ethiopian Public Hospitals
Authors Gamessa TW , Abebe ST , Abate LD, Abo MK, Mekonnen AA , Tadesse ZK , Woyesa AF, Obse RB, Ibrahim MA, Simegn GL
Received 21 February 2022
Accepted for publication 13 May 2022
Published 19 May 2022 Volume 2022:14 Pages 405—413
DOI https://doi.org/10.2147/CEOR.S363376
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Samer Hamidi
Tadesse Waktola Gamessa,1 Samuel Tadesse Abebe,1 Lemlem Degafu Abate,1 Megersa Kebede Abo,1 Alemu Abibi Mekonnen,1 Zerihun Ketema Tadesse,1 Addisu Fayera Woyesa,1 Regasa Bayisa Obse,1 Mahdi Abdella Ibrahim,1 Gizeaddis Lamesgin Simegn2
1Pharmaceticals and Medical Equipment Directorate, Ministry of Health, Addis Ababa, Ethiopia; 2School of Biomedical Engineering, Jimma Institute of Technology, Jimma University, Jimma, Ethiopia
Correspondence: Tadesse Waktola Gamessa, Pharmaceuticals and Medical Equipment Directorate, Ministry of Health, Addis Ababa, Ethiopia, Tel +251910201915, Email [email protected]
Background: Planning and budgeting of medical devices allow a healthcare institution to properly use funds, acquire quality and efficient medical devices, and improve healthcare service delivery. The lack of proper policy in the procurement and management of medical devices causes inappropriate usage of funds and impedes the quality of a product. This study aimed to identify the current practices and gaps in the planning and budgeting of medical devices in Ethiopian public hospitals. In this study, an assessment was conducted in all regional public hospitals to assess the current status of medical device management, identify the gaps, and provide suggestions for areas of improvement.
Methods: A descriptive cross-sectional design was used for the study assessment where a structured data collection tool was utilized to collect data. A multi-stage stratified random sampling proportionate to size technique was employed for the sampling of public hospitals in all regions of Ethiopia. The collected data was analyzed using SPSS version 26 software.
Results: The availability of medical equipment development plans, budgeting, and spare parts procurement plans were found to be below 50% in public hospitals. It was also noted that 40.3% of hospitals do not prepare medical device technical specifications during procurement orders. Moreover, the engagement of biomedical engineers/technicians in the planning and procurement of medical devices was found to be below 50%.
Conclusion: This assessment showed that there is a need for improvement in the development of procurement plans and preparation of technical specifications for medical devices in Ethiopian public hospitals. Developing policies and strategies for the proper use of funds in the procurement of medical devices, involving biomedical engineering professionals in the planning, procurement and use of medical devices could help to improve the quality, optimized utilization and efficiency of medical devices and ultimately enhance healthcare service delivery.
Keywords: biomedical engineering professionals, budgeting, medical device, planning, public hospitals
Introduction
A medical device is a substantial resource in the health care delivery system.1,2 It is an essential asset in the prevention, diagnosis, treatment, and monitoring of illnesses and diseases, and rehabilitation of patients.3–5 The medical device is a subset of healthcare technology whose management cycle runs from planning, assessment, and budgeting to decommissioning and disposal.3,6,7 Medical devices require careful planning of recurrent operating budgets for sustainable operation.8 Appropriate policies, guidelines, and strategies are needed at each level of healthcare delivery for proper management and use of medical devices.9 According to World Health Organization (WHO) recommendation, policies and strategies are required for medical device management as part of any national health plan.10,11 Medical device policies and strategies are important to ensure access to safe, effective, and high-quality medical devices with the ultimate goal of improving healthcare systems and service delivery.12–14 In this regard, the Ethiopian Health Services Guideline (EHSTG II) indicates that each health facility should have policies, guidelines, and development plans for medical device procurement, acquisition, installation, maintenance, and disposal.15
Planning and assessment are the first main phases of the healthcare technology management cycle followed by budgeting and financing for medical devices.8,16,17 As part of planning and assessment, the EHSTG II recommends the hospitals establish a model medical equipment list for their essential healthcare services . The model medical equipment list describes the types and quantities of medical equipment required by the hospital to provide each service with minimum requirements as per national standards. WHO also recommends the health sectors develop core medical equipment and technology lists that are commonly considered important or necessary for specific preventive, diagnostic, treatment, or rehabilitation procedures.18,19 WHO has also developed the list of Essential In Vitro Diagnostics (IVDs) and provided recommendations to incorporate priority medical devices for procurement, reimbursement, and for supplementing universal health coverage.20 The IVDs help the success of universal health coverage by improving “access to good-quality, affordable in vitro diagnostics (IVDs) that allows health providers to make timely diagnoses and offer the most appropriate treatment.21 Besides, the healthcare facilities shall have medical equipment development plan (EDP) based on the model equipment list and medical equipment inventory, which is a plan to define goals for acquisition, maintenance, and replacement of equipment in the short term and long term.22 The medical equipment development plan helps the overall planning and budgeting of medical devices.
To enhance and devise a system for improving medical device management at the national level, the existing gaps and challenges at hospital levels should be identified. Even though the Ministry of Health (MOH) of Ethiopia continuously supports the health facilities with the supply and management of medical devices, there is no evidence-based information and data that indicate the gaps and problems of public health facilities. In this regard, MOH has conducted a national medical device baseline survey in 82 hospitals in 2020. This survey was limited to collecting and addressing only a few medical device indicators (availability of functional medical devices, percentage of health facilities with updated medical device inventory, percentage of health facilities with scheduled preventive maintenance, percentage of health facilities with functional medical equipment management committee, percentage of installed medical device, availability of medical device as per national standard and percentage of biomedical positions filled at health facilities).23
To find the underlying gaps in the current practices of public hospitals in planning and budgeting medical devices and make recommendations on areas of improvement, it was important to conduct this assessment. Hence, a comprehensive study to assess the status and practices of the medical device in health facilities that help devise evidence-based decisions and systems is required. In this area, the authors could not find any published literature regarding the planning and budgeting of medical devices in Ethiopian public hospitals at a national level. Therefore, this study identified the existing practices, gaps, and challenges in the planning and budgeting of medical devices and recommended potential areas of improvement and interventions.
Methods
Data Collection Methods
A descriptive cross-sectional design was used for this study. A structured data collection tool was used to collect the data. The descriptive data assessment was used to explore the availability of medical device policies, guidelines, strategies, development plans, annual plans, budgets, and involvement of Biomedical engineers/technicians in medical device planning and technical specification in public hospitals.
Sampling Technique and Sampling Distribution
For this assessment, all Ethiopian regions and the two city councils were included. A multi-stage stratified random sampling proportionate to size technique was employed. Stratifications were based on the level of public hospitals (Primary, General, and Referral). The proportion of facilities at different levels was determined using the maximum number of the population expected to be served according to the Ethiopian Health Sector Transformation Plan, 2015/16-2019/20. Accordingly, 14 referrals, 26 general and 23 primary hospitals were included. Regional distribution was made based on proportionate to the number of facilities available in each region and city council.
Measurements and Analysis
The analysis explores the availability and practices of public hospitals on medical device planning, budgeting, and technical specification during procurement. The percentage and frequency of the collected data were analyzed using SPSS version 26 software.
Data Quality Assurance Process
The data collection team developed an assessment methodology, which includes the scope, sampling, design, and data collection instruments. 9 data collectors and 3 supervisors were recruited and trained on the data collection instrument and assessment approach. All of them were Biomedical Engineers who had subject matter expertise and experience in the field of data collection. The supervisors supported data collectors to supervise spot-checking and overall field data collection. When data collectors encounter any challenges and questions, the supervisors were at their disposal to address the issues. The assessment core team checked the collected data for clarity, completeness, and appropriateness. The team also verified the accuracy of records by checking unexpected results or outliers. The field data collection was conducted from December 2019 to January 2020.
Results
Biomedical Engineering Structures
The assessment report identified that there are four different structures available at public hospitals; directorate, case team, department, and unit. Among the assessed hospitals, 19 (30%) hospitals reported that they do not have any organizational structure for biomedical engineering. Despite the availability of various structures at hospitals, there are no clear structural distinctions between them regarding human resources, organogram, and responsibilities. The available biomedical engineering structures are illustrated in Figure 1.
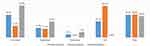 |
Figure 1 Biomedical engineering structures available at public hospitals. |
Availability of Medical Device Policies and Strategies
The assessment results showed that hospital-specific medical device policies and strategies are available in 79.4% of public hospitals at a national level. Most of the general hospitals (88.5%) reported the availability of policies and strategies followed by referral hospitals (85.7%) and primary hospitals with the least availability (65.2%). Despite the availability of medical device policies and strategies in the assessed hospitals, there is no medical devices national policy and strategy that the authors could know at the time of the assessment. The detailed results of medical device management policies and strategies in public hospitals are illustrated in Figure 2.
 |
Figure 2 Percentage of public hospitals that have medical device management policies and strategies. |
Availability of Medical Equipment Development Plans
At a national level, less than 50% of public hospitals were found to have a medical equipment development plan. Referral hospitals take the majority (71.4%) followed by general hospitals (46.2%) by having medical device development plans. Only 39.1% of the primary hospitals were found to have a development plan. Medical equipment development plan helps to define goals for acquisition, maintenance, and equipment replacement in the short and long terms. Figure 3 demonstrates the assessment results for the availability of medical equipment development plans in public hospitals.
 |
Figure 3 Percentage of public hospitals that have medical device development. |
Availability of Medical Device Procurement Planning
At the national level, the percentage of public hospitals with a planning system for medical device procurement was found to be 54%. About 50% of referrals, 53.8% of the general, and 56.5% of primary hospitals have medical device procurement plans. It is highly recommended for any hospital to have need-based assessment and procurement plans to run the essential healthcare services. Figure 4 shows the summarized assessment results for the availability of medical device procurement planning.
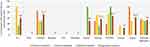 |
Figure 4 Availability of medical device planning system for procurement in public hospitals. |
Availability of Medical Device Procurement Budgeting
Only 49.2% of hospitals reported to have a regular budget for procuring medical devices at a national level. In this regard, referral hospitals (57.1%) have better budget allocation followed by primary hospitals (52.2%) and general hospitals (42.3%). Before and during the purchase of medical devices, it is important to have a budgeted plan and capital that enable the equipment to be used through its life span. The assessment results for medical device budgeting in Ethiopian public hospitals are demonstrated in Figure 5.
 |
Figure 5 Percentages of public hospitals that have a dedicated budget for procurement of medical device. |
Magnitude of Biomedical Engineering Professionals Involved in Planning and Procurement of Medical Device
Biomedical engineering (BME) professionals are health professionals who are responsible for medical device overall management in healthcare institutions. However, the assessment indicated that only 49.2% of public hospitals involve them in medical device planning, budgeting, and procurement. Figure 6 demonstrates the assessment results in public hospitals.
 |
Figure 6 Percentage of public hospitals that involve biomedical engineers/technicians in planning, budgeting, and procurement of medical device. |
Magnitude of Biomedical Engineering Professionals Involved in the Development of Medical Device Specification
According to our assessment, only 58.7% of public hospitals prepare medical device specifications during procurement; 92.9% of referrals, 65.4% of general, and 30.4% of primary hospitals prepare medical device specifications and involve BME professionals in their procurement plan. All hospitals in Addis Ababa and Harari have specification trends for procuring medical devices and participate in BME professionals in the specification of the medical device. Hospitals in Tigray, Oromia, and SNNPR have a relatively better experience than other regions in this regard. The hospitals’ experience in specification development is shown in Figure 7. The report shows that BME participates in the preparation of specifications in all hospitals that have developed medical device specifications. The percentage of BME professionals’ involvement in medical device specification is shown in Figure 8.
 |
Figure 7 Percentage of medical device specification experience of public hospitals. |
 |
Figure 8 Percentage of biomedical engineers and technicians’ involvement in medical device specification preparation. |
Medical Device Spare Parts and Accessories Procurement Plans
According to this national assessment, only 38.1% of public hospitals have medical device spare parts and accessories procurement plans. About 57.1% of referrals, 50% of the general, and 13% of primary hospitals have medical device spare parts and accessories procurement plans. All public hospitals in Addis Ababa have spare parts and accessories procurement plans. Spare parts and accessories are essential components required to maintain the functionality of a medical device for its life span which need proper planning and an adequate budget. The trends in medical device spare parts and accessories procurement plans in public hospitals are shown in Figure 9.
 |
Figure 9 Percentage of hospitals having medical device spare parts and accessories procurement plan. |
Discussions
This study aimed to assess the current status of the medical device management system, identify gaps, and provide areas of improvement suggestions for policymakers and managers for efficient use of the medical devices and ultimately improving service delivery in Ethiopian public hospitals. The assessment addressed the management cycle of a medical device including planning, budgeting, technical specification preparation, and procurement aspects.24,25 The roles of biomedical engineers in medical device management were also assessed. Engaging biomedical engineers in the planning and management of medical devices is important.26–29 However; the results of the assessment showed that the involvement of biomedical engineers and technicians in planning and budgeting medical devices is still very low. Even though there is no national policy for medical device management, 79.4% of public hospitals reported that they have a medical device management policy and strategy within their institution. This result indicates that hospitals develop their guiding policy and strategy for the planning and management of medical devices regardless of national policy with alignment to EHSTG II guidelines.
The medical equipment development plan (EDP) needs to be designed to address medical devices’ current stock status, need assessment, and short-term and long-term procurement plans.15 This plan is the basis for medical devices’ annual procurement and budget planning. However, only 49.2% of hospitals have developed EDP for their medical device. According to this assessment, the Ethiopian public hospitals have huge gaps in planning for medical device procurement. An appropriate procurement plan for medical device improves optimum utilization, minimizes resource loss, and ultimately enhance healthcare delivery.30,31 About 46% of public hospitals do not have a medical device annual procurement plan for their healthcare services. Planning an appropriate budget for purchase and management is very important for life long use and management of medical devices.32,33 However, the study indicated that 50.8% of hospitals do not have a dedicated budget for the purchase of a medical device.
It was also noted that 40.3% of hospitals do not prepare medical device technical specifications during procurement orders. Only the list of the medical devices to be purchased is sent to the office responsible for procurement without detailed technical requirements. These are misleading for the purchaser and affect the quality of medical devices and services at large.34 This assessment has shown that there is also a significant gap in planning for medical device spare parts procurement. To sustain the life span of the equipment and maintain it regularly, planning and budgeting for spare parts are essential.32 On contrary, the report indicated that 61.9% of public hospitals do not have any plan for medical device spare parts procurement. All public hospitals that have medical device procurement planning and technical specification reported that they actively engage biomedical engineers and technicians in planning activities.
The assessment covered the whole regions of Ethiopia, and the sampling distribution of hospitals was adequate to solicit representative data. A descriptive cross-sectional approach was used for the data collection techniques. The assessment employed data collectors that have relevant experiences in the medical device management sector. Lack of organized data in the hospitals, and retrieving data from the paper-based documentation system were challenging during data collection.
Developing and implementing proper planning and budgeting practices for the procurement and management of medical devices are required to overcome the acquisition of poor-quality medical devices, the unequal distribution of devices, unbalanced allocation of resources and to improve the quality and efficiency of the medical device for delivery of quality healthcare service. The hospitals need to make the right plans and develop technical specifications by engaging biomedical engineering professionals that significantly improve procurement practices. It is strongly recommended for hospitals to plan and budget not only for the procurement of medical devices but also for the spare parts and accessories needed to run the equipment over its life span.
Conclusions
This assessment was targeted to identify the gaps and challenges of Ethiopian public hospitals in the planning and procurement of medical devices. The assessment provides preliminary information on the availability of proper planning and budgeting for medical device procurement. The availability of medical equipment development plans, budgeting, and spare parts were found to be below 50% in public hospitals. Moreover, this assessment showed that there are no proper medical device procurement plans and technical specification preparations. Developing policies, directives, and strategies for the proper use of the medical device at the national level, and strengthening hospitals with an electronic-based medical device management information system, involving Biomedical Engineering professionals at all levels, including medical device planning, procurement, management, and usage must improve the functionality of the medical device and ultimately to improve the service delivery in health care facilities. Each public hospital should establish a strong planning system and sufficient budgeting for the procurement and management of the medical device. Hospitals must prepare customized medical device technical specifications based on the health services they provide. Though this assessment can serve as a baseline national study in medical device planning and procurement, conducting additional assessments in specific medical device management and usage procedures will be significant to get a more in-depth understanding of the underlying causes of specific deficiencies and improve decision making.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study is funded by the Ministry of Health, Ethiopia.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. Decommissioning Medical Devices, WHO Medical Device Technical Series. World Health Organization; 2019.
2. Friebel R, Molloy A, Leatherman S, Dixon J, Bauhoff S, Chalkidou K. Achieving high-quality universal health coverage: a perspective from the National Health Service in England. BMJ Glob Heal. 2018;3(6):e000944. doi:10.1136/BMJGH-2018-000944
3. World Health Organization. Global Atlas of Medical Devices. World Health Organization; 2017.
4. Sabet Sarvestani A, Sienko KH, Sprumont D, Sankoh O, Tanner M, Elger B. Medical device landscape for communicable and non-communicable diseases in low-income countries. Global Health. 2018;14(1):1–6. doi:10.1186/S12992-018-0355-8/FIGURES/2
5. M. Medicines and Healthcare Products Regulatory Agency. Devices in practice - checklists for using medical devices; 2014.
6. Anderson DM, Cronk R, Best L, et al. Budgeting for environmental health services in healthcare facilities: a ten-step model for planning and costing. Int J Environ Res Public Health. 2020;17(6):2075. doi:10.3390/IJERPH17062075
7. Marks IH, Thomas H, Bakhet M, Fitzgerald E. Medical equipment donation in low-resource settings: a review of the literature and guidelines for surgery and anaesthesia in low-income and middle-income countries. BMJ Glob Heal. 2019;4(5):e001785. doi:10.1136/BMJGH-2019-001785
8. Temple-bird C, Kawohl W, Lenel A, Temple-bird C. Guide 2 how to plan and budget for your healthcare technology; 2005.
9. K. International. Medical devices 2030: making a power play to avoid the commodity trap; 2018.
10. World Health Organization. Development of Medical Device Policies WHO Medical Device Technical Series. World Health Organization; 2011.
11. Marešová P, Klímová B, Honegr J, Kuča K, Ibrahim WNH, Selamat A. Medical device development process, and associated risks and legislative aspects-systematic review. Front Public Heal. 2020;8:308. doi:10.3389/FPUBH.2020.00308/BIBTEX
12. C. and I. N. F. Trust. Medical devices policy; 2021.
13. Lingg M, Mansilla AD, Durán-Arenas L, Wyss K. The regulation, assessment, and management of medical devices in Mexico: how do they shape the quality of delivered healthcare? Saf Heal. 2017;3(1):1–10. doi:10.1186/S40886-017-0055-8
14. Zamzam AH, Abdul Wahab AK, Azizan MM, Satapathy SC, Lai KW, Hasikin K. A systematic review of medical equipment reliability assessment in improving the quality of healthcare services. Front Public Heal. 2021;9:1426. doi:10.3389/FPUBH.2021.753951/BIBTEX
15. E. Ministry of Health. Ethiopian hospital services transformation guidelines; 2016:133.
16. Bagherpour M, Erjaee A. The role of project management office in public health: a new approach for establishment in Iran. Iran J Public Health. 2017;46(3):433–434.
17. Corciovă C, Andriţoi D, Luca C. A modern approach for maintenance prioritization of medical equipment. Oper Manag-Emerg Trend Digit Era. 2020. doi:10.5772/INTECHOPEN.92706
18. World Health Organization. Core Medical Equipment. World Health Organization; 2011:59.
19. World Health Organization. Interagency List of Priority Medical Devices for Essential Interventions for Reproductive, Maternal, Newborn and Child Health. World Health Organization; 2016.
20. World Health Organization. First WHO Model List of Essential in vitro Diagnostics; 2019.
21. World Health Organization. Priority Medical Devices List for the COVID-19 Response and Associated Technical Specifications: Interim Guidance, 19 November 2020. World Health Organization; 2020.
22. E. Ministry of Health. Ethiopian Hospital Services, Transformation Guidelines, Vol. 1. USAID; 2016:133.
23. Federal Ministry of Health. National pharmacy service, pharmaceuticals supply chain and medical device management monitoring and evaluation framework; 2019:68.
24. Kirwin E, Round J, Bond K, McCabe C. A conceptual framework for life-cycle health technology assessment. Value Heal. 2022. doi:10.1016/J.JVAL.2021.11.1373
25. Gutiérrez-Ibarluzea I, Chiumente M, Dauben HP. The life cycle of health technologies. Challenges and ways forward. Front Pharmacol. 2017;8:14. doi:10.3389/FPHAR.2017.00014
26. David Y, Jahnke EG. Planning medical technology management in a hospital. Glob Clin Eng J. 2018;(1):23–32. doi:10.31354/GLOBALCE.V0I1.23
27. World Health Organization. Human Resources for Medical Devices: The Role of Biomedical Engineers. World Health Organization; 2017.
28. Arab-Zozani M, Imani A, Doshmangir L, Dalal K, Bahreini R. Assessment of medical equipment maintenance management: proposed checklist using Iranian experience. Biomed Eng Online. 2021;20(1):1–23. doi:10.1186/S12938-021-00885-5/TABLES/1
29. Bui LM, Thi Thu Phung H, Ho Thi T-T, et al. Recent findings and applications of biomedical engineering for COVID-19 diagnosis: a critical review. Bioengineered. 2021;12(1):8594–8613. doi:10.1080/21655979.2021.1987821
30. Diaconu K, Chen YF, Cummins C, Jimenez Moyao G, Manaseki-Holland S, Lilford R. Methods for medical device and equipment procurement and prioritization within low- and middle-income countries: findings of a systematic literature review. Global Health. 2017;13(1):1–16. doi:10.1186/S12992-017-0280-2/TABLES/8
31. Moyimane MB, Matlala SF, Kekana MP. Experiences of nurses on the critical shortage of medical equipment at a rural district hospital in South Africa: a qualitative study. Pan Afr Med J. 2017;28. doi:10.11604/PAMJ.2017.28.100.11641
32. Bektemur G, Muzoglu N, Arici MA, Karaaslan MK. Cost analysis of medical device spare parts. Pakistan J Med Sci. 2018;34(2):472. doi:10.12669/PJMS.342.14245
33. Desmond S, Gavaghan M, Howe T, et al. A systematic assessment of budget impact models for medical devices. Value Heal. 2018;21:S179. doi:10.1016/J.JVAL.2018.04.1191
34. Sumana G, Aswal DK, Aswal DK. Importance of standards in biomedical device and its role in strengthening the healthcare sector. Front Nanotechnol. 2021;3:27. doi:10.3389/FNANO.2021.622804/BIBTEX
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
