Back to Journals » Drug Design, Development and Therapy » Volume 14
Pinocembrin Ameliorates Cognitive Impairment Induced by Vascular Dementia: Contribution of Reelin-dab1 Signaling Pathway
Authors Kang ZC, Wang HG, Yang YL, Zhao XY, Zhou QM, Yang YL, Yang JY, Du GH
Received 10 February 2020
Accepted for publication 12 June 2020
Published 4 September 2020 Volume 2020:14 Pages 3577—3587
DOI https://doi.org/10.2147/DDDT.S249176
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Manfred Ogris
Ze-Chun Kang,1,2 Hai-Gang Wang,2 Yu-Lin Yang,2 Xiao-Yue Zhao,2 Qi-Meng Zhou,2 Ying-Lin Yang,2 Jing-Yu Yang,1,* Guan-Hua Du1,2,*
1Department of Pharmacology, Shenyang Pharmaceutical University, Shenyang City, Liaoning Province, People’s Republic of China; 2Beijing Key Laboratory of Drug Target and Screening Research, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jing-Yu Yang
Department of Pharmacology, Shenyang Pharmaceutical University, Shenyang City, Liaoning Province, People’s Republic of China
Email [email protected]
Guan-Hua Du
Beijing Key Laboratory of Drug Target and Screening Research, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China
Email [email protected]
Background: As a substrate of apoER2, Reelin has been verified to exert neuroprotection by preventing memory impairment. Pinocembrin is the most abundant natural flavonoid found in propolis, and it has been used to exert neuroprotection, blood–brain barrier protection, anti-oxidation, and inflammation diminishing, both in vitro and in vivo. However, the roles and molecular mechanisms of pinocembrin in neurobehavioral outcomes and neuronal repair after vascular dementia are still under investigation.
Purpose: To explore the role of pinocembrin in the involvement of the Reelin-dab1 signaling pathway in improving memory impairment, both in cell culture and animals experiments.
Material and Methods: Behavioral tests were conducted on day 48 to confirm the protection of pinocembrin against cognitive impairment. Cell and molecular biology experiments demonstrated that the Reelin-dab1 pathway mediates the underlying mechanism of cognitive improvement by pinocembrin.
Results: It was showed that pinocembrin alleviated learning and memory deficits induced by vascular dementia, by inducing the expression of Reelin, apoER2, and p-dab1 in the hippocampus. The expression of Reelin and p-dab1 was both inhibited following Reelin RNA interference in SH-SY5Y prior to oxygen glucose deprivation (OGD) injury, suggesting that Reelin played a core role in pinocembrin’s effect on OGD in vitro.
Conclusion: Pinocembrin improves the cognition via the Reelin-dab1 signaling pathway.
Keywords: pinocembrin, Reelin, vascular dementia, hippocampus, cognitive impairment, signaling pathway
Introduction
Vascular dementia (VD) characterized by cognitive deficits is the second most frequent etiology of dementia. The pathogenesis of VD remains not fully understood, leading to the lack of a cure. Therefore, a therapeutic target needs to be identified, which allows for the alleviation of cognitive impairment, aiming to prevent or treat VD using novel pharmacological agents. Reelin, an extracellular matrix molecule mostly investigated as a critical factor in controlling the migration and laminar arrangement of neurons in various structures, including the spinal cord, neocortex, cerebellum, and hippocampus during brain development, is involved in many neuropsychiatric and neurodegenerative diseases with clinical symptoms of cognitive deficits, including schizophrenia, bipolar disorder, depression, epilepsy, and autism.1–7 In addition, the signaling pathways of Reelin seem to be particularly susceptible to neurodegenerative diseases, potentially contributing to their pathogenesis.8
Pinocembrin (5,7-dihydroxyflavanone, also designated as pino) is the most abundant natural flavonoid identified in propolis, honey, and several plants including wild marjoram and ginger roots.9–11 In 1992, Pinocembrin was extracted from Eriodictyon californicum for the first time, and over the past decade, many reports have showed its protective ability against ischemic brain injury with a wide treatment time window.12 Pinocembrin has been used to exert neuroprotection,13 blood–brain barrier protection,14 anti-oxidation,13,15 and inflammation diminishing16 both in vitro and in vivo. It has also been reported to be involved in neuroprotection against Chronic cerebral hypoperfusion (CCH) by brain mitochondria.17
This study aimed to explore the roles and molecular mechanisms of pinocembrin in neurobehavioral outcomes and neuronal repair after VD induction in rats.
Materials and Methods
Agents and Drugs
Pinocembrin (≥99%, tested by HPLC) was performed as sterile powder for injection by synthesis and processing at the Department of New Drug Development, Institute of Materia Medica, Chinese Academy of Medical Sciences. Prior to being used, it was dissolved in 0.9% NaCl. SH-SY5Y cells were purchased commercially from American Type Culture Collection.
Animals and Experimental Design
In this experiment, we established vascular dementia models by bilateral common carotid artery ligation by referring to studies by Liao and Zhu.18,19 Ten percent chloral hydrate (4mL/kg) was used for anesthetization of male Wistar rats (6 to 8 weeks old, 180 to 200g, Vital River Company, Beijing, China), after anesthetization, the bilateral carotid arteries were exposed through a cervical incision and occluded using silk sutures. During the surgical procedure, the use of a heating pad prevented hypothermia. The rats which were operated named as operative group. The rats in the control group (sham group) were sham-operated, which involved the bilateral exposure of the common carotid arteries.
From postoperative day 24 to day 28, all the rats, including rats in the sham and operative groups, were given the screening trial 3 times daily. The trail was a part of Morris water maze test (MWM) and we considered the time the rats to find the submerged platform as the escape latency.20 The average of three escape latency values in rats from each operative group (value 1) and total mean escape latency in rats from the sham group (value 2) on the day 5 were detected. Screening ratio (SR) was identified as an index for judging the cognitive impairment for each operative rats.SR = (value1−value 2)/value 2. The rat was identified as a cognitive impairment when SR was >0.2. Those operative group rats with an SR >0.2 were randomly assigned to 4 groups: (1) 2VO (Cognitive impairment induced by permanent ligation of bilateral common carotid artery) rats treated with normal saline (2VO group); (2) 2VO rats treated with pinocembrin 1 mg/kg (pino-1group); (3) 2VO rats treated with pinocembrin 3 mg/kg (pino-3group); and (4) 2VO rats treated with pinocembrin 10 mg/kg (pino-10 group). Each group consisted of 12 rats.
From post-surgical day 29, the rats were given a daily intravenous injection (tail vein) of either pinocembrin (1, 3.10 mg/kg) or vehicle (normal saline, 0.9%NaCl), until the end of the experiment on day 56. From day 48 after surgery, all the rats were given the step down passive avoidance test. From day 50 after surgery, all the rats (sham group,2 VO group, pino-1 group, pino-3 group and pino-10 group) were given the MWM test (Figure 1).
 |
Figure 1 Experimental design, time-line of pinocembrin administration and behavioral tasks (n = 12 per group). Abbreviation: MWM, Morris water maze. |
All animals were kept in at 25°C and humidity of 65%, and provided with water and food ad libitum. All animal protocols were strictly on basis of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The Experimental Animal Administration Committee of the Peking Union Medical College approved all protocols and experimental designs.
Morris Water Maze Test
The rats’ pre- and post-treatment cognitive function was evaluated using the MWM as previously described. Briefly, the protocol consisted of three trials per day for five consecutive days, with each trial randomly started from one of the four quadrants. The experiment was started by putting rats into the water toward walls of pool. The rats were then permitted to swim until they could reach the hidden platform (the maximum swimming time was 60s). The video camera was used to record the escape latencies and paths of swimming. If the rat failed to escape within 60s, the researcher guided rats to the platform and kept them on it for 10 s prior to the next experiment. At day 6, a probe experiment was performed as follows: the platform was removed from the pool and the rats were permitted to swim freely for 60s. The percentage of time spent in the target quadrant was recorded and used as a measure of spatial memory.
Step Down Passive Avoidance Test
The step down test consisted of placing the rats on an elevated platform placed close to the wall of an arena. The rats learned to associate the exploration of the adjacent compartment with an aversive stimulus, consisting of a foot shock delivered through the floor grid (10 electric shocks, 20ms/0.5mA/5Hz). If the rats were exposed once more to the same environment, they would increase the latency before stepping down onto the platform or avoid stepping down. The time to avoid stepping down was recorded as latencies. If the rats stepped down onto the platform, we considered that these rats had an error. A recall trial was performed 24h after the acquisition trial, in a similar manner to the acquisition trial, but with recording the Error number made by each rat in 300s.
Measurement of Plasma Reelin Levels
Whole blood was collected into microcentrifuge tubes using a sterile capillary tube, according to the manufacturer’s instructions. Plasma was collected by centrifuging the blood samples at 2500×g for 15 min at 4°C, then stored at −80°C. The cortex and hippocampus were immediately weighed and homogenized on ice with tissue homogenizer in the PBS buffer. The tissue homogenates were centrifuged at 12,000 g at 4°C for 10 min, and the supernatants were used to detect Reelin concentration. Reelin concentrations were measured using a rat Reelin immunoassay kit (Abcam, Cambridge, MA, USA).
Tissue Preparation
On day 56 after surgery, 6 rats from each group were anesthetized using 10% chloral hydrate and perfused with 0.9% saline. The hippocampus, cerebral cortex were isolated for Western blotting trials and enzyme-linked immunosorbent assay (ELISA), and these specimens were kept in liquid nitrogen. Six rats from each group were anesthetized using 10% chloral hydrate and perfused with 4% paraformaldehyde solution. The cerebral tissues were fixed with 4% paraformaldehyde solution for 24 hours. Brain tissue was dehydrated with ethanol at different concentration gradients, rendered transparent by xylene and embedded in paraffin, after which pathological sections were cut and stained with hematoxylin-eosin staining (HE).
In vitro Cell Viability Assays
We used the SH-SY5Y under oxygen glucose deprivation (OGD) condition as the model in vitro to simulate the neuron damage in VD.21 The cells in 96-well were cultured in DMEM medium containing 10% FBS (fetal bovine serum) at 5% CO2 and 37°C. When the confluency of cells reached to 85%, 0.5% of serum was used to starve cells overnight prior to pretreatment with various concentrations of pinocembrin for 24 hours. The cells were then exposed to DMEM without glucose and oxygen for 24 hours. Cell viability was determined using methyl thiazolyl tetrazolium assays (Sigma Aldrich, Inc). The absorbance was determined at 490nm.
siRNA Transfection
SH-SY5Y cells with a concentration of 1×105/mL were cultured in six-well plates. The cells were cultured for 24 hours and the confluency of cells was 60–70%. After being washed with siRNA transfection medium (included in the siRNA reagent system from Santa Cruz Biotechnology, sc-36,868), the cells were incubated with Reelin siRNA (Santa Cruz Biotechnology, sc-42,208) for 6 hours. The cells with transfection of Reelin siRNA were cultured with fresh medium for one day. The medium was changed and cells were cultured for another 36 hours, and then retreated for 24 hours with pinocembrin. Following this, Western blotting was performed for cells. The siRNA transfection protocol was completed with the standards of biological safety and security procedures.
Western Blot
The tissues of hippocampal were homogenized in lysis buffer (Beyotime, China) (50mM HEPES, pH7.4, 150mM NaCl, 1mM β-glycerophosphate, 3mMDTT, 3mMNaVO3, 1mM EDTA, 1Mmegta, 1NaF, 1mM Phenylmethylsulfonyl fluoride, 1% TritonX and 1×protease and phosphatase inhibitors cocktail) and centrifuged (12,000 rpm) at 4°C for 15 min. Protein lysates (40µg) were electrophoresed on 8% or 6% sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis (PAGE) gels, then transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, USA). At room temperature, 50 g/L nonfat milk was used to block the cell membranes for 1 hour, then the membranes were cultured overnight at 4°C with Anti-Reelin antibody [G10] (ab78540), Anti-apolipoprotein E receptor 2 (ApoER2) antibody (ab204112), Phospho-Dab1 (Tyr220) Antibody (#3327). Next, the membranes were incubated using horseradish peroxidase – conjugated goat anti-rabbit IgG (1:1000 dilution; Beyotime, China) for 60 min at room temperature. Images of blots were visualized with Western Lightning Plus-ECL (Thermo Fisher Scientific, USA). Band densities were calculated withβ-actin as internal reference (1:1000 dilution; Beyotime, China).
Statistical Analysis
Data analyses were performed with the SPSS 22.0 software package. The measurement data were expressed as the mean ± standard deviation (Mean±SD). Independent t-test was adopted for comparison between the two groups and the One-way or two-way analysis of variance (ANOVA) for comparison among multiple groups. Chi-square test (χ2 test) was adopted for comparison between the two groups as to enumeration data expressed as the percentage or case (n). P<0.05 was considered statistically different.
Results
Pinocembrin Alleviates VD-Induced Learning and Memory Deficits
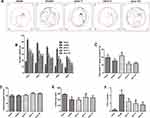
VD is characterized by progressive neuronal damage with learning and memory deficits. Training trials on the MWM were used to assess place or spatial learning. During those trials, VD rats traveled longer than those in the sham group, whereas rats treated with pinocembrin traveled shorter than those treated with saline (Figure 2A). Compared with those in the sham group, saline-treated rats had longer escape latencies from day 1 to day 5 as shown in Figure 2B. The pino-3 group had a significantly shorter escape latency than saline-treated rats (P < 0.05). In the probe trail, the pino-1 group spent significantly longer in the target quadrant compared to saline-treated rats (P < 0.05), indicating that pinocembrin can alleviate learning and memory deficits (Figure 2C). To determine the influence of the swimming capacity on escape latency, the swimming speed was calculated for each group. There were no significant differences among groups regarding speed of swimming (P > 0.05; Figure 2D).
The step down type passive avoidance test was performed to investigate whether pinocembrin affects the rats’ behavior. It came out that pinocembrin reduces the number of errors (P < 0.05; Figure 2E), decreasing the latency to step down (P < 0.05; Figure 2F) (Figure 2AF).
Pinocembrin Attenuates Neuronal Damage in the Rat’s Hippocampus
To evaluate the protective effect of pinocembrin on the hippocampal neurons injured by 2VO surgery, the brain sections obtained from each group were stained with hematoxylin and eosin. In the sham group, CA1 neurons were aligned with a clear cytoplasm and nuclei, whereas in the 2VO group, CA1 neurons exhibited an irregular arrangement and focal necrosis. In the groups treated with pinocembrin, the neurons were significantly more regular than that in the 2VO group. Among these groups, the strongest protective effect of CA1 neurons was observed in the group treated with 3mg/kg pinocembrin (Figure 3).
Pinocembrin Ameliorates the Level of Reelin in Plasma, Cerebral Cortex, and Hippocampus
In the present study, ELISA was used to evaluate the level of Reelin in plasma, the cerebral cortex, and the hippocampus. As shown in Table 1, VD was associated with a decreased level of Reelin VD in plasma, the cerebral cortex, and the hippocampus (all P < 0.05), which was mitigated by treatment with pinocembrin (all P < 0.05).
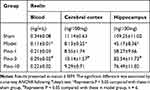 |
Table 1 Effects of Pinocembrin on the Level of Reelin in Plasma, the Cerebral Cortex, and the Hippocampus |
Pinocembrin Increases Protein Expression of the Reelin-dab1 signal Pathway in the Hippocampus
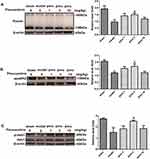
As shown in Figure 4A, 8 weeks after surgery, the protein expression of Reelin in the hippocampus was significantly downregulated compared with the sham group (P < 0.05). The downregulation was mitigated by pinocembrin, which facilitated the expression of Reelin in the hippocampus (P < 0.05). As shown in Figure 4B and C, VD is associated with the downregulation of apoER2 (the best-characterized Reelin receptors-2) and p-dab1 (Reelin’s intracellular adaptor protein-1) expression (both P < 0.05), which is mitigated by pinocembrin administration (both P < 0.05).(Figure 4AC)
Pinocembrin Activates the Reelin-dab1 signaling Pathway in vitro
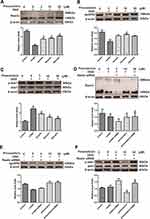
To further investigate the role of the Reelin-dab1 signaling pathway, we examined the protective role of pinocembrin in SH-SY5Y injury induced by OGD. As seen in Figure 5A, the expression of Reelin in SH-SY5Y is downregulated by OGD and significantly elevated by pinocembrin (P < 0.05). The results in Figure 5B showed a decrease in apoER2 expression presented by the OGD group, which was mitigated by pinocembrin administration (P < 0.05). Although the change tendency of p-dab1 level expression after OGD in SH-SY5Y was different from that in the hippocampus, the reversing action of pinocembrin was similar (P < 0.05, Figure 5C).
To further investigate the core role of Reelin on the effect pinocembrin on VD, we tested the silencing efficiency of Reelin siRNA in SH-SY5Y. The results revealed that Reelin siRNA reduced Reelin expression by 4695% in the presence or absence of pinocembrin, respectively (Figure 5D). However, there was no significant difference for the expression of apoER2 after blocking of the RELN gene between groups, as shown in Figure 5E (P > 0.05). The phosphorylation of dab1 occurred downstream of Reelin signaling since, under Reelin RNA interference, pinocembrin increased the phosphorylation of dab1 compared with the OGD group, as shown in Figure 5F (P < 0.05). (Figure 5AF)
Discussion
Several studies have demonstrated that pinocembrin can be used to treat diseases including stroke, Alzheimer’s disease (AD) and VD.22,23 In this study, we hypothesized that the mechanisms underlying the effect of pinocembrin on VD may be associated with a decreased damage of hippocampal neurons and up-regulation of the Reelin-dab1signaling pathway. Our results demonstrated the following: (1) pinocembrin reduced impairment of memory induced by 2VO through decreasing the escape latency and extending the mean time spent in the target quadrant in the MWM, without an increase in swimming speed; (2) pinocembrin improved the impaired learning ability by reducing the number of errors and decreasing the latency to step down in the step down type passive avoidance test; (3) pinocembrin reduced hippocampal neuronal damage, as evaluated by morphological observations; (4) pinocembrin enhanced the expression of Reelin and apoER2 and increased the phosphorylation of dab1 in 2VO rats and OGD SH-SY5Ycells; (5) pinocembrin had no effect on OGD SH-SY5Y cells, which mediated RELN gene silencing. In the present study, pinocembrin was used at 1, 3, and 10mg/kg doses in animal experiment. Taken together, our findings suggest that pinocembrin enhances cognitive impairment via the reduction of hippocampal neuronal damage, and the mechanism of pinocembrin on improving cognitive impairment is related to the Reelin-dab1 signaling pathway.
VD is a clinical syndrome of the acquired loss of cognitive skills, which poses a great burden on older individuals and their families. In contrast to ischemic stroke caused by a sudden reduction of the blood flow to the corresponding brain regions, VD is caused by a gradual decrease in the cerebral blood flow, bringing about memory impairment and progressing to dementia. The 2VO rat model is a typical animal model to investigate the cognitive impairments associated with VD.24 The hippocampal blood flow drops significantly after 2VO surgery, with an obvious cognitive deficit occurring at 4 weeks.25 One of the main clinical features of VD is the cognitive decline and the MWM is the most extensively used behavior trial to evaluating memory and learning in rodents.26 In the present study, 2VO rats showed a cognitive impairment in the first stage of the MWM test. In the second stage, pinocembrin-treated rats showed decreased escape latency and spent significantly longer in the target quadrant, without an improvement of the swimming ability. The step down test is commonly used to assess long-term memory in rodents and in the present study, pinocembrin-treated rats showed less steps down than 2VO rats. The hippocampus is a crucial brain region in the central nervous system (CNS), which is responsible for learning and memory. A previous review has provided powerful evidence that damage to the hippocampus causes learning and memory impairments.27 In the present study, HE staining showed 2VO-associated degeneration of hippocampal neurons, which was alleviated by pinocembrin. This result was consistent with the results from the MWM and the step down test.
The above results confirmed that treatment with pinocembrin alleviates cognitive impairment and relieves neuronal damage. We strongly suggest a link between cognition improvement and the Reelin-dab1 signaling pathway. The suggestions were proved by the following: (1) pinocembrin exerts an inducing effect on the expression of Reelin and apoER2, up-regulating dab1 in the hippocampus and in SH-SY5Y; (2) silencing of the RELN gene suppressed dab1 phosphorylation.
Reelin is a large, secreted extracellular matrix glycoprotein, and firstly detected from the developing brain tissues. Reelin is secreted by the Cajal-Retzius neurons in the marginal zone of developing cerebral cortex and hippocampus into the circulation. It plays a key role in learning and memory by the adult brain. The expression and function of Reelin in the brain have been widely studied. Furthermore, the expression of Reelin in other tissues such as blood has also been analyzed. Telese et al used global nuclear run-on sequencing to show that Reelin-induced cortical neurons leaded to the rapid activation of a transcriptome involvement of learning and memory and illuminated that down-regulation expression in schizophrenic patients could help to the development of cognitive impairment of this disease.28 However, the correlation of Reelin with VD has not yet been reported. As shown in Table 1, in the present study, ELISA revealed that Reelin level significantly decreased in the blood of 2VO rats. The level of Reelin increased with pinocembrin administration. The results of hippocampus determined by ELISA were consistent with those of Western blotting (Figure 3A), indicating that the protein expression of Reelin was stronger in pinocembrin-treated than saline-treated rats.
The Reelin pathway was previously reported to exert a key role in the adult brain by promoting learning and memory,29,30 which makes it a key candidate pathway for VD investigation. A study31 by Wu et al showed that Reelin-deficient mice exhibited increased stroke size, suggesting that Reelin plays a neuroprotective role in cerebral ischemia. Scholars have discovered that apoER2 plays a role as a signal transducer factor in the signaling pathway induced by Reelin through protein-to-protein interaction based on Reelin’s motif identified by its dab1. Therefore, revealing the molecular mechanism underlying the effect of pinocembrin on learning and memory occurs via the Reelin-dab1 signal pathway is very meaningful. As shown by our results, pinocembrin can effectively promote the expression of Reelin and the phosphorylation of dab1, although the expression of apoER2 is not significantly altered. It confirms Reelin’s core role in the Reelin-dab1 signal pathway. However, there are still some deficiencies in this study. Vascular dementia and vascular cognitive impairment caused by vascular dementia are senile diseases, and age is a major risk factor for them.32 Many studies in gerontics have revealed that the response to ischemia varies greatly with age, especially in terms of cognitive endpoints.33,34 In our study, we established vascular dementia models with 6–8-week old rats by bilateral common carotid artery ligation, so the cognitive impairment occurred for a short period of time, and the change of cognitive impairment may be slightly different from the physiological and pathological process of senile patients with vascular dementia. Thus, in the future, the role of Pinocembrin in ameliorating cognitive impairment induced by vascular dementia is still needed to be furtherly confirmed through modified methods of animal experiments and clinical trials. All in all, using behavioral testing, cell culture and RNA silencing, we uncovered that pinocembrin administration ameliorate cognitive deficits resulting from the bilateral carotid arteries occlusion, and also uncovered that pinocembrin attenuated neuronal damage was related to Reelin-dab1 signaling pathway. Reelin is very important for memory, so we come to the conclusion that Reelin is likely beneficial for memory.
Conclusion
All in all, using behavioral testing, cell culture and RNA silencing, we uncovered that pinocembrin administration ameliorate cognitive deficits resulting from the bilateral carotid arteries occlusion, and we also uncovered that pinocembrin attenuated neuronal damage was related to Reelin-dab1 signaling pathway. Reelin is very important for memory, so we come to the conclusion that Reelin is likely beneficial for memory.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bertelli D, Papotti G, Bortolotti L, Marcazzan GL, Plessi M. (1)H-NMR simultaneous identification of health-relevant compounds in propolis extracts. Phytochem Anal. 2012;23(3):260–266. doi:10.1002/pca.1352
2. Danert FC, Zampini C, Ordonez R, Maldonado L, Bedascarrasbure E, Isla MI. Nutritional and functional properties of aqueous and hydroalcoholic extracts from Argentinean propolis. Nat Prod Commun. 2014;9(2):167–170.
3. Eichenbaum H. The role of the hippocampus in navigation is memory. J Neurophysiol. 2017;117(4):1785–1796. doi:10.1152/jn.00005.2017
4. Farkas E, Luiten PG, Bari F. Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res Rev. 2007;54(1):162–180. doi:10.1016/j.brainresrev.2007.01.003
5. Forster E, Bock HH, Herz J, Chai X, Frotscher M, Zhao S. Emerging topics in Reelin function. Eur J Neurosci. 2010;31(9):1511–1518. doi:10.1111/j.1460-9568.2010.07222.x
6. Forster E, Zhao S, Frotscher M. Laminating the hippocampus. Nat Rev Neurosci. 2006;7(4):259–267. doi:10.1038/nrn1882
7. Gao M, Zhu SY, Tan CB, Xu B, Zhang WC, Du GH. Pinocembrin protects the neurovascular unit by reducing inflammation and extracellular proteolysis in MCAO rats. J Asian Nat Prod Res. 2010;12(5):407–418. doi:10.1080/10286020.2010.485129
8. Guang HM, Du GH. Protections of pinocembrin on brain mitochondria contribute to cognitive improvement in chronic cerebral hypoperfused rats. Eur J Pharmacol. 2006;542(13):77–83. doi:10.1016/j.ejphar.2006.04.054
9. Herring A, Donath A, Steiner KM, et al. Reelin depletion is an early phenomenon of Alzheimer’s pathology. J Alzheimers Dis. 2012;30(4):963–979. doi:10.3233/JAD-2012-112069
10. Hu C, Wang P, Zhang S, et al. Neuroprotective effect of melatonin on soluble Abeta1-42-induced cortical neurodegeneration via Reelin-Dab1 signaling pathway. Neurol Res. 2017;39(7):621–631. doi:10.1080/01616412.2017.1312805
11. Izuta H, Shimazawa M, Tazawa S, Araki Y, Mishima S, Hara H. Protective effects of Chinese propolis and its component, chrysin, against neuronal cell death via inhibition of mitochondrial apoptosis pathway in SH-SY5Y cells. J Agric Food Chem. 2008;56(19):8944–8953. doi:10.1021/jf8014206
12. Lee GH, D’Arcangelo G. New insights into Reelin-mediated signaling pathways. Front Cell Neurosci. 2016;10:122. doi:10.3389/fncel.2016.00122
13. Liang J, Yu Y, Wang B, et al. Ginsenoside Rb1 attenuates oxygen-glucose deprivation-induced apoptosis in SH-SY5Y cells via protection of mitochondria and inhibition of AIF and cytochrome c release. Molecules. 2013;18(10):12777–12792. doi:10.3390/molecules181012777
14. Liu R, Li JZ, Song JK, et al. Pinocembrin improves cognition and protects the neurovascular unit in Alzheimer related deficits. Neurobiol Aging. 2014;35(6):1275–1285. doi:10.1016/j.neurobiolaging.2013.12.031
15. Lussier AL, Weeber EJ, Rebeck GW. Reelin proteolysis affects signaling related to normal synapse function and neurodegeneration. Front Cell Neurosci. 2016;10:75. doi:10.3389/fncel.2016.00075
16. Massaro CF, Katouli M, Grkovic T, et al. Anti-staphylococcal activity of C-methyl flavanones from propolis of Australian stingless bees (Tetragonula carbonaria) and fruit resins of Corymbia torelliana (Myrtaceae). Fitoterapia. 2014;95:247–257. doi:10.1016/j.fitote.2014.03.024
17. Meng F, Liu R, Gao M, et al. Pinocembrin attenuates blood-brain barrier injury induced by global cerebral ischemia-reperfusion in rats. Brain Res. 2011;1391:93–101. doi:10.1016/j.brainres.2011.03.010
18. Liao W, Xue Z, Wang X, et al. Metabolic profiling deciphering the potential targets of Yi-Gan San against vascular dementia in rat. Brain Res. 2020;1727:146512. doi:10.1016/j.brainres.2019.146512
19. Zhu NW, Yin XL, Lin R, et al. Possible mechanisms of lycopene amelioration of learning and memory impairment in rats with vascular dementia. Neural Regen Res. 2020;15(2):332–341. doi:10.4103/1673-5374.265565
20. Collaer ML, Hindmarsh PC, Pasterski V, Fane BA, Hines M. Reduced short term memory in congenital adrenal hyperplasia (CAH) and its relationship to spatial and quantitative performance. Psychoneuroendocrinology. 2016;64:164–173. doi:10.1016/j.psyneuen.2015.11.010
21. Li N, Gu Z, Li Y, Fu X, Wang J, Bai H. A modified bilateral carotid artery stenosis procedure to develop a chronic cerebral hypoperfusion rat model with an increased survival rate. J Neurosci Methods. 2015;255:115–121. doi:10.1016/j.jneumeth.2015.08.002
22. Qin L, Tu W, Sun X, Zhang J, Chen Y, Zhao H. Retardation of neurobehavioral development and reelin down-regulation regulated by further DNA methylation in the hippocampus of the rat pups are associated with maternal deprivation. Behav Brain Res. 2011;217(1):142–147. doi:10.1016/j.bbr.2010.10.018
23. Rogers JT, Rusiana I, Trotter J, et al. Reelin supplementation enhances cognitive ability, synaptic plasticity, and dendritic spine density. Learn Mem. 2011;18(9):558–564. doi:10.1101/lm.2153511
24. Shi LL, Chen BN, Gao M, et al. The characteristics of therapeutic effect of pinocembrin in transient global brain ischemia/reperfusion rats. Life Sci. 2011;88(1112):521–528. doi:10.1016/j.lfs.2011.01.011
25. Telese F, Ma Q, Perez PM, et al. LRP8-Reelin-regulated neuronal enhancer signature underlying learning and memory formation. Neuron. 2015;86(3):696–710. doi:10.1016/j.neuron.2015.03.033
26. Vorhees CV, Williams MT. Reprint of “Value of water mazes for assessing spatial and egocentric learning and memory in rodent basic research and regulatory studies. Neurotoxicol Teratol. 2015;52(Pt A):93–108. doi:10.1016/j.ntt.2015.06.002
27. Zhang H, Lai Q, Li Y, Liu Y, Yang M. Learning and memory improvement and neuroprotection of Gardenia jasminoides (Fructus gardenia) extract on ischemic brain injury rats. J Ethnopharmacol. 2017;196:225–235. doi:10.1016/j.jep.2016.11.042
28. Pujadas L, Rossi D, Andres R, et al. Reelin delays amyloid-beta fibril formation and rescues cognitive deficits in a model of Alzheimer’s disease. Nat Commun. 2014;5:3443. doi:10.1038/ncomms4443
29. Wasser CR, Herz J. Reelin: Neurodevelopmental architect and homeostatic regulator of excitatory synapses. J Biol Chem. 2017;292(4):1330–1338. doi:10.1074/jbc.R116.766782
30. Won SJ, Kim SH, Xie L, et al. Reelin-deficient mice show impaired neurogenesis and increased stroke size. Exp Neurol. 2006;198(1):250–259. doi:10.1016/j.expneurol.2005.12.008
31. Wu CX, Liu R, Gao M, et al. Pinocembrin protects brain against ischemia/reperfusion injury by attenuating endoplasmic reticulum stress induced apoptosis. Neurosci Lett. 2013;546:57–62. doi:10.1016/j.neulet.2013.04.060
32. Wolters FJ, Ikram MA. Epidemiology of Vascular Dementia. Arterioscler Thromb Vasc Biol. 2019;39(8):1542–1549. doi:10.1161/ATVBAHA.119.311908
33. Santos MAO, Bezerra LS, Correia CDC, Bruscky IS. Neuropsychiatric symptoms in vascular dementia: epidemiologic and clinical aspects. Dement Neuropsychol. 2018;12(1):40–44. doi:10.1590/1980-57642018dn12-010006
34. Maestre GE, Mena LJ, Melgarejo JD, et al. Incidence of dementia in elderly latin americans: results of the maracaibo aging study. Alzheimers Dement. 2018;14(2):140–147. doi:10.1016/j.jalz.2017.06.2636
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.