Back to Journals » Infection and Drug Resistance » Volume 12
Pivmecillinam compared to other antimicrobials for community-acquired urinary tract infections with Escherichia coli, ESBL-producing or not – a retrospective cohort study
Authors Jansåker F , Boel JB , Thønnings S, Hertz FB , Hansen KH, Frimodt-Møller N, Knudsen JD
Received 19 March 2019
Accepted for publication 10 May 2019
Published 13 June 2019 Volume 2019:12 Pages 1691—1702
DOI https://doi.org/10.2147/IDR.S209255
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Joachim Wink
Filip Jansåker,1,2 Jonas Bredtoft Boel,3 Sara Thønnings,1,2 Frederik Boëtius Hertz,3 Katrine Hartung Hansen,2 Niels Frimodt-Møller,2 Jenny Dahl Knudsen1,2
1Department of Clinical Microbiology, Hvidovre Hospital, University of Copenhagen, 2650 Hvidovre, Denmark; 2Department of Clinical Microbiology, Rigshospitalet, University of Copenhagen, 2100 Copenhagen, Denmark; 3Department of Clinical Microbiology, Herlev and Gentofte Hospital, University of Copenhagen, 2730 Herlev, Denmark
Objectives: To compare the therapeutic effect of pivmecillinam and other common oral antibiotics for community-acquired urinary tract infections (UTIs) caused by Extended Spectrum Beta-Lactamase (ESBL)- or non-ESBL-producing Escherichia coli.
Methods: Retrospective cohort study from 2010 to mid-2016 with data from the regional Laboratory Database and three national databases on antibiotic prescriptions, hospital admission, and mortality, respectively. Primary care patients (≥18 years) empirically treated for UTI caused by non-ESBL- or ESBL-producing E. coli (non-ESBL and ESBL E. coli) were included. Seven antibiotics, commonly used empirically for UTI, were investigated. Treatment failure measured as the redemption of a new antibiotic prescription or admission to hospital due to UTI. Cox proportional hazard ratios and adjusted risk differences along with 95% confidence intervals were calculated for 14 and 30 days, respectively.
Results: Thirty-six thousand two hundred and ninety-three (95.7%) and 1624 (4.3%) cases were included in the non-ESBL and ESBL groups, respectively. Male sex, high age, ESBL production, and resistance to empirical therapy were found to independently increase the risk of treatment failure. Compared to pivmecillinam, ciprofloxacin had significantly lower treatment failure for non-ESBL E. coli, but significantly higher treatment failure in ESBL E. coli. There was no significant difference between nitrofurantoin and pivmecillinam.
Conclusion: All antibiotics seem to have a higher risk of treatment failure for UTI caused by ESBL-producing E. coli as compared to non-ESBL-producing E. coli. At present, nitrofurantoin and pivmecillinam seem to be the most relevant orally available therapies for E. coli UTI. Local resistance data should guide which of the two that should be the contemporary first-line option.
Keywords: UTI, ESBL, E. coli, pivmecillinam, nitrofurantoin, cohort
Introduction
Urinary tract infection (UTI) is a prevalent bacteriological infection in humans, commonly caused by Escherichia coli.1,2 The rate of UTI caused by E. coli with Extended Spectrum Beta-Lactamase production (ESBL E. coli) has increased over recent decades and the effect of antibiotic treatment, especially the oral options, is gradually becoming limited.3–6
In vitro studies have shown a clear majority of ESBL E. coli to be susceptible to the beta-lactam antibiotic mecillinam (amdinocillin), but with an increased MIC compared to non-ESBL E. coli. This has made mecillinam an interesting therapeutic candidate for infections caused by ESBL E. coli.7–9 Several studies have shown oral prodrug pivmecillinam (pivoxil amdinocillin) to be a safe and effective agent in the treatment of UTI.10,11 Even in countries where it is widely used, there continues to be low rates of resistance.5,12,13 Clinical studies on pivmecillinam´s effectiveness against UTI caused by ESBL E. coli are few, with a limited number of patients, and conflicting results.14–16 There are no comparative studies with other antibiotics for UTI caused by ESBL E. coli, where nitrofurantoin would be interesting due to the low prevalence of resistance in ESBL E. coli.9,17 Thus, this study was designed to compare the therapeutic effect of pivmecillinam and other common oral antibiotics for UTIs caused by ESBL and non-ESBL E. coli, respectively. We present the evaluation of a large dataset and comprehensive comparisons of different oral antibiotics for UTI prescribed at the debut of the E. coli bacteriuria; with follow-up on hospital admission and new antibiotic prescriptions for UTI, but clinical data were not available.
Material and methods
Study design
A retrospective cohort study was conducted on unique cases of UTI caused by non-ESBL and ESBL E. coli, empirically treated with pivmecillinam or other common antibiotics for UTI. The study was pre-approved by the National eHealth Authority Denmark (FSEID-00002684/DST nr: 706,471).
Setting
The study was conducted at the Departments of Clinical Microbiology (DCM), Copenhagen University Hospital, Hvidovre, and Herlev, Denmark. Data were collected between the 1st of January 2010 and 30th of September 2016, from the Capital Region of Denmark (Copenhagen), corresponding to a catchment area of approximately 1.8 million inhabitants. Visits to the general practitioners and following laboratory tests are free of charge for the patients as part of the public tax-financed Healthcare system in Denmark. Bacteriological data from urine samples, sent for investigation at one of the DCMs from primary care patients, were extracted through the common laboratory system of the DCMs. Data on these patients´ collection of antibiotic prescriptions, hospital admission and mortality were collected from Statistic Denmark’s national databases. All data were linked using the unique civil registration number given to all Danish citizens.18
Databases
For this study, the following four databases were used: The Laboratory Database for the DCMs (Capital Region of Demark) containing detailed information on pathogens and date of microbiological samples beside patient identifications; The Danish Registry of Medicinal Product Statistics containing detailed information on all dispensed drug prescriptions from Danish pharmacies using the Anatomical Therapeutic Chemical system; The Danish National Patient Registry containing information on every hospital admission and discharge; and finally, The Danish National Cause of Death Register containing date and cause of death.
Participants
The study included men and women ≥18 years, with significant bacteriuria for E. coli (ie, ≥103 colony forming units, cfu/mL) and to whom a simultaneous oral empirical antibiotic for UTI had been redeemed. The available oral antibiotics in Denmark at the time were pivmecillinam, sulfamethizole, nitrofurantoin, ciprofloxacin, trimethoprim, pivampicillin or amoxicillin. Patients were stratified according to empirical treatment and ESBL production (ie, ESBL and non-ESBL). Exclusion criteria were previous significant bacteriuria within the last month, prior inclusion in the study (ESBL cases were the primary inclusion criteria and included before non-ESBL), prescription of long-term prophylactic UTI antibiotic therapy or no prescription to commonly used antibiotics for acute UTI within four days before and after the date of obtaining the urine sample. Specimens are generally not routinely obtained for uncomplicated UTI, which is why it is reasonable to assume that participants had more complicated UTI. However, clinical symptoms and comorbidity were unavailable, and thus, the precise distribution of uncomplicated and complicated UTI is unknown.
Outcome definitions
Clinical treatment failure was defined as the redemption of any new prescription of antibiotics for UTIs or admission to hospital due to UTI at 14 and 30 days, respectively. The unadjusted and adjusted model for known factors at the time of prescription (ie, sex and age) demonstrated the outcome of empiric therapy. Antibiotics that were not exclusively recommended for UTI in Denmark (ie, ciprofloxacin, trimethoprim or an aminopenicillin) needed to have an indication for UTI on the new prescription to be used in the outcome analyses as treatment failure. Antibiotics that were exclusively recommended for UTI in Denmark (ie, pivmecillinam, sulfamethizole and nitrofurantoin) were considered treatment failure if no other indication was written on the new prescription.
Microbiological data
During the study period, all the urine samples had been secured at a general practitioner’s office and transported in containers (Urine-Monovette® with boric acid, Sarstedt, Nümbrecht, Germany) once daily on workdays to the DCMs to be analyzed and logged in the common laboratory database routinely. Each urine sample was processed either automated (DCM, Hvidovre) or manually (DCM, Herlev), according to the routine methods used at each laboratory. In brief, 10 μL of urine was spread on each of CHROM-agarTM and 5% blood agar and incubated at 37°C for 20 hrs. Significant growth of E. coli (103 cfu/mL) was identified at the species level by MALDI-TOF MS (Bruker, Germany). Antimicrobial susceptibility testing was performed by Disk Diffusion Test Methodology as described by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) and interpreted according to EUCAST clinical breakpoints using Oxoid disks (Basingstoke, UK) on Mueller–Hinton agar plates.19–21 Strains with a zone diameter for cefpodoxime of ≤24 mm, for ceftazidime of ≤24 mm or for cefotaxime of ≤24 mm were considered ESBL-screening test-positive. ESBL strains were phenotypically confirmed by either the MAST test, performed as a combined-disk method, using disks containing cefpodoxime ± ESBL and/or AmpC inhibitors (MAST®, Merseyside, UK) or as a combined-disk method using disks containing ceftazidime ±ESBL or AmpC inhibitors and cefotaxime ±ESBL and/or AmpC inhibitors (Rosco NeoSensitabs, Rosco, Taastrup, Denmark) at the participating laboratories.22,23
Statistical analysis
Characterization of the study population included medians and interquartile ranges (IQRs) and frequency tables. Event-free survival was calculated from the time of collection of the urine sample until clinical treatment failure, death or the last day of follow-up, whichever came first. Due to multiple pairwise comparisons, only results with a p-value of less than 0.01 were considered significant. HR were calculated using Cox proportional hazard regression analysis along with 99% CIs for 14 days and 30 days of clinical treatment failure, respectively. The outcomes were adjusted for potential confounders such as age (as a continuous variable) and sex. The adjusted model for these factors known at the time of prescription was regarded to demonstrate the outcome of empiric therapy. However, since resistance varies between countries and Denmark is a low-resistance country,13 resistance was included in a second adjusted model to investigate a more generalizable empirical outcome. Adjusted risk difference was calculated post hoc for significant findings using the R package stdReg (version 3.0.0). A sensitivity analysis was performed to test the robustness of the definition of clinical treatment failure. For this analysis, we removed the restriction regarding the indications on the prescriptions, thereby including all prescriptions on the six antibiotics (Tables S2 and S3).
Study size
Denmark has generally low prevalence of ESBL E. coli (eg, 4% in 2016) and as many years as possible was included to get as substantial a study size as possible. Few ESBL E. coli were seen in Denmark before 2010, and we only had access to sufficient data in the civil registry databases from Statistic Denmark up until September 2016.13,23
Results
Around 125 270 episodes were identified with bacteriuria caused by E. coli from patients aged ≥18 years. After applying the exclusion criteria, 36 293 cases remained, of which 1624 (4.3%) were infected with ESBL E. coli (Figure 1).
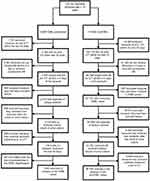 | Figure 1 Flow diagram of included patients with E. coli bacteriuria. |
Descriptive data
Patients in the non-ESBL group were significantly younger than in the ESBL group (52 [IQR 31–71] versus 58 [IQR 35–75] years, p<0.001). The proportion of females was higher in the non-ESBL cases compared to the ESBL cases (89.5% vs 87.2%, p=0.003). Pivmecillinam was the most common empirical treatment in both groups. Patients receiving nitrofurantoin, ciprofloxacin and trimethoprim were significantly older compared to those who received pivmecillinam (Table 1).
 | Table 1 Descriptive characteristics of the 37 917 cases of bacteriuria caused by E. coli |
Antibiotic resistance data
For all antibiotics, prevalence of resistance was considerably higher in the ESBL group compared to the non-ESBL group (Table 1). Nitrofurantoin showed significantly lower frequency of resistance (2.9%) compared to mecillinam (10.7%) in the ESBL group (p<0.001), as all other antibiotics here studied showed significantly higher resistances (eg, ciprofloxacin [67.1%] and trimethoprim [57.6%]). Resistance to mecillinam was 2.5% for non-ESBL E. coli, and even lower for nitrofurantoin (0.8%; p<0.001) and amoxicillin-clavulanic acid (2.1%; p<0.001), but not all isolates were tested.
Clinical outcome
Increasing age (HR 1.01 [99% CI 1.01–1.01, p<0.001]) and male sex (HR 1.74 [99% CI 1.61–1.86, p<0.001]) were significant risk factors for clinical treatment failure. There was a greater proportion of ESBL cases with clinical treatment failure within 14 and 30 days compared to the non-ESBL cases, when looking at all new antibiotic prescriptions, prescription of a new type of antibiotic and hospital admissions due to UTI (Table 2, Figure 2). ESBL production also increased the risk of treatment failure significantly for the commonly used antibiotics: pivmecillinam, ciprofloxacin, sulfamethizole and trimethoprim. Resistance to the empirically prescribed antibiotic also significantly increased the risk of treatment failure.
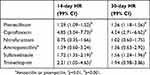 | Table 2 Failure of empirical treatment for ESBL versus non-ESBL (reference)-producing E. coli UTI (adjusted for age and sex) |
 | Figure 2 Empirical treatment success probability over time for E. coli UTI (adjusted for age and sex) “Resistant” denounces resistance toward the antibiotic used for treatment. |
The probability of clinical success over time can be seen in Figure 2. For cases with susceptible isolates, there was a relatively high probability of clinical success compared to the resistant isolates for all antibiotics. The same tendency was seen for non-ESBL versus ESBL isolates, although the difference was smaller. For non-ESBL isolates, there was a higher risk of treatment failure with aminopenicillin, sulfamethizole or trimethoprim compared to nitrofurantoin, pivmecillinam and ciprofloxacin, respectively. For ESBL isolates, nitrofurantoin followed by pivmecillinam had a higher probability of clinical success compared to the other four investigated antibiotics. For the resistant isolates, trimethoprim had the lowest chance of clinical success and pivmecillinam the highest, whereas for susceptible isolates nitrofurantoin had the lowest probability of success and ciprofloxacin the highest.
When studying the different antibiotic’s effect on non-ESBL cases compared to pivmecillinam (adjusted for sex and age; Table 3), a significantly increased risk of clinical treatment failure within 14 and 30 days was observed for amoxicillin/pivampicillin and sulfamethizole, respectively. The 30-day risk difference was 15.0% (99% CI 9.1 to 21.0) and 9.2% (99% CI 7.7 to 10.6), respectively. The difference was eliminated when adjusting for resistance (Table 3). Compared to pivmecillinam, there was no significant difference in risk of clinical treatment failure for ciprofloxacin, nitrofurantoin and trimethoprim; however, when adjusting for resistance, there was a significant difference between ciprofloxacin and pivmecillinam (HR 0.63 [99% CI 0.51 to 0.78, p<0.001]). The risk difference at day 30 when adjusting for resistance was −10.2% (99% CI −14.6 to −5.75). Unadjusted risk of treatment failures for each antibiotic compared to pivmecillinam can be viewed in Table S1.
 | Table 3 Failure of empirical treatment for non-ESBL- and ESBL-producing E. coli UTI (adjusted for age, sex and resistance) |
For ESBL cases, the risk of treatment failure (adjusted for age and sex) within 30 days was significantly lower for pivmecillinam compared to ciprofloxacin and sulfamethizole. When adjusting for resistance, the significant advantages of pivmecillinam over other antibiotics disappeared.
The sensitivity analysis (Tables S2 and S3) showed similar results as the primary analysis and thereby showing that the definition of clinical treatment failure was robust to changes in regard to prescriptions included.
Discussion
This is to our knowledge the largest cohort study on the effect of commonly used antibiotics for UTI caused by non-ESBL and ESBL E. coli. Treatment failure was significantly correlated to ESBL production, high age, male sex and resistance to empirical antibiotic. The recommended threshold of 20% for resistance prevalence to change empirical antibiotic was seen only for nitrofurantoin, pivmecillinam and ciprofloxacin for non-ESBL E. coli, but only for nitrofurantoin and pivmecillinam in ESBL E. coli (67.1% ciprofloxacin resistance).2 Based on the resistance patterns correlated with the clinical outcome, we suggest that nitrofurantoin and pivmecillinam currently should be the first-line antibiotics for UTI caused by E. coli and most likely all lower UTI since the vast majority (80–90%) is caused by E. coli.1,2 The locally recommended option should be the one with the currently lowest resistance prevalence, least selective for resistance and least adverse effects.
Empirical treatment with ciprofloxacin was found to be significantly inferior to pivmecillinam for lower UTI caused by ESBL E. coli, but superior to pivmecillinam for non-ESBL E. coli when adjusting for resistance. This finding is in concordance with a randomized controlled trial where the fluoroquinolone was shown superior to pivmecillinam for older women with uncomplicated UTI.24 Nevertheless, considering the risk of development of resistance as well as long-term adverse reactions, fluoroquinolones should be saved for more severe infections and not be used empirically in lower UTI.25
Nitrofurantoin seems to show better effect for ESBL cases, even when adjusting for the higher resistance in the pivmecillinam group. However, the difference was not significant. A randomized controlled trial comparing pivmecillinam to nitrofurantoin for UTI is much warranted. Preferably as a non-inferiority trial using the widely used 10% margin.26 However, we believe this study provides evidence that nitrofurantoin and pivmecillinam should continue as first-line recommendations for UTI, especially in regions with high prevalence of ESBL E. coli – but local resistance patterns should guide the choice. In Denmark, pivmecillinam has been the main treatment for UTI during the study period and thus with a higher resistance compared to nitrofurantoin, especially in ESBL E. coli (p<0.001), with nitrofurantoin as the second choice, or as first choice in patients allergic to penicillins.
Even though aminopenicillins, trimethoprim and sulfamethizole should not be used empirically for UTI in Denmark due to resistance above the 20% threshold,2 several patients received these antibiotics (especially the latter). The outcome was clearly affected by resistance; however, the significant inferiority compared to pivmecillinam disappeared when adjusting for the resistance. This means that all the mentioned antibiotics can be considered equally effective for treatment of lower UTI, if a urine culture has confirmed susceptibility towards the drug.
A large placebo-controlled study has demonstrated that about 25% of uncomplicated UTI are self-limited within 14 days.27 In Figure 2, all antibiotics have a much higher clinical success probability compared to the self-limited effect, even if the E. coli is resistant to the prescribed antibiotic (except for trimethoprim). This is probably because of the high accumulation of the antibiotics in the urine.28 Nonsusceptibility to mecillinam has also been shown to not necessarily correlate with treatment failure.29 In our study, resistance to the empirically prescribed antibiotic results in a worse outcome for all antibiotics, including pivmecillinam. However, pivmecillinam seems to demonstrate the lowest prevalence of treatment failure when the isolates are resistant to the empirically prescribed antibiotic.
Limitations
The study has some important limitations. Firstly, no information on comorbidity or clinical data on the patients were retrieved. However, it is not recommended to culture sporadic uncomplicated UTI since it is considered unnecessary in the diagnosis of uncomplicated UTI.30–32 Thus, we assume that the population is skewed toward more complicated infections. Secondly, pivmecillinam was by far the most used antibiotic and that combined the low prevalence of ESBL E. coli resulted in a low number of ESBL cases treated with the other antibiotics. This might be the reason why a significant inferiority for ESBL cases compared to non-ESBL cases could not be demonstrated for nitrofurantoin (n=85) and aminopenicillins (n=25), as for the other antibiotics. Thirdly, fosfomycin, an antibiotic that also has low resistance in ESBL E. coli,9,33 was not available in Denmark during the study period. Nevertheless, nitrofurantoin has recently shown to be superior to fosfomycin for UTI, in a population with low proportions of ESBL E. coli.34 Lastly, failure of treatment rested alone on the redemption of a new prescription of an antibiotic commonly used for the treatment of UTI (with an indication for UTI stated on the prescription) or admission to hospital because of UTI; both clinical and microbiological outcomes are missing. Since clinical data were lacking, we cannot know if all patients had treatment failure due to a true UTI or because of other reasons (ie, intolerance/allergy to initial prescription, non-compliance, loss of prescription, change in prescription due to resistance, etc.). Thus, the considered treatment failures are likely overestimated in this register study and cannot be fully compared with prospective controlled clinical trials.
Despite the above-described limitations that come with a retrospective database study, this kind of methodology to measure the clinical outcome of antibiotic therapy in UTIs has been used before and is continuously being used.15,35,36 In addition, we believe this study has several major strengths that render the findings robust and useful: it was population-based including a very high number of unique patients during a long time period, it compares pivmecillinam and several antibiotics not compared before (neither for non-ESBL nor ESBL E. coli), and the data used are highly validated.
Conclusion
In our population, pivmecillinam and nitrofurantoin seem to have an overall high efficacy compared to other commonly used antibiotics and should at least be considered as equal first-line options for UTI caused by E. coli, especially in areas where ESBL prevalence and resistance to other more commonly used antibiotics are high. Local resistance data should guide which of the two should be the contemporary first-line option.
Résumé box
E. coli UTI is one of the most common infections in humans. The increased prevalence of ESBL-producing E. coli has significantly limited oral antibiotic treatment options. In this study, commonly used oral treatment options were found to be inferior in E. coli UTI when ESBL production is present. Pivmecillinam and nitrofurantoin seem to be the currently best oral option for E. coli UTI, and local resistance data should guide which of the two that should be the contemporary first-line option.
Acknowledgments
Preliminary results from this study were presented at ECCMID 2018, Madrid. Lilly og Herbert Hansens Fond (Bogense, Denmark) financed the study by impartial/non-commercial funding. The funding agent had no role in designing and conducting the study, analysis and interpretation of data, or writing and approval of the manuscript. FJ and NFM received a research grant from JPIAMR during the study period.
Author contributions
Filip Jansåker and Jonas Bredtoft Boel are co-primary authors. All authors take responsibility for the integrity of the data and the accuracy of the data analysis and have approved the final version of the manuscript. Concept: FJ. Design and planning: all authors. Access, acquisition and analysis of data: FJ, JBB, and JDK. Statistical analysis: JBB. Interpretation of data: all authors. Drafting of the manuscript: FJ, JBB and JDK. Critical revision of the manuscript for important intellectual content: ST, FBH, KHH, JDK and NFM. Administrative support: FBH, ST and KHH. Obtained funding: FJ. Guarantors and supervision: NFM and JDK. All authors agree to be accountable for all aspects of the work. The primary authors and supervisors attest that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Disclosure
All authors affirm that this manuscript was conducted honestly, accurately, and transparently. The authors report no conflicts of interest in this work.
References
1. Nicolle LE. Urinary tract infection. Crit Care Clin. 2013;29(3):699–715. doi:10.1016/j.ccc.2013.03.014
2. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103–e120. doi:10.1093/cid/ciq257
3. Pitout JD, Laupland KB. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis. 2008;8(3):159–166. doi:10.1016/S1473-3099(08)70041-0
4. Livermore DM. Fourteen years in resistance. Int J Antimicrob Agents. 2012;39(4):283–294. doi:10.1016/j.ijantimicag.2011.12.012
5. Kahlmeter G, Poulsen HO. Antimicrobial susceptibility of Escherichia coli from community-acquired urinary tract infections in Europe: the ECO.SENS study revisited. Int J Antimicrob Agents. 2012;39(1):45–51. doi:10.1016/j.ijantimicag.2011.09.013
6. Kahlmeter G, Ahman J, Matuschek E. Antimicrobial resistance of Escherichia coli causing uncomplicated urinary tract infections: a European update for 2014 and comparison with 2000 and 2008. Infect Dis Ther. 2015;4(4):417–423. doi:10.1007/s40121-015-0095-5
7. Tarnberg M, Ostholm-Balkhed A, Monstein HJ, Hallgren A, Hanberger H, Nilsson LE. In vitro activity of beta-lactam antibiotics against CTX-M-producing Escherichia coli. Eur J Clin Microbiol Infect Dis. 2011;30(8):981–987. doi:10.1007/s10096-011-1183-4
8. Thomas K, Weinbren MJ, Warner M, Woodford N, Livermore D. Activity of mecillinam against ESBL producers in vitro. J Antimicrob Chemother. 2006;57(2):367–368. doi:10.1093/jac/dki451
9. Zykov IN, Sundsfjord A, Smabrekke L, Samuelsen O. The antimicrobial activity of mecillinam, nitrofurantoin, temocillin and fosfomycin and comparative analysis of resistance patterns in a nationwide collection of ESBL-producing Escherichia coli in Norway 2010–2011. Infect Dis. 2016;48(2):99–107. doi:10.3109/23744235.2015.1087648
10. Graninger W. Pivmecillinam–therapy of choice for lower urinary tract infection. Int J Antimicrob Agents. 2003;22 Suppl 2:73–78.
11. Nicolle LE. Pivmecillinam in the treatment of urinary tract infections. J Antimicrob Chemother. 2000;46 Suppl 1:
12. Schito GC, Naber KG, Botto H, et al. The ARESC study: an international survey on the antimicrobial resistance of pathogens involved in uncomplicated urinary tract infections. Int J Antimicrob Agents. 2009;34(5):407–413. doi:10.1016/j.ijantimicag.2009.04.012
13.
14. Jansaker F, Frimodt-Moller N, Sjogren I, Dahl Knudsen J. Clinical and bacteriological effects of pivmecillinam for ESBL-producing Escherichia coli or Klebsiella pneumoniae in urinary tract infections. J Antimicrob Chemother. 2014;69(3):769–772. doi:10.1093/jac/dkt404
15. Soraas A, Sundsfjord A, Jorgensen SB, Liestol K, Jenum PA. High rate of per oral mecillinam treatment failure in community-acquired urinary tract infections caused by ESBL-producing Escherichia coli. PLoS One. 2014;9(1):e85889. doi:10.1371/journal.pone.0085889
16. Bollestad M, Grude N, Solhaug S, et al. Clinical and bacteriological efficacy of pivmecillinam treatment for uncomplicated urinary tract infections caused by ESBL-producing Escherichia coli: a prospective, multicentre, observational cohort study. J Antimicrob Chemother. 2018;73:2503–2509. doi:10.1093/jac/dky230
17. Auer S, Wojna A, Hell M. Oral treatment options for ambulatory patients with urinary tract infections caused by extended-spectrum-beta-lactamase-producing Escherichia coli. Antimicrob Agents Chemother. 2010;54(9):4006–4008. doi:10.1128/AAC.01760-09
18. Schmidt M, Pedersen L, Sorensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541–549. doi:10.1007/s10654-014-9930-3
19. Aspevall O, Hallander H, Gant V, Kouri T. European guidelines for urinalysis: a collaborative document produced by European clinical microbiologists and clinical chemists under ECLM in collaboration with ESCMID. Clin Microbiol Infect. 2001;7(4):173–178.
20.
21. Azuz S, Gertsen JB, Rasmussen SS, Jensen CS, Jansaker EF, Fuglsang-Damgaard D. Behind the scenes in urinalysis: to report or not to report. Infect Dis. 2017;49(9):712–715. doi:10.1080/23744235.2017.1318218
22. EUCAST guidelines for detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance. Available from:
23. Hansen DS, Schumacher H, Hansen F, et al. Extended-spectrum beta-lactamase (ESBL) in Danish clinical isolates of Escherichia coli and Klebsiella pneumoniae: prevalence, beta-lactamase distribution, phylogroups, and co-resistance. Scand J Infect Dis. 2012;44(3):174–181. doi:10.3109/00365548.2011.632642
24. Nicolle LE, Madsen KS, Debeeck GO, et al. Three days of pivmecillinam or norfloxacin for treatment of acute uncomplicated urinary infection in women. Scand J Infect Dis. 2002;34(7):487–492. doi:10.1080/00365540110080728
25. Kaur K, Fayad R, Saxena A, et al. Fluoroquinolone-related neuropsychiatric and mitochondrial toxicity: a collaborative investigation by scientists and members of a social network. J Community Support Oncol. 2016;14(2):54–65. doi:10.12788/jcso.0167
26.
27. Ferry SA, Holm SE, Stenlund H, Lundholm R, Monsen TJ. Clinical and bacteriological outcome of different doses and duration of pivmecillinam compared with placebo therapy of uncomplicated lower urinary tract infection in women: the LUTIW project. Scand J Prim Health Care. 2007;25(1):49–57. doi:10.1080/02813430601183074
28. Frimodt-Moller N. Correlation between pharmacokinetic/pharmacodynamic parameters and efficacy for antibiotics in the treatment of urinary tract infection. Int J Antimicrob Agents. 2002;19(6):546–553.
29. Monsen TJ, Holm SE, Ferry BM, Ferry SA. Mecillinam resistance and outcome of pivmecillinam treatment in uncomplicated lower urinary tract infection in women. APMIS. 2014;122(4):317–323. doi:10.1111/apm.12147
30. Bent S, Nallamothu BK, Simel DL, Fihn SD, Saint S. Does this woman have an acute uncomplicated urinary tract infection? JAMA. 2002;287(20):2701–2710. doi:10.1001/jama.287.20.2701
31. Colgan R, Williams M. Diagnosis and treatment of acute uncomplicated cystitis. Am Fam Physician. 2011;84(7):771–776.
32. Grigoryan L, Trautner BW, Gupta K. Diagnosis and management of urinary tract infections in the outpatient setting: a review. JAMA. 2014;312(16):1677–1684. doi:10.1001/jama.2014.12842
33. Wilson DT, May DB. Potential role of fosfomycin in the treatment of community-acquired lower urinary tract infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Am J Ther. 21768870. doi:10.1097.
34. Huttner A, Kowalczyk A, Turjeman A, et al. Effect of 5-day nitrofurantoin vs single-dose fosfomycin on clinical resolution of uncomplicated lower urinary tract infection in women: a randomized clinical trial. JAMA. 2018;319(17):1781–1789. doi:10.1001/jama.2018.3627
35. Bjerrum L, Dessau RB, Hallas J. Treatment failures after antibiotic therapy of uncomplicated urinary tract infections. A prescription database study. Scand J Prim Health Care. 2002;20(2):97–101.
36. Montelin H, Forsman KJ, Tangden T. Retrospective evaluation of nitrofurantoin and pivmecillinam for the treatment of lower urinary tract infections in men. PLoS One. 2019;14(1):e0211098. doi:10.1371/journal.pone.0211098
Supplementary materials
 | Table S1 Unadjusted empirical treatment failure for nonESBL- and ESBL-producing E. coli UTI |
 | Table S2 Failure of empirical treatment for ESBL versus non-ESBL (reference) -producing E. coli UTI (adjusted for age and sex) |
 | Table S3 Failure of empirical treatment for non-ESBL- and ESBL-producing E. coli UTI (adjusted for age, sex, and resistance). |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
