Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Placenta-Derived Exosomes and Gestational Diabetes Mellitus
Received 27 February 2022
Accepted for publication 21 April 2022
Published 4 May 2022 Volume 2022:15 Pages 1391—1404
DOI https://doi.org/10.2147/DMSO.S363226
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Xuemin Liu, Hui Qiu
Department of Obstetrics and Gynecology, Shengjing Hospital of China Medical University, Shenyang, Liaoning Province, 110004, People’s Republic of China
Correspondence: Hui Qiu, Email [email protected]
Abstract: Gestational diabetes mellitus (GDM) refers to different degrees of glucose metabolism abnormalities that occur or be first discovered during pregnancy. It is closely related to many adverse pregnancy outcomes. Placenta-specific exosomes are one kind of extracellular vesicles which are only produced by the placenta. These exosomes participate in many physiological and pathological processes of the body through the contained RNA, lipids, proteins, and DNA. In gestational diabetes, the placental exosomes play an important role in the occurrence and development of gestational diabetes through regulating insulin resistance, inflammatory factors, and endothelial cell dysfunction. In this review, we will discuss the generation, changes, and mechanism of placenta-specific exosomes in GDM, as well as their prospects as a predictive and therapeutic target for gestational diabetes.
Keywords: pregnancy, gestational diabetes, exosome, placenta
Introduction
Gestational Diabetes Mellitus (GDM) refers to different degrees of glucose metabolism abnormalities that occur or be first discovered during pregnancy.1 It is generally considered to be insulin resistance caused by pancreatic β-cell dysfunction.2 The frequency of gestational diabetes mellitus at collaborating centers was 17.8% according to the International Association of Diabetes in Pregnancy Study Group criteria.3 GDM not only increases the risk of gestational hypertension, premature delivery, premature rupture of membranes, fetal malformations, dystocia, cesarean section, etc., but also increases the risk of metabolic syndrome, type 2 diabetes, and cardiovascular disease in offspring.4–6 Early diagnosis, intervention, and treatment can improve the prognosis of gestational diabetes. At present, the pathogenesis of gestational diabetes is not fully understood. Possible mechanisms include genetics, insulin resistance, autoimmunity, and the release of inflammatory factors.7–9 Clarifying the pathogenesis of gestational diabetes is of great significance to its diagnosis and treatment. During pregnancy, the placenta participates in the regulation of the endocrine system by secreting a variety of substances including exosomes. The role of placenta-derived exosomes in the etiology and progression of complications of pregnancy is still in a formative stage. This article summarizes the research progress of placenta-derived exosomes and GDM for providing new ideas for the pathogenesis and prevention of GDM.
Placenta and GDM
The placenta is an important organ for the exchange of substances between the fetus and the mother. The placental barrier can also shield the fetus from harmful substances; In addition, the placenta synthesizes a variety of enzymes, hormones, and cytokines to maintain normal pregnancy.10 It is estimated that 69% of all human proteins are expressed in the placenta and most of them are associated with pregnancy, estrogen biosynthetic, and metabolic pathways.11 During pregnancy, the placenta secretes many insulin-resistant hormones such as placental prolactin, estrogen, progesterone, and adrenal cortex hormones.12 As pregnancy progresses, the secretion of placental hormones gradually increases, making pregnancy a physiological insulin resistance state. Glucose metabolism is characterized by increased glycogen production and decreased utilization of glucose by tissues, which smooths the transfer of nutrition to the fetus.13–16 When facing insulin resistance, normal pregnant women can secrete enough insulin to supply the body’s needs, while a small number of pregnant women with defective β cell function cannot secrete so much insulin, unable to overcome insulin resistance, leading to gestational diabetes. At the same time, maternal hyperglycemia affects the structure of the placenta and the distribution of blood vessels and increases maternal and fetal complications.17–20 Therefore, the placenta plays an important role in the occurrence and development of gestational diabetes.
Exosomes
Exosomes are one kind of extracellular vesicles (EVs). Extracellular vesicles are derived from membranes in almost all cells and participate in the regulation of human functions through the contained RNA, lipids, proteins, and DNA. Based on their source, morphology, and the way they are released into the extracellular environment, extracellular vesicles are divided into three types including apoptotic bodies, exosomes, and microvesicles (MV).21,22 Exosomes are 50–120 nm in diameter and can uniquely reflect the phenotype of their parent cell. They were first discovered in 1981 by Trams et al.23 In 1984, Harding and Stahl described the release of small vesicles and tubules from rat reticulocytes.24 Exosomes are present in almost all biological fluids such as blood, saliva, lymph, amniotic fluid, milk, lachrymal, and mammary gland secretions etc.25 Methods to isolate exosomes include differential centrifugation, density gradient centrifugation, size exclusion chromatography, filtration, polymer-based precipitation, immunological separation, and isolation by sieving.26–28 As an important intercellular signal transduction carrier, exosomes work by interacting with the recipient cells.29 After being taken up, exosomes release their contents into the new host cells and exert their biological functions. The endocytosis process is achieved through phagocytosis or receptor and raft-mediated endocytosis.30–33 Adhesion is another form of interaction between exosomes and target cells, which is facilitated by the transmembrane proteins on the surface of the exosomes.34,35 Through these methods, exosomes participate in the regulation of a variety of normal physiology and diseases.
Placenta-Derived Exosomes (PdE)
Many exosomes are only expressed in the human placenta, the so-called placenta-derived exosomes. These placenta-derived exosomes carry signals in the form of RNA,36,37 proteins,38–40 lipids,41 and DNA.42 During pregnancy, exosomes are released from the placenta into the maternal blood circulation and participate in placental development and maternal immune tolerance.CD63 is a widely accepted exosomal marker. Placenta-derived exosomes can be differentiated from other exosomes by the presence of placenta-specific proteins or miRNAs such as placental alkaline phosphatase (PLAP).43 The total amount and the specific placenta-derived exosomes could be determined by quantum dots coupled with CD63 and PLAP antibodies, respectively.44 The exosomes are identified in maternal plasma as early as 6 weeks of pregnancy. The number of exosomes and the concentration of placenta-derived exosomes in maternal circulation increased significantly with the progression of pregnancy, with maximum numbers at term.45–47 Some miRNA clusters are specific to trophoblasts.36,48 The placenta-specific miRNA clusters are C19MC and C14MC. Morales-Prieto et al37 explored that 34 of 46 miRNAs belonging to C14MC were downregulated in the third-trimester trophoblasts, while 46 out of 47 miRNAs belonging to C19MC were upregulated. The known function of placental exosomes in normal pregnancy are as follows:
Maternal-Fetal Communication
High levels of miRNAs can be found in the maternal blood during pregnancy and rapid decline in the first 24h postpartum, which suggests that there is a miRNA-based maternal-fetal communication.49,50 One study showed that placental miRNA can traffic to the maternal circulation with compartment-specific expression and that maternal miRNA can traffic to the placenta and even into the fetal compartment.51 Exosomal trafficking and function were also demonstrated by injecting fetal cell-derived fluorescently labeled exosomes into pregnant mice and by using genetically engineered mice in which fetal and maternal exosomes could be distinguished.52,53 These studies suggest that miRNAs are involved in the communication between the placenta and the fetal-maternal compartment.
Transfer Gene Information to Target Cells
Exosomes include many kinds of molecules, of which miRNAs are the most concerned. It is estimated that more than half of human gene expression is regulated by miRNAs.54 C19MC-derived miRNAs are expressed in human placental trophoblasts and secreted into the maternal circulation via exosomes where they can target maternal tissues.55,56 Fetal exosomes can also reach maternal gestational tissues. Foetal lung-derived C4BPA plays a role in birth timing determination. C4BPA can bind to CD40 of placental villous trophoblast to promote p100 processing to p52 and then activate the NF-κB pathway in the placenta, which contribute to the timing of birth.57,58 Bioinformatic analysis suggests that MIR517A is possibly participating in tumor necrosis factor-mediated signaling.56 The exosomal miR-512-3p participates in human trophoblast functions by targeting PPP3R1, encoding a regulatory subunit of calcineurin.59 BeWo exosomal miR-517a-3p was internalized into Jurkat cells and subsequently suppressed the expression of PRKG1 in recipient Jurkat cells.60 Besides miRNAs, transfer RNAs (tRNA) have been identified in syncytiotrophoblast-derived extracellular vesicles which alter gene expression in target cells. Most tRNAs within syncytiotrophoblast extracellular vesicles were 5’-tRNA halves.61 This suggests a novel mechanism for maternal-fetal signaling in normal pregnancy.
Inhibition of Maternal Immune Tolerance
During pregnancy, the maternal immune system needs to be in a state of tolerance to maintain the survival and growth of trophoblasts. The placenta-derived exosomes are immunosuppressive and promote fetal allograft survival by influencing many mechanisms.62 Decidual macrophages play an important role in this process. Trophoblasts produce a variety of components to induce decidual macrophages to differentiate into M2 types. Trophoblast-derived exosomes increased monocyte migration and produced large amounts of cytokines such as interleukin (IL)-1β, IL-6, Serpin-E1, granulocyte colony-stimulating factor, granulocyte/ monocyte colony-stimulating factor, and TNF-α.63 Fas-induced T cell apoptosis is the main mechanism of immune tolerance. Exosomes from term-delivering pregnancies are significantly higher and exhibit greater suppression of CD3-zeta and JAK3.64 These placenta-derived membrane fragment isolates are capable of inducing FasL-mediated apoptosis and down-regulating CD3-zeta expression, which may contribute to the immune tolerance of the fetu.65,66 Placental exosomes carrying Fas ligand (FasL) and TRAIL mediate the immune privilege of the fetus by transmitting apoptosis signals during pregnancy.67 The increase in th2 cell secretion and the decrease in th1 cytokine secretion can also protect the fetus from the maternal immune system. Exosomes derived from villous cytotrophoblasts (VCT) reduced the production of Th1 cytokines in PBMCs, which was mediated by exosome-associated syncytin-2.68 NK cell receptor NKG2D was expressed and secreted via placental exosomes, which down-modulated the cognate receptor expression and might be a possible fetal immune escape mechanism.69,70 These results indicate that trophoblast-derived exosomes play an important role in maternal adaptation to pregnancy and fetal immune tolerance.
Regulate Angiogenesis and Endothelial Cell Migration
Remodeling of uterine spiral arteries by extravillous trophoblast cells is fundamental for pregnancy. This process requires invasion and differentiation of trophoblast cell.71,72 Placenta-derived exosomes contain biologically active proteins that can interact with the maternal endothelium and regulate their function.73,74 These exosomes can also induce extravillous cytotrophoblast cell invasion and proliferation in a time-and dose-dependent manner.46 It has been reported that trophoblast-derived MMP-12 mediates elastolysis, induces disruption of uterine vascular smooth muscle cell architecture, and favors extravillous trophoblast invasion during uterine spiral artery remodeling.75,76 Exosomes derived from human term placental tissue mesenchymal stem cells stimulated both endothelial tube formation and migration and enhanced angiogenesis-related gene expression.77 These changes induced by exosomes are critical for the normal growth and development of the fetus.
Placental Barrier
Placenta is the primary barrier between the maternal and fetus, which can protect the fetus from virus infection in the mother’s body.78 The specific mechanism is not fully elucidated. The exosomes produced and released by placental trophoblasts play an important role.79 The microRNAs contained in placental exosomes restrict viral infections in autocrine and paracrine manners without depending on type III IFN signaling.80–83 Autophagy is a conserved vacuole/lysosome-mediated degradation pathway for clearing and recycling cellular components, which is involved in limiting inflammation signals upon virus invasion.84–86 When the placenta is infected with a certain type of virus, placental trophoblasts release a large number of exosomes, which deliver their miRNAs to maternal, fetal, or placental cells to alter gene expression, eventually inducing autophagy, followed by virus degradation.87–89 At least three members of the C19MC family (miR517-3p, miR516b-5p, miR512-3p) exhibit these potent antiviral effects against RNA and DNA viruses through strongly inducing autophagy.80 Based on this antiviral feature, vitro-constructed miRNAs have been used as a vaccine or therapeutic target against SARS-CoV-2.90,91
Changes and Mechanisms of pdE in GDM
It was reported that the plasma concentration of total and placenta-derived exosomes was higher in GDM compared with the normal pregnancy matched by gestational age even during early pregnancy. However, the ratio of placental exosomes to total exosomes was lower in GDM pregnancies.92 Hyperglycemia and hypoxia are risk factors for metabolic complications during pregnancy. In order to test the effect of extracellular glucose concentration on exosomal signaling, first-trimester primary trophoblast cells were incubated under different concentrations of glucose and oxygen. The results showed that glucose (25 mM) significantly increased the release of exosomes from trophoblast cells at all oxygen tensions tested. The released exosomes significantly increased the release of all cytokines from human umbilical vein endothelial cells except IL-2 and IL-10.93 High glucose increased the release of exosomes from HUVECs, and the increased exosomes mimicked some of the effects of high glucose.94 Hypoxia (ie 1% O2) promotes the release and activity of cytotrophoblast- exosomes, thereby promoting extravillous trophoblasts invasion and proliferation.46,95 Hypoxia also increases the release of exosomes from placental mesenchymal stem cells, placental microvascular endothelial cells migration, and tube formation.96 These changes may contribute to placental vascular adaptation to low oxygen tension under both physiological and pathological conditions. The number of exosomes in the maternal blood circulation is closely related to BMI. 12–25% of exosomes in the maternal circulation come from the placenta. The contribution of placental exosomes to the total exosomal population decreases with higher maternal BMI across gestation. This study established that maternal BMI is an important factor affecting exosomal changes.97 Obesity increases the expression of some exosomal miRNAs in mice, including miR-192, miR-122, miR-27a-3p, and miR-27b-3p. Exosomes isolated from obese mice induce glucose intolerance and insulin resistance in lean mice.98 Adipose tissue exosomes in GDM increased the expression of glucose metabolism-related genes in placental cells. The up-regulated genes are associated with glycolysis, gluconeogenesis, glycogen production, and degradation. These suggested adipose tissue mediated the changes in placental function in GDM by inducing placental exosomes.99 The studies of placental exosomes in GDM are shown in Table 1.
 |
Table 1 The Studies of Placental Exosomes in Gestational Diabetes Mellitus |
Functions and Mechanisms of pdE in GDM
Exosomes contain numerous RNAs and transfer them between cells or organs, thereby establishing intercellular or interorgan communication. It has been identified that many mRNAs, lncRNA, and circRNAs are differentially expressed in umbilical cord blood exosomes of GDM patients.100 Bioinformatic analysis showed that the exosomal proteins in GDM are mainly associated with energy production, inflammation, and metabolism.101 The protein-protein interaction network revealed that the differentially expressed mRNAs were associated with the glucagon signaling pathway. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) biological pathway analyses demonstrated that exosomal circRNAs and lncRNAs parental genes are involved in the regulation of the metabolic process, growth, and development were significantly enriched in umbilical cord blood of GDM. Most of the exosomal circRNAs and lncRNAs harbored GDM-related microRNA binding sites. These results showed that exosomal mRNAs, lncRNAs, and circRNAs are aberrantly expressed in the umbilical cord blood of GDM patients and play potential roles in GDM development and fetus growth.102–104
Placental Exosomes and Insulin Resistance
Ten miRNAs (miR‒122-5p; miR‒132-3p; miR‒1323; miR‒136-5p; miR‒182-3p; miR‒210-3p; miR‒29a-3p; miR‒29b-3p; miR‒342-3p, and miR-520h) showed significantly higher levels in placenta of GDM cases. Bioinformatics analysis showed that these miRNAs are involved in insulin secretion/regulation and glucose transport in pregnant women.105 Exosomes isolated from placental explants from normal and GDM pregnancies show different miRNA profiles. Placental exosomes from GDM pregnancies decreased insulin-stimulated migration and glucose uptake in primary skeletal muscle cells obtained from normal pregnancies. However, placental exosomes from NGT increase migration and glucose uptake in skeletal muscle of diabetic subjects. Placental exosomes might have a role in the changes in insulin sensitivity in normal and GDM pregnancies.106 Mice infused with GDM small extracellular vesicles (sEVs) have attenuated glucose-stimulated insulin secretion, muscle basal insulin signaling, and insulin responsiveness, and are more likely to develop glucose intolerance. This result suggests sEVs can regulate maternal glucose homeostasis in pregnancy and contribute to the development of GDM.107 Tu et al108 reported that miR-409-5p was highly expressed in the serum of GDM patients and it is positively correlated with insulin resistance index (HOMA-IR). Qi et al109 found that the expression of miR-185 was down-regulated in serum and placenta of GDM patients and is negatively correlated with HOMA-IR. miR-140-3p overexpression also contributes to the defective placental IR signaling in patients with GDM.110 Dipeptidyl peptidase IV (DPPIV) can regulate glucose-dependent insulin secretion by breaking down GLP-1. DPPIV-bound syncytiotrophoblast-derived extracellular vesicles were significantly increased in the circulation of GDM pregnancies.111 The cytokine tumor necrosis factor-alpha (TNF-alpha) has been implicated in the pathogenesis of insulin resistance in Type 2 diabetes mellitus via regulating glucose, lipid metabolism, and insulin resistance.112 Placental tissues from patients with GDM release greater amounts of TNF-alpha under conditions of high glucose,113 which may be related to the placental miRNA.114 The expression of miR-143, mitochondrial complexes were significantly decreased in A2GDM (controlled by medication) placentae, while human placental lactogen levels, expression of glycolytic enzymes, GLUT1, and mTOR signaling were significantly increased compared with A1GDM (controlled by diet).115 Overexpression of miR-494 improved insulin secretion and total insulin content, while in GDM, the miR-494 level was significantly decreased.116 These results suggest that placental exosomes promote the occurrence and development of GDM by regulating insulin resistance.
Placental Exosomes and Inflammation
Placental exosomes may contribute to maternal systemic inflammation during pregnancy. The exosomes of obese women increased the release of IL-6, IL-8, and TNF-α from endothelial cells.97 Exosomes isolated from GDM pregnancies can also significantly increase the release of proinflammatory cytokines from endothelial cells.92 Utilizing the BeWo cell line and whole placental explants, Holder et al117 demonstrated that macrophage exosomes were actively transported into the human placenta and induced the placenta to release proinflammatory cytokines. Exosome-encapsulated miR-6869-5p is significantly downregulated in placenta-derived macrophages of GDM patients, which may contribute to maintaining placental microenvironment balance by preventing inflammation.118 MiR-657 can promote the generation of inflammatory cytokines (IL-6 and TNF-α) and activation of NF-κB. The expression of miR-657 was increased in patients with GDM, which contributes to the pathogenesis of GDM via the IL-37/NF-κB signaling axis.114 The expression levels of miR-875-5p are downregulated in patients with GDM.119 Fu et al120 found that miR-875-5p regulated IR and inflammation by targeting TXNRD1 in GDM rats. Trophoblast-derived exosomes have been demonstrated to induce macrophages to synthesize and release pro-inflammatory factors through fibronectin.121 Upregulation of miR-518d may contribute to the pathology of the development of GDM, via an effect on the regulation of proliferator-activated receptor-α (PPARα) expression.122 We also found that miR-518d negatively regulates the expression of PPARα and triggers the nuclear transport process of NF-κB and phosphorylation of pathway-associated proteins leading to an inflammatory response and the development of GDM.123
Placental Exosomes and Endothelial Cell Dysfunction
Placental exosomes can also regulate placental and fetal membrane endothelial dysfunction in gestational diabetes mellitus.124 High glucose increased endothelial wound healing and the expression of P~Ser1177-eNOS, hCAT-1, VEGF, and ICAM-1 by increasing the release of exosomes from HUVECs. Exosomes were isolated from HUVECs incubated with basal glucose reverted the effect of high glucose on endothelial cells.94 Rocío Salsoso found that ExN (Normal pregnancy -exosomes) and ExGDM (GDM-exosomes) cargo have differential effects in HUVECs. ExN restores GDM-reduced wound recovery but ExGDM delays wound recovery in normal pregnancies. ExN restores GDM-upregulated L-arginine/NO/p44/42mapk signaling but ExGDM increase it and ROS in normal pregnancies. Foetoplacental endothelium-derived exosomes maintain a GDM phenotype in HUVECs.125 The cell adhesion molecules (CAMs) promote attachment and trans-endothelial migration of leukocytes. Díaz-Pérez et al126 identified a reduction of ICAM-1 protein in fetoplacental endothelial cells in GDM pregnancy, which may be a kind of protection to avoid leukocyte transmigration into the placenta.MiR-101 is up-regulated by hyperglycemia and contributes to some of the defects of the umbilical cord vein (HUVECs) through the target gene EZH2 in GDM.127 These studies show that placental exosomes are associated with fetoplacental endothelial dysfunction in gestational diabetes mellitus.
Placental Exosomes and Fetus Growth
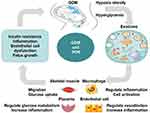
15–45% of newborns of women with gestational diabetes mellitus (GDM) are macrosomia. We know that the main cause of macrosomia in GDM is hyperglycemia and the increased insulin resistance of the mother,128 but the molecular mechanism is not very clear.MicroRNAs have been identified to regulate placental development and fetal growth.129,130 There was a significant positive correlation between the ratio of placental-derived to total exosomes and birth weight percentile. The contribution of placental exosomes to the total exosome concentration was significantly decreased in FGR cases compared to controls.131 143 miRNAs were differentially expressed in the plasma samples from pregnant women with fetal macrosomia compared with the controls.132 Li et al133 demonstrated that miR-508-3p was up-regulated and may contribute to macrosomia through enhancing the EGFR-PI3K-Akt signaling pathway. Jiang et al134 showed that the expression level of placental miR-21 was significantly upregulated in serum samples of macrosomia. High levels of miR21 expression and low levels of miR143 expression could predict the risk for macrosomia.135,136 The interaction of two miRNAs affects the risk of macrosomia through the mitogen-activated protein kinases signaling pathway. The low expression of miR-16 and miR-21 in the placenta is associated with small gestational age (SGA) status.137 MiR-17-92 clusters contribute to macrosomia development by regulating the cell cycle pathway and can also be used as a predictive biomarker for macrosomia.138 Some lncRNAs were aberrantly expressed in the umbilical cord blood from GDM macrosomia, which suggested lncRNAs might also play a role in fetal development.139 The functions and mechanisms of placenta-derived exosomes in GDM are shown in Figure 1.
PdE as Predictive Markers and Therapeutic Targets for GDM
Placental exosomes were significantly higher in pregnancies complicated by GDM than in normal pregnancies, Gestational age and pregnancy outcomes were the main factors of exosome concentration. Maternal body mass index, glucose concentration, and fetal body weight were also correlated with the concentration of placental exosomes, suggesting that exosomes may be involved in maternal metabolic adaptation to pregnancy.140,141 Rahimi et al142 detected dysregulation of Drosha, Dicer, and DGCR8 in GDM patients which are major enzymes in the miRNA biogenesis process. Therefore, they favor the hypothesis that miRNAs are involved in the development of GDM. Cao et al143 found that the expression of plasma microRNA-16-5p, −17-5p, −20a-5p from GDM women were significantly upregulated compared with non-GDM women. From these studies, it can be concluded that miRs are involved in the pathogenesis of GDM and have potential as diagnostic biomarkers for disease development.144–146 It is reported that circulating early–mid-pregnancy (range 7–23 weeks of gestation) miR-155-5p and −21-3p levels were positively associated with GDM.147 The expression levels of three miRNAs (miR-132, miR-29a, and miR-222) were significantly decreased in GDM women compared with the controls at 16–19 gestational weeks. Serum miRNAs could be candidate biomarkers for predicting GDM.148–150 First-trimester cf DNA (cDNA from placental exosome) levels are associated with GDM.151 Placental exosomes isolated from the urine of GDM women show a differential profile expression of microRNAs across gestation, suggesting that urine is a potential biological fluid for the research of pathological conditions during pregnancy.152
Given the role of miRNA in the mechanism of the occurrence and development of GDM, it is expected to use as a target to develop treatments for GDM.miR-21 can reverse high glucose and high insulin-induced IR in 3T3-L1 adipocytes, which may be a new therapeutic target for metabolic diseases.153 There are many ways to regulate miRNA levels in vivo, of which anti-miRs are the most widely used approaches.154 MiRNAs are small and comprise a known sequence, which makes anti-miRs have the potential to become a new class of drugs. At present, the research on this aspect is mostly in animal experiments, and the application of anti-miRs in gestational diabetes needs further study.
Conclusion
In gestational diabetes, hypoxia, high glucose, and BMI affect the production of placental exosomes, which in turn regulate insulin resistance, inflammatory factors, and endothelial cell dysfunction. These factors work together to promote the occurrence and development of diabetes and fetal growth and development. The diagnosis of gestational diabetes is very late, and there is still a lack of effective predictive methods. The content of placental exosomes can change in early pregnancy, and the detection of exosomes is expected to become an effective method for predicting GDM. Placental exosomes contain a variety of components. Except for mRNA, there are relatively few studies on other components of exosomes. The function and mechanism of most placental exosomes are still unclear. Clarifying the mechanism of exosomes in gestational diabetes is of great significance to its early prevention, diagnosis, and treatment.
Disclosure
We declare that we have no conflict of interest.
References
1. Kautzky-Willer A, Harreiter J, Winhofer-Stöckl Y, et al. [Gestational diabetes mellitus (Update 2019)]. Wien Klin Wochenschr. 2019;131(Suppl 1):91–102. German. doi:10.1007/s00508-018-1419-8
2. Plows JF, Stanley JL, Baker PN, Reynolds CM, Vickers MH. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19(11):3342. doi:10.3390/ijms19113342
3. Sacks DA, Hadden DR, Maresh M, et al. Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel-recommended criteria: the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Diabetes Care. 2012;35(3):526–528. doi:10.2337/dc11-1641
4. Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991–2002.
5. Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25(10):1862–1868. doi:10.2337/diacare.25.10.1862
6. Dabelea D, Pettitt DJ. Intrauterine diabetic environment confers risks for type 2 diabetes mellitus and obesity in the offspring, in addition to genetic susceptibility. J Pediatr Endocrinol Metab. 2001;14(8):1085–1091. doi:10.1515/jpem-2001-0803
7. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(Suppl1):S67–S74. doi:10.2337/dc13-S067
8. Haller-Kikkatalo K, Uibo R. Clinical recommendations for the use of islet cell autoantibodies to distinguish autoimmune and non-autoimmune gestational diabetes. Clin Rev Allergy Immunol. 2016;50(1):23–33. doi:10.1007/s12016-014-8461-8
9. Korkmazer E, Solak N. Correlation between inflammatory markers and insulin resistance in pregnancy. J Obstet Gynaecol. 2015;35(2):142–145. doi:10.3109/01443615.2014.948408
10. Gude NM, Roberts CT, Kalionis B, King RG. Growth and function of the normal human placenta. Thromb Res. 2004;114(5–6):397–407. doi:10.1016/j.thromres.2004.06.038
11. Uhlén M, Fagerberg L, Hallström BM, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419. doi:10.1126/science.1260419
12. Napso T, Yong H, Lopez-Tello J, Sferruzzi-Perri AN. The role of placental hormones in mediating maternal adaptations to support pregnancy and lactation. Front Physiol. 2018;9:1091. doi:10.3389/fphys.2018.01091
13. Constância M, Angiolini E, Sandovici I, et al. Adaptation of nutrient supply to fetal demand in the mouse involves interaction between the Igf2 gene and placental transporter systems. Proc Natl Acad Sci U S A. 2005;102(52):19219–19224. doi:10.1073/pnas.0504468103
14. Dilworth MR, Kusinski LC, Cowley E, et al. Placental-specific Igf2 knockout mice exhibit hypocalcemia and adaptive changes in placental calcium transport. Proc Natl Acad Sci U S A. 2010;107(8):3894–3899. doi:10.1073/pnas.0911710107
15. Lain KY, Catalano PM. Metabolic changes in pregnancy. Clin Obstet Gynecol. 2007;50(4):938–948. doi:10.1097/GRF.0b013e31815a5494
16. Barbour LA, Shao J, Qiao L, et al. Human placental growth hormone causes severe insulin resistance in transgenic mice. Am J Obstet Gynecol. 2002;186(3):512–517. doi:10.1067/mob.2002.121256
17. Huynh J, Dawson D, Roberts D, Bentley-Lewis R. A systematic review of placental pathology in maternal diabetes mellitus. Placenta. 2015;36(2):101–114. doi:10.1016/j.placenta.2014.11.021
18. Ladfors L, Shaat N, Wiberg N, Katasarou A, Berntorp K, Kristensen K. Fetal overgrowth in women with type 1 and type 2 diabetes mellitus. PLoS One. 2017;12(11):e0187917. doi:10.1371/journal.pone.0187917
19. Nelson SM, Coan PM, Burton GJ, Lindsay RS. Placental structure in type 1 diabetes: relation to fetal insulin, leptin, and IGF-I. Diabetes. 2009;58(11):2634–2641. doi:10.2337/db09-0739
20. Meng Q, Shao L, Luo X, et al. Expressions of VEGF-A and VEGFR-2 in placentae from GDM pregnancies. Reprod Biol Endocrinol. 2016;14(1):61. doi:10.1186/s12958-016-0191-8
21. Simpson RJ, Lim JW, Moritz RL, Mathivanan S. Exosomes: proteomic insights and diagnostic potential. Expert Rev Proteomics. 2009;6(3):267–283. doi:10.1586/epr.09.17
22. Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis, and function. Nat Rev Immunol. 2002;2(8):569–579. doi:10.1038/nri855
23. Trams EG, Lauter CJ, Salem N, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta. 1981;645(1):63–70. doi:10.1016/0005-2736(81)90512-5
24. Harding C, Heuser J, Stahl P. Endocytosis and intracellular processing of transferrin and colloidal gold-transferrin in rat reticulocytes: demonstration of a pathway for receptor shedding. Eur J Cell Biol. 1984;35(2):256–263.
25. György B, Szabó TG, Pásztói M, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68(16):2667–2688. doi:10.1007/s00018-011-0689-3
26. Dragovic RA, Collett GP, Hole P, et al. Isolation of syncytiotrophoblast microvesicles and exosomes and their characterization by multicolour flow cytometry and fluorescence Nanoparticle Tracking Analysis. Methods. 2015;87:64–74. doi:10.1016/j.ymeth.2015.03.028
27. Greening DW, Xu R, Ji H, Tauro BJ, Simpson RJ. A protocol for exosome isolation and characterization: evaluation of ultracentrifugation, density-gradient separation, and immunoaffinity capture methods. Methods Mol Biol. 2015;1295:179–209.
28. Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;30:3–22. doi:10.1002/0471143030.cb0322s30
29. Mitchell MD, Peiris HN, Kobayashi M, et al. Placental exosomes in normal and complicated pregnancy. Am J Obstet Gynecol. 2015;213(4 Suppl):S173–181. doi:10.1016/j.ajog.2015.07.001
30. Feng D, Zhao WL, Ye YY, et al. Cellular internalization of exosomes occurs through phagocytosis. Traffic. 2010;11(5):675–687. doi:10.1111/j.1600-0854.2010.01041.x
31. Escrevente C, Keller S, Altevogt P, Costa J. Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer. 2011;11:108. doi:10.1186/1471-2407-11-108
32. Svensson KJ, Christianson HC, Wittrup A, et al. Exosome uptake depends on ERK1/2-heat shock protein 27 signaling and lipid Raft-mediated endocytosis negatively regulated by caveolin-1. J Biol Chem. 2013;288(24):17713–17724. doi:10.1074/jbc.M112.445403
33. Tian T, Zhu YL, Hu FH, Wang YY, Huang NP, Xiao ZD. Dynamics of exosome internalization and trafficking. J Cell Physiol. 2013;228(7):1487–1495. doi:10.1002/jcp.24304
34. Munich S, Sobo-Vujanovic A, Buchser WJ, Beer-Stolz D, Vujanovic NL. Dendritic cell exosomes directly kill tumor cells and activate natural killer cells via TNF superfamily ligands. Oncoimmunology. 2012;1(7):1074–1083. doi:10.4161/onci.20897
35. Nolte-’t Hoen EN, Buschow SI, Anderton SM, Stoorvogel W, Wauben MH. Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood. 2009;113(9):1977–1981. doi:10.1182/blood-2008-08-174094
36. Noguer-Dance M, Abu-Amero S, Al-Khtib M, et al. The primate-specific microRNA gene cluster (C19MC) is imprinted in the placenta. Hum Mol Genet. 2010;19(18):3566–3582. doi:10.1093/hmg/ddq272
37. Morales-Prieto DM, Chaiwangyen W, Ospina-Prieto S, et al. MicroRNA expression profiles of trophoblastic cells. Placenta. 2012;33(9):725–734. doi:10.1016/j.placenta.2012.05.009
38. Tong M, Kleffmann T, Pradhan S, et al. Proteomic characterization of macro-, micro-and nano-extracellular vesicles derived from the same first-trimester placenta: relevance for feto-maternal communication. Hum Reprod. 2016;31(4):687–699. doi:10.1093/humrep/dew004
39. Baig S, Kothandaraman N, Manikandan J, et al. Proteomic analysis of human placental syncytiotrophoblast microvesicles in preeclampsia. Clin Proteomics. 2014;11(1):40. doi:10.1186/1559-0275-11-40
40. Salomon C, Yee S, Scholz-Romero K, et al. Extravillous trophoblast cells-derived exosomes promote vascular smooth muscle cell migration. Front Pharmacol. 2014;5:175. doi:10.3389/fphar.2014.00175
41. Baig S, Lim JY, Fernandis AZ, et al. Lipidomic analysis of human placental syncytiotrophoblast microvesicles in adverse pregnancy outcomes. Placenta. 2013;34(5):436–442. doi:10.1016/j.placenta.2013.02.004
42. Orozco AF, Jorgez CJ, Ramos-Perez WD, et al. Placental release of distinct DNA-associated micro-particles into maternal circulation: reflective of gestation time and preeclampsia. Placenta. 2009;30(10):891–897. doi:10.1016/j.placenta.2009.06.012
43. Burkova EE, Grigor’eva AE, Bulgakov DV, et al. Extra purified exosomes from human placenta contain an unpredictable small number of different major proteins. Int J Mol Sci. 2019;20(10):2434. doi:10.3390/ijms20102434
44. Kshirsagar SK, Alam SM, Jasti S, et al. Immunomodulatory molecules are released from the first trimester and term placenta via exosomes. Placenta. 2012;33(12):982–990. doi:10.1016/j.placenta.2012.10.005
45. Sarker S, Scholz-Romero K, Perez A, et al. Placenta-derived exosomes continuously increase in maternal circulation over the first trimester of pregnancy. J Transl Med. 2014;12:204. doi:10.1186/1479-5876-12-204
46. Salomon C, Kobayashi M, Ashman K, Sobrevia L, Mitchell MD, Rice GE. Hypoxia-induced changes in the bioactivity of cytotrophoblast-derived exosomes. PLoS One. 2013;8(11):e79636. doi:10.1371/journal.pone.0079636
47. Jin J, Menon R. Placental exosomes: a proxy to understand pregnancy complications. Am J Reprod Immunol. 2018;79(5):e12788. doi:10.1111/aji.12788
48. Landgraf P, Rusu M, Sheridan R, et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129(7):1401–1414. doi:10.1016/j.cell.2007.04.040
49. Ng EK, Tsui NB, Lau TK, et al. mRNA of placental origin is readily detectable in maternal plasma. Proc Natl Acad Sci U S A. 2003;100(8):4748–4753. doi:10.1073/pnas.0637450100
50. Chim SS, Shing TK, Hung EC, et al. Detection and characterization of placental microRNAs in maternal plasma. Clin Chem. 2008;54(3):482–490. doi:10.1373/clinchem.2007.097972
51. Chang G, Mouillet JF, Mishima T, et al. Expression and trafficking of placental microRNAs at the feto-maternal interface. FASEB J. 2017;31(7):2760–2770. doi:10.1096/fj.201601146R
52. Sheller-Miller S, Lei J, Saade G, Salomon C, Burd I, Menon R. Feto-maternal trafficking of exosomes in murine pregnancy models. Front Pharmacol. 2016;7:432. doi:10.3389/fphar.2016.00432
53. Sheller-Miller S, Choi K, Choi C, Menon R. Cyclic-recombinase-reporter mouse model to determine exosome communication and function during pregnancy. Am J Obstet Gynecol. 2019;221(5):
54. Sayed D, Abdellatif M. MicroRNAs in development and disease. Physiol Rev. 2011;91(3):827–887. doi:10.1152/physrev.00006.2010
55. Donker RB, Mouillet JF, Chu T, et al. The expression profile of C19MC microRNAs in primary human trophoblast cells and exosomes. Mol Hum Reprod. 2012;18(8):417–424. doi:10.1093/molehr/gas013
56. Luo SS, Ishibashi O, Ishikawa G, et al. Human villous trophoblasts express and secrete placenta-specific microRNAs into maternal circulation via exosomes. Biol Reprod. 2009;81(4):717–729. doi:10.1095/biolreprod.108.075481
57. Ithier MC, Parobchak N, Yadava S, Cheng J, Wang B, Rosen T. Fetal lung C4BPA induces p100 processing in human placenta. Sci Rep. 2019;9(1):5519. doi:10.1038/s41598-019-42078-0
58. Di Stefano V, Wang B, Parobchak N, Roche N, Rosen T. RelB/p52-mediated NF-κB signaling alters histone acetylation to increase the abundance of corticotropin-releasing hormone in human placenta. Sci Signal. 2015;8(391):ra85. doi:10.1126/scisignal.aaa9806
59. Kurashina R, Kikuchi K, Iwaki J, Yoshitake H, Takeshita T, Takizawa T. Placenta-specific miRNA (miR-512-3p) targets PPP3R1 encoding the calcineurin B regulatory subunit in BeWo cells. J Obstet Gynaecol Res. 2014;40(3):650–660. doi:10.1111/jog.12217
60. Kambe S, Yoshitake H, Yuge K, et al. Human exosomal placenta-associated miR-517a-3p modulates the expression of PRKG1 mRNA in Jurkat cells. Biol Reprod. 2014;91(5):129. doi:10.1095/biolreprod.114.121616
61. Cooke WR, Cribbs A, Zhang W, et al. Maternal circulating syncytiotrophoblast-derived extracellular vesicles contain biologically active 5’-tRNA halves. Biochem Biophys Res Commun. 2019;518(1):107–113. doi:10.1016/j.bbrc.2019.08.015
62. Mincheva-Nilsson L, Baranov V. The role of placental exosomes in reproduction. Am J Reprod Immunol. 2010;63(6):520–533. doi:10.1111/j.1600-0897.2010.00822.x
63. Atay S, Gercel-Taylor C, Suttles J, Mor G, Taylor DD. Trophoblast-derived exosomes mediate monocyte recruitment and differentiation. Am J Reprod Immunol. 2011;65(1):65–77. doi:10.1111/j.1600-0897.2010.00880.x
64. Taylor DD, Akyol S, Gercel-Taylor C. Pregnancy-associated exosomes and their modulation of T cell signaling. J Immunol. 2006;176(3):1534–1542. doi:10.4049/jimmunol.176.3.1534
65. Gercel-Taylor C, Connor SM, Lam GK, Taylor DD. Shed membrane fragment modulation of CD3-zeta during pregnancy: link with induction of apoptosis. J Reprod Immunol. 2002;56(1–2):29–44. doi:10.1016/S0165-0378(02)00025-6
66. Sabapatha A, Gercel-Taylor C, Taylor DD. Specific isolation of placenta-derived exosomes from the circulation of pregnant women and their immunoregulatory consequences. Am J Reprod Immunol. 2006;56(5–6):345–355. doi:10.1111/j.1600-0897.2006.00435.x
67. Stenqvist AC, Nagaeva O, Baranov V, Mincheva-Nilsson L. Exosomes secreted by human placenta carry functional Fas ligand and TRAIL molecules and convey apoptosis in activated immune cells, suggesting exosome-mediated immune privilege of the fetus. J Immunol. 2013;191(11):5515–5523. doi:10.4049/jimmunol.1301885
68. Lokossou AG, Toudic C, Nguyen PT, et al. Endogenous retrovirus-encoded Syncytin-2 contributes to exosome-mediated immunosuppression of T cells†. Biol Reprod. 2020;102(1):185–198. doi:10.1093/biolre/ioz124
69. Hedlund M, Stenqvist AC, Nagaeva O, et al. Human placenta expresses and secretes NKG2D ligands via exosomes that down-modulate the cognate receptor expression: evidence for immunosuppressive function. J Immunol. 2009;183(1):340–351. doi:10.4049/jimmunol.0803477
70. Mincheva-Nilsson L, Nagaeva O, Chen T, et al. Placenta-derived soluble MHC class I chain-related molecules down-regulate NKG2D receptor on peripheral blood mononuclear cells during human pregnancy: a possible novel immune escape mechanism for fetal survival. J Immunol. 2006;176(6):3585–3592. doi:10.4049/jimmunol.176.6.3585
71. Kam EP, Gardner L, Loke YW, King A. The role of trophoblast in the physiological change in decidual spiral arteries. Hum Reprod. 1999;14(8):2131–2138. doi:10.1093/humrep/14.8.2131
72. Abbas Y, Turco MY, Burton GJ, Moffett A. Investigation of human trophoblast invasion in vitro. Hum Reprod Update. 2020;26(4):501–513. doi:10.1093/humupd/dmaa017
73. Tong M, Chamley LW. Placental extracellular vesicles and feto-maternal communication. Cold Spring Harb Perspect Med. 2015;5(3):a023028. doi:10.1101/cshperspect.a023028
74. Salomon C, Torres MJ, Kobayashi M, et al. A gestational profile of placental exosomes in maternal plasma and their effects on endothelial cell migration. PLoS One. 2014;9(6):e98667. doi:10.1371/journal.pone.0098667
75. Harris LK, Smith SD, Keogh RJ, et al. Trophoblast- and vascular smooth muscle cell-derived MMP-12 mediates elastolysis during uterine spiral artery remodeling. Am J Pathol. 2010;177(4):2103–2115. doi:10.2353/ajpath.2010.100182
76. Harris LK, Gabor Than IF. Award lecture: transformation of the spiral arteries in human pregnancy: key events in the remodeling timeline. Placenta. 2011;32(Suppl 2):S154–S158. doi:10.1016/j.placenta.2010.11.018
77. Komaki M, Numata Y, Morioka C, et al. Exosomes of human placenta-derived mesenchymal stem cells stimulate angiogenesis. Stem Cell Res Ther. 2017;8(1):219. doi:10.1186/s13287-017-0660-9
78. Arora N, Sadovsky Y, Dermody TS, Coyne CB. Microbial vertical transmission during human pregnancy. Cell Host Microbe. 2017;21(5):561–567. doi:10.1016/j.chom.2017.04.007
79. Ouyang Y, Mouillet JF, Coyne CB, Sadovsky Y. Review: placenta-specific microRNAs in exosomes - good things come in nano-packages. Placenta. 2014;35:S69–73. doi:10.1016/j.placenta.2013.11.002
80. Delorme-Axford E, Donker RB, Mouillet JF, et al. Human placental trophoblasts confer viral resistance to recipient cells. Proc Natl Acad Sci U S A. 2013;110(29):12048–12053. doi:10.1073/pnas.1304718110
81. Ander SE, Diamond MS, Coyne CB. Immune responses at the maternal-fetal interface. Sci Immunol. 2019;4(31):eaat6114. doi:10.1126/sciimmunol.aat6114
82. Bayer A, Lennemann NJ, Ouyang Y, et al. Type III interferons produced by human placental trophoblasts confer protection against zika virus infection. Cell Host Microbe. 2016;19(5):705–712. doi:10.1016/j.chom.2016.03.008
83. Bayer A, Delorme-Axford E, Sleigher C, et al. Human trophoblasts confer resistance to viruses implicated in perinatal infection. Am J Obstet Gynecol. 2015;212(1):
84. Choi Y, Bowman JW, Jung JU. Autophagy during viral infection - a double-edged sword. Nat Rev Microbiol. 2018;16(6):341–354. doi:10.1038/s41579-018-0003-6
85. Delorme-Axford E, Bayer A, Sadovsky Y, Coyne CB. Autophagy as a mechanism of antiviral defense at the maternal-fetal interface. Autophagy. 2013;9(12):2173–2174. doi:10.4161/auto.26558
86. He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93.
87. Delorme-Axford E, Sadovsky Y, Coyne CB. The placenta as a barrier to viral infections. Annu Rev Virol. 2014;1(1):133–146. doi:10.1146/annurev-virology-031413-085524
88. Sadovsky Y, Mouillet JF, Ouyang Y, Bayer A, Coyne CB. The function of trophomirs and other MicroRNAs in the human placenta. Cold Spring Harb Perspect Med. 2015;5(8):a023036. doi:10.1101/cshperspect.a023036
89. Mouillet JF, Ouyang Y, Bayer A, Coyne CB, Sadovsky Y. The role of trophoblastic microRNAs in placental viral infection. Int J Dev Biol. 2014;58(2–4):281–289. doi:10.1387/ijdb.130349ys
90. Abdel-Ghany S, Sabit H. microRNA-based vaccination and treatment for COVID-19. Curr Trends Vaccine Vaccinol. 2020;3(1):109.
91. Marchi R, Sugita B, Centa A, et al. The role of microRNAs in modulating SARS-CoV-2 infection in human cells: a systematic review. Infect Genet Evol. 2021;91:104832. doi:10.1016/j.meegid.2021.104832
92. Salomon C, Scholz-Romero K, Sarker S, et al. Gestational diabetes mellitus is associated with changes in the concentration and bioactivity of placenta-derived exosomes in maternal circulation across gestation. Diabetes. 2016;65(3):598–609. doi:10.2337/db15-0966
93. Rice GE, Scholz-Romero K, Sweeney E, et al. The effect of glucose on the release and bioactivity of exosomes from first trimester trophoblast cells. J Clin Endocrinol Metab. 2015;100(10):E1280–E1288. doi:10.1210/jc.2015-2270
94. Sáez T, de Vos P, Kuipers J, Sobrevia L, Faas MM. Fetoplacental endothelial exosomes modulate high d-glucose-induced endothelial dysfunction. Placenta. 2018;66:26–35. doi:10.1016/j.placenta.2018.04.010
95. Grace T, Dominic G, Vyjayanthi K, et al. Oxygen tension regulates the miRNA profile and bioactivity of exosomes released from extravillous trophoblast cells – liquid biopsies for monitoring complications of pregnancy. PLoS One. 2017;12(3):e0174514. doi:10.1371/journal.pone.0174514
96. Salomon C, Ryan J, Sobrevia L, et al. Exosomal signaling during hypoxia mediates microvascular endothelial cell migration and vasculogenesis. PLoS One. 2013;8(7):e68451. doi:10.1371/journal.pone.0068451
97. Elfeky O, Longo S, Lai A, Rice GE, Salomon C. Influence of maternal BMI on the exosomal profile during gestation and their role on maternal systemic inflammation. Placenta. 2017;50:60–69. doi:10.1016/j.placenta.2016.12.020
98. Castaño C, Kalko S, Novials A, Párrizas M. Obesity-associated exosomal miRNAs modulate glucose and lipid metabolism in mice. Proc Natl Acad Sci U S A. 2018;115(48):12158–12163. doi:10.1073/pnas.1808855115
99. Jayabalan N, Lai A, Ormazabal V, et al. Adipose tissue exosomal proteomic profile reveals a role on placenta glucose metabolism in gestational diabetes mellitus. J Clin Endocrinol Metab. 2019;104(5):1735–1752. doi:10.1210/jc.2018-01599
100. He X, Kuang G, Wu Y, Ou C. Emerging roles of exosomal miRNAs in diabetes mellitus. Clin Transl Med. 2021;11(6):e468. doi:10.1002/ctm2.468
101. Jayabalan N, Lai A, Nair S, et al. Quantitative proteomics by SWATH-MS suggest an association between circulating exosomes and maternal metabolic changes in gestational diabetes mellitus. Proteomics. 2019;19(1–2):e1800164. doi:10.1002/pmic.201800164
102. Cao M, Zhang L, Lin Y, et al. Circular RNA expression profiles in umbilical cord blood exosomes from normal and gestational diabetes mellitus patients. Biosci Rep. 2020;40(11). doi:10.1042/BSR20201946
103. Cao M, Zhang L, Lin Y, et al. Differential mRNA and long noncoding RNA expression profiles in umbilical cord blood exosomes from gestational diabetes mellitus patients. DNA Cell Biol. 2020;39(11):2005–2016. doi:10.1089/dna.2020.5783
104. Yan L, Feng J, Cheng F, et al. Circular RNA expression profiles in placental villi from women with gestational diabetes mellitus. Biochem Biophys Res Commun. 2018;498(4):743–750. doi:10.1016/j.bbrc.2018.03.051
105. Gillet V, Ouellet A, Stepanov Y, et al. miRNA profiles in extracellular vesicles from serum early in pregnancies complicated by gestational diabetes mellitus. J Clin Endocrinol Metab. 2019;104(11):5157–5169. doi:10.1210/jc.2018-02693
106. Nair S, Jayabalan N, Guanzon D, et al. Human placental exosomes in gestational diabetes mellitus carry a specific set of miRNAs associated with skeletal muscle insulin sensitivity. Clin Sci (Lond). 2018;132(22):2451–2467. doi:10.1042/CS20180487
107. James-Allan LB, Rosario FJ, Barner K, et al. Regulation of glucose homeostasis by small extracellular vesicles in normal pregnancy and gestational diabetes. FASEB J. 2020;34(4):5724–5739. doi:10.1096/fj.201902522RR
108. Tu C, Wang L, Tao H, Gu L, Zhu S, Chen X. Expression of miR-409-5p in gestational diabetes mellitus and its relationship with insulin resistance. Exp Ther Med. 2020;20(4):3324–3329. doi:10.3892/etm.2020.9049
109. Qi S, Wang X. Decreased expression of miR-185 in serum and placenta of patients with gestational diabetes mellitus. Clin Lab. 2019;65(12). doi:10.7754/Clin.Lab.2019.190445
110. Zhao C, Zhao C, Zhao H. Defective insulin receptor signaling in patients with gestational diabetes is related to dysregulated miR-140 which can be improved by naringenin. Int J Biochem Cell Biol. 2020;128:105824. doi:10.1016/j.biocel.2020.105824
111. Kandzija N, Zhang W, Motta-Mejia C, et al. Placental extracellular vesicles express active dipeptidyl peptidase IV; levels are increased in gestational diabetes mellitus. J Extracell Vesicles. 2019;8(1):1617000. doi:10.1080/20013078.2019.1617000
112. Kirwan JP, Hauguel-de Mouzon S, Lepercq J, et al. TNF-alpha is a predictor of insulin resistance in human pregnancy. Diabetes. 2002;51(7):2207–2213. doi:10.2337/diabetes.51.7.2207
113. Coughlan MT, Oliva K, Georgiou HM, Permezel JM, Rice GE. Glucose-induced release of tumour necrosis factor-alpha from human placental and adipose tissues in gestational diabetes mellitus. Diabet Med. 2001;18(11):921–927. doi:10.1046/j.1464-5491.2001.00614.x
114. Wang P, Wang H, Li C, et al. Dysregulation of microRNA-657 influences inflammatory response via targeting interleukin-37 in gestational diabetes mellitus. J Cell Physiol. 2019;234(5):7141–7148. doi:10.1002/jcp.27468
115. Muralimanoharan S, Maloyan A, Myatt L. Mitochondrial function and glucose metabolism in the placenta with gestational diabetes mellitus: role of miR-143. Clin Sci (Lond). 2016;130(11):931–941. doi:10.1042/CS20160076
116. He Y, Bai J, Liu P, et al. miR-494 protects pancreatic β-cell function by targeting PTEN in gestational diabetes mellitus. EXCLI J. 2017;16:1297–1307. doi:10.17179/excli2017-491
117. Holder B, Jones T, Sancho Shimizu V, et al. Macrophage exosomes induce placental inflammatory cytokines: a novel mode of maternal-placental messaging. Traffic. 2016;17(2):168–178. doi:10.1111/tra.12352
118. Wang P, Ma Z, Wang Z, Wang X, Zhao G, Wang Z. MiR-6869-5p induces M2 polarization by regulating PTPRO in gestational diabetes mellitus. Mediators Inflamm. 2021;2021:6696636. doi:10.1155/2021/6696636
119. Zamanian Azodi M, Rezaei-Tavirani M, Rezaei-Tavirani M, Robati RM. Gestational diabetes mellitus regulatory network identifies hsa-miR-145-5p and hsa-miR-875-5p as potential biomarkers. Int J Endocrinol Metab. 2019;17(3):e86640. doi:10.5812/ijem.86640
120. Fu S, Fu S, Ma X, Yang X, Ling J. miR‑875‑5p regulates IR and inflammation via targeting TXNRD1 in gestational diabetes rats. Mol Med Rep. 2021;23(5):303. doi:10.3892/mmr.2021.11942
121. Atay S, Gercel-Taylor C, Taylor DD. Human trophoblast-derived exosomal fibronectin induces pro-inflammatory IL-1β production by macrophages. Am J Reprod Immunol. 2011;66(4):259–269. doi:10.1111/j.1600-0897.2011.00995.x
122. Zhao C, Zhang T, Shi Z, Ding H, Ling X. MicroRNA-518d regulates PPARα protein expression in the placentas of females with gestational diabetes mellitus. Mol Med Rep. 2014;9(6):2085–2090. doi:10.3892/mmr.2014.2058
123. Qiu H, Liu X, Yao S, Zhou J, Zhang X, Du J. Regulation and mechanism of miR-518d through the PPARα-mediated NF-κB pathway in the development of gestational diabetes mellitus. J Diabetes Res. 2020;2020:7019597. doi:10.1155/2020/7019597
124. Sáez T, de Vos P, Sobrevia L, Faas MM. Is there a role for exosomes in foetoplacental endothelial dysfunction in gestational diabetes mellitus. Placenta. 2018;61:48–54. doi:10.1016/j.placenta.2017.11.007
125. Sáez T, Salsoso R, Leiva A, et al. Human umbilical vein endothelium-derived exosomes play a role in foetoplacental endothelial dysfunction in gestational diabetes mellitus. Biochim Biophys Acta Mol Basis Dis. 2018;1864(2):499–508. doi:10.1016/j.bbadis.2017.11.010
126. Díaz-Pérez FI, Hiden U, Gauster M, et al. Post-transcriptional down regulation of ICAM-1 in feto-placental endothelium in GDM. Cell Adh Migr. 2016;10(1–2):18–27. doi:10.1080/19336918.2015.1127467
127. Floris I, Descamps B, Vardeu A, et al. Gestational diabetes mellitus impairs fetal endothelial cell functions through a mechanism involving microRNA-101 and histone methyltransferase enhancer of zester homolog-2. Arterioscler Thromb Vasc Biol. 2015;35(3):664–674. doi:10.1161/ATVBAHA.114.304730
128. Kc K, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab. 2015;66(Suppl 2):14–20. doi:10.1159/000371628
129. Mouillet JF, Chu T, Hubel CA, Nelson DM, Parks WT, Sadovsky Y. The levels of hypoxia-regulated microRNAs in plasma of pregnant women with fetal growth restriction. Placenta. 2010;31(9):781–784. doi:10.1016/j.placenta.2010.07.001
130. Rodosthenous RS, Burris HH, Sanders AP, et al. Second trimester extracellular microRNAs in maternal blood and fetal growth: an exploratory study. Epigenetics. 2017;12(9):804–810. doi:10.1080/15592294.2017.1358345
131. Miranda J, Paules C, Nair S, et al. Placental exosomes profile in maternal and fetal circulation in intrauterine growth restriction - liquid biopsies to monitoring fetal growth. Placenta. 2018;64:34–43. doi:10.1016/j.placenta.2018.02.006
132. Ge Q, Zhu Y, Li H, Tian F, Xie X, Bai Y. Differential expression of circulating miRNAs in maternal plasma in pregnancies with fetal macrosomia. Int J Mol Med. 2015;35(1):81–91. doi:10.3892/ijmm.2014.1989
133. Li J, Song L, Zhou L, et al. A microRNA signature in gestational diabetes mellitus associated with risk of macrosomia. Cell Physiol Biochem. 2015;37(1):243–252. doi:10.1159/000430349
134. Jiang H, Wu W, Zhang M, et al. Aberrant upregulation of miR-21 in placental tissues of macrosomia. J Perinatol. 2014;34(9):658–663. doi:10.1038/jp.2014.58
135. Jiang H, Wen Y, Hu L, Miao T, Zhang M, Dong J. Serum microRNAs as diagnostic biomarkers for macrosomia. Reprod Sci. 2015;22(6):664–671. doi:10.1177/1933719114561557
136. Zhang JT, Cai QY, Ji SS, et al. Decreased miR-143 and increased miR-21 placental expression levels are associated with macrosomia. Mol Med Rep. 2016;13(4):3273–3280. doi:10.3892/mmr.2016.4892
137. Maccani MA, Padbury JF, Marsit CJ. miR-16 and miR-21 expression in the placenta is associated with fetal growth. PLoS One. 2011;6(6):e21210. doi:10.1371/journal.pone.0021210
138. Li J, Chen L, Tang Q, et al. The role, mechanism and potentially novel biomarker of microRNA-17-92 cluster in macrosomia. Sci Rep. 2015;5:17212. doi:10.1038/srep17212
139. Shi Z, Zhao C, Long W, Ding H, Shen R. Microarray expression profile analysis of long non-coding RNAs in umbilical cord plasma reveals their potential role in gestational diabetes-induced macrosomia. Cell Physiol Biochem. 2015;36(2):542–554. doi:10.1159/000430119
140. Nakahara A, Elfeky O, Garvey C, Guanzon D, Longo SA, Salmon C. Exosome profiles for normal and complicated pregnancies—a longitudinal study [3O]. Obstet Gynecol. 2019;133:162. doi:10.1097/01.AOG.0000558864.31601.aa
141. Nair S, Ormazabal V, Lappas M, McIntyre HD, Salomon C. Extracellular vesicles and their potential role inducing changes in maternal insulin sensitivity during gestational diabetes mellitus. Am J Reprod Immunol. 2021;85(2):e13361. doi:10.1111/aji.13361
142. Rahimi G, Jafari N, Khodabakhsh M, Shirzad Z, Dogaheh HP. Upregulation of microRNA processing enzymes Drosha and Dicer in gestational diabetes mellitus. Gynecol Endocrinol. 2015;31(2):156–159. doi:10.3109/09513590.2014.969700
143. Cao Y, Jia Y, Xing B, Shi D, Dong X. Plasma microRNA-16-5p, −17-5p and −20a-5p: novel diagnostic biomarkers for gestational diabetes mellitus. J Obstetr Gynaecol Res. 2017;43(6):974–981. doi:10.1111/jog.13317
144. Pillar N, Yoffe L, Hod M, Shomron N. The possible involvement of microRNAs in preeclampsia and gestational diabetes mellitus. Best Pract Res Clin Obstet Gynaecol. 2015;29(2):176–182. doi:10.1016/j.bpobgyn.2014.04.021
145. Zhu Y, Tian F, Li H, Zhou Y, Lu J, Ge Q. Profiling maternal plasma microRNA expression in early pregnancy to predict gestational diabetes mellitus. Int J Gynaecol Obstet. 2015;130(1):49–53. doi:10.1016/j.ijgo.2015.01.010
146. Hocaoglu M, Demirer S, Senturk H, Turgut A, Komurcu-Bayrak E. Differential expression of candidate circulating microRNAs in maternal blood leukocytes of the patients with preeclampsia and gestational diabetes mellitus. Pregnancy Hypertens. 2019;17:5–11. doi:10.1016/j.preghy.2019.04.004
147. Wander PL, Boyko EJ, Hevner K, et al. Circulating early- and mid-pregnancy microRNAs and risk of gestational diabetes. Diabetes Res Clin Pract. 2017;132:1–9. doi:10.1016/j.diabres.2017.07.024
148. Zhao C, Dong J, Jiang T, et al.Early second-trimester serum MiRNA profiling predict gestational diabetes mellitus. PLoS One. 2011. doi:10.1371/journal.pone.0023925
149. Sørensen AE, van Poppel M, Desoye G, et al. The predictive value of miR-16, −29a and −134 for early identification of gestational diabetes: a nested analysis of the DALI cohort. Cells. 2021;10(1):170. doi:10.3390/cells10010170
150. Yoffe L, Polsky A, Gilam A, et al. Early diagnosis of gestational diabetes mellitus using circulating microRNAs. Eur J Endocrinol. 2019;181(5):565–577. doi:10.1530/EJE-19-0206
151. Thurik FF, Lamain‐de Ruiter M, Javadi A, et al.Absolute first trimester cell-free DNA levels and their associations with adverse pregnancy outcomes. Prenatal Diag. 2016;36:1104–11.
152. Herrera-van Oostdam AS, Toro-Ortíz JC, López JA, et al. Placental exosomes isolated from urine of patients with gestational diabetes exhibit a differential profile expression of microRNAs across gestation. Int J Mol Med. 2020;46(2):546–560. doi:10.3892/ijmm.2020.4626
153. Ling HY, Hu B, Hu XB, et al. MiRNA-21 reverses high glucose and high insulin induced insulin resistance in 3T3-L1 adipocytes through targeting phosphatase and tensin homologue. Exp Clin Endocrinol Diabetes. 2012;120(9):553–559. doi:10.1055/s-0032-1311644
154. van Rooij E, Purcell AL, Levin AA. Developing microRNA therapeutics. Circ Res. 2012;110(3):496–507. doi:10.1161/CIRCRESAHA.111.247916
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.