Back to Journals » Blood and Lymphatic Cancer: Targets and Therapy » Volume 13
PI3k Inhibitors in NHL and CLL: An Unfulfilled Promise
Authors Bou Zeid N , Yazbeck V
Received 6 September 2022
Accepted for publication 22 January 2023
Published 8 March 2023 Volume 2023:13 Pages 1—12
DOI https://doi.org/10.2147/BLCTT.S309171
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Wilson Gonsalves
Naji Bou Zeid,1 Victor Yazbeck2
1Hôtel-Dieu de France Hospital, Saint Joseph University of Beirut, Beirut, Lebanon; 2Massey Cancer Center, Virginia Commonwealth University, Richmond, VA, USA
Correspondence: Victor Yazbeck, Virginia Commonwealth University, 401 College Street, Box 980035, Richmond, VA, 23298, USA, Tel +1-804-628-2073, Fax +1-804-828-5941, Email [email protected]
Abstract: Phosphatidylinositol 3-kinases (PI3Ks) are a family of intracellular signal transducer enzymes that can attach a phosphate group to the 3’-hydroxyl of the inositol moiety of membrane-embedded phosphatidylinositol (PI). PI3Ks have been shown to play important roles in cell proliferation, growth, survival, motility, and metabolism. Nonetheless, the PI3K pathway has also shown to be overactivated in several tumors, particularly B-cell malignancies. In recent years, the PI3K signaling pathway has become the major focus of substantial drug discovery and development efforts. Selective (PI3K) inhibitors have been approved for the treatment of chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL), and indolent non-Hodgkin lymphomas (iNHL), such as follicular lymphoma and marginal-zone lymphoma. Four selective PI3K inhibitors have received accelerated FDA approvals for the treatment of patients with relapsed/refractory (R/R) CLL and/or iNHL based mainly on single-arm Phase II studies: Idelalisib (PI3K-δ inhibitor), copanlisib (dual PI3K-α and PI3K-δ inhibitor), duvelisib (dual PI3K-γ and PI3K-δ inhibitor), and umbralisib (dual PI3Kδ and CK1ϵ inhibitor). Conversely, recent interim results of randomized control trials (RCTs) involving some of these agents, showed a worrisome trend of decrease in overall survival (OS), and an increase in fatal and severe adverse effects, in comparison with patients in the control arms. Consequently, the class of PI3K inhibitors came under scrutiny, with an FDA expert panel voting on April 21, 2022, recommending that future FDA approvals of PI3K inhibitors be supported by randomized data, rather than single-arm data only, and further discontinuing the use of almost all the PI3K inhibitors in hematologic malignancies. As we believe further research is needed to help potentialize PI3K inhibitors by improving their safety profiles, this mini-review aims at revisiting the clinical successes, the failures, and the promising aspect of this class of drugs, while presenting possible ways that could benefit its successful development.
Keywords: phosphatidylinositol-3 kinases, hematologic malignancies, clinical trials, safety profile, accelerated approval
Introduction
The phosphatidylinositol-3 kinases (PI3K) pathway is overactivated in several malignancies, and therefore developing small molecules targeting this pathway has long been of clinical interest for a plethora of neoplasms.1 While preclinical efficacy has been shown across several tumor types, early promising clinical activity was observed in B-cell malignancies particularly Chronic lymphocytic leukemia (CLL) and indolent Non-Hodgkin Lymphomas (iNHL),2 with four PI3K inhibitors having received initial FDA approval for the treatment of these hematological malignancies, mostly based on single-arm phase II trials that have shown improvement in surrogate endpoints: overall response rates (ORR) and progression-free survival (PFS).
However, interim results of randomized control trials (RCTs) involving some of these agents showed a worrisome trend of decrease in overall survival (OS), and an increase in fatal and severe adverse effects, in comparison with patients in the control arms. In view of these results, a broader investigation into the toxicity of PI3K inhibitors as a class of drugs was launched, and the clinical trials involving their use were put on hold. Given these recent developments that prompted the re-evaluation of this class of drugs following concerns about their safety profiles, this led to our current investigation, which aims to review the indications and uses of PI3K inhibitors in iNHL and CLL, their clinical development and their safety profiles. Our mini-review also aims to discuss pre-clinical and clinical testing strategies to safely develop PI3K inhibitors in the future.
Chronic Lymphocytic Leukemia
Chronic lymphocytic leukemia (CLL) is the most common adult leukemia in Western countries. Incidence in Europe and in the United States are similar and ranges between 4 and 6 cases per 100.000 person per year.3 CLL is characterized by the clonal expansion and accumulation of mature CD5+ B cells in the peripheral blood, bone marrow, and secondary lymphoid organs. The presentation and clinical course of CLL is variable. CLL is distinguishable from small lymphocytic lymphoma (SLL) only by its leukemic presentation. Treatment indication is based on the presence of active disease.4 Despite high response rates to initial treatment, relapse is common and relapsed or refractory (R/R) disease is often characterized by resistance to chemotherapy.5
Recent advances in the field include the identification of frequent mutations, genetic alterations and signaling pathways providing a comprehensive understanding of CLL pathogenesis. These advances played a key role in the development and approval of novel agents, including novel kinase inhibitors targeting the B-cell receptor (BCR) pathway such as Bruton-Tyrosine kinase inhibitors (Ibrutinib, acalabrutinib, zanubrutinib) and PI3K inhibitors (Idelalisib, duvelisib), and the B-cell lymphoma 2 antagonist (venetoclax).6 For example, in CLL patients with del(17p)/TP53 mutation who are not eligible for other targeted therapies, idelalisib plus rituximab was considered a first-line therapy, and a second-line therapy in patients without del(17p)/TP53 mutation in whom first-line treatment did not work.7 Furthermore, PI3Ki as part of the BCR signal inhibitors (BCRi) have been included in the algorithm for managing high-risk R/R CLL using allogeneic hematopoietic stem cell transplantation (alloSCT).8 Although, the indications for alloSCT have significantly decreased for patients with R/R CLL given the efficacy of these novel agents, but it remains a valid consideration for several young patients with del17p/TP53 mutation. While both BCRi had exhibited promising clinical activity, PI3Ki drug development has been hampered by toxicity, especially in the frontline setting. However, it was not until recently, that we had the results of ASCEND: the first progressive trial to compare the efficacy of these different classes of BCRi (BTKi and PI3Ki) in patients with R/R CLL. The ASCEND trial is a Phase III randomized trial of Acalabrutinib vs control arm using Idelalisib plus Rituximab (IR) or Bendamustine plus Rituximab (BR) at the investigator’s choice in patients with R/R CLL. In this trial, acalabrutinib demonstrated superior outcome with an improvement in progression-free survival: median PFS NR for acalabrutinib vs 16.5 mo for the control arm with HR 0.31, 95% CI (0.2–0.49). The estimated 12-month PFS was 88% (95% CI, 81% to 92%) for acalabrutinib and 68% (95% CI, 59% to 75%) for control arm. Also, acalabrutinib showed a more favorable safety profile with serious adverse events occurring in 29% of the patients on acalabrutinib vs 56% with IR, and 26% with BR.9
Non-Hodgkin Lymphomas
Lymphomas are a heterogenous group of cancers of the lymphatic system, divided into two distinct groups that can affect any organ in the body: Hodgkin lymphoma (HL) and non-Hodgkin lymphomas (NHL).10 The latter11 constitutes more than 90% of the lymphomas, and is subdivided into different subtypes, ranging from the most indolent to the most aggressive.12 Follicular lymphoma (FL) is the most common (70%) indolent NHL (iNHL), with an estimated 15,000 new cases in the US annually.13 Marginal zone lymphoma is the second most common form (~25%) of indolent NHL. Patients may initially present with asymptomatic disease, however, most patients with FL have advanced stage disease at diagnosis, and the strongest determinant of survival is progression of disease within 2 years following diagnosis or initiation of first-line treatment.14,15 For marginal-zone lymphoma (MZL), long-term outcome also appears less favorable in patients with progression of disease within 2 years of initiating therapy.16
Asymptomatic patients are typically managed with observation approach until they manifest symptoms that warrant treatment. Treatment for patients with FL or MZL varies widely from “watch and wait”, the use of single agent anti-CD20 monoclonal antibodies (mAB), to the use of chemo-immunotherapy. However, novel therapies have recently been FDA-approved for the treatment of these iNHL,17 including agents that target the (BCR) pathway: Bruton-Tyrosine kinase inhibitors (ibrutinib, zanubrutinib), and the phosphatidylinositol 3-kinases inhibitors (idelalisib, duvelisib, copanlisib, and umbralisib), the subject of our review.
Phosphatidylinositol 3-Kinases Pathway
Cellular membranes are assembled as phospholipid bilayers, with some of them containing an inositol group that can be modified by several distinct kinases (PIK), with each one of them showing specificity for phosphorylating a particular hydroxyl of inositol.
Phosphatidylinositol 3-kinase (PI3K) attaches a phosphate group to the 3’-hydroxyl of the inositol moiety of membrane-embedded phosphatidylinositol (PI). Several PI3Ks have been discovered, but the most important one is the PI3K that phosphorylates PIP2 to PIP3 by adding a phosphate group to the inositol group that already has phosphate groups attached to the 4’- and 5’-hydroxyl groups of inositol trisphosphate.
PI3Ks are implicated in the regulation of different signaling pathways and have been shown to play important roles in cell proliferation, growth, survival, motility, and metabolism (Figure 1). PI3K are divided into three subclasses based on structure, regulation, and lipid substrate specificity. Of these, four different catalytic paralogues (α, β, δ, and γ) compose the class I PI3K,1 including a regulatory subunit and a catalytic subunit. While PI3Kα and PI3Kβ are ubiquitously expressed in various tissues, PI3Kγ is expressed in T lymphocytes, and PI3K δ is limited to B lymphocytes and its precursors. Furthermore, PI3Kα and PI3Kδ are responsible for the development of normal B lymphocyte, with mice deficient in PI3Kδ showing defect in their humoral response, the further development of colitis and a decrease in their B lymphocytes (B1, B2 and marginal zone).18,19
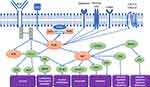 |
Figure 1 Phosphoinositide 3-kinases pathways interactions in B-cell lymphoid malignancies. Abbreviations: AKT—protein kinase B (PKB); AS160—Akt substrate of 160 kDa; BAD—BCL2 associated agonist of cell death; BCR—B cell receptor; BTK—Bruton’s tyrosine kinase; CXCL12—C-X-C motif chemokine 12; CXCL13—C-X-C motif chemokine 13; CD19 –– Cluster Differentiation 19; CD40L –– Cluster Differentiation 40 ligand; ERK—extracellular signal-regulated kinase; FOXO—forkhead box protein; GSK3—glycogen synthase kinase 3; LYN—tyrosine-protein kinase Lyn; MAPK –– Mitogen-activated protein kinase; MDM2—mouse double minute 2 homolog; NF-κB—nuclear factor kappa-light-chain-enhancer of activated B cells; p706K—p706 kinase; NFAT ––Nuclear factor of activated T-cells; PI3K—phosphoinositide 3-kinases; PIP2—phosphatidylinositol 4,5-bisphosphate; PIP3—phosphatidylinositol 3,4,5-triphosphate; PKC –– Protein kinase C; SYK—spleen tyrosine kinase; PLCγ2—phospholipase C γ2; PTEN—phosphatase and tensin homolog; mTOR—mammalian target of rapamycin; PRAS40—proline-rich Akt substrate of 40 kDa. Notes: Modifyied with permission from Phillips TJ, Michot JM, Ribrag V. Can Next-Generation PI3K Inhibitors Unlock the Full Potential of the Class in Patients With B-Cell Lymphoma? Clinical Lymphoma Myeloma and Leukemia. 2021:21(1);8-20.e3.© 2020 The Authors. Published by Elsevier Inc. Creative Commons CC-BY-NC-ND license.49 Hus I, Puła B, Robak T. PI3K Inhibitors for the Treatment of Chronic Lymphocytic Leukemia: Current Status and Future Perspectives. Cancers (Basel). 2022 Mar 18;14(6):1571. Copyright © 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).20 |
The PI3K Pathway in B Cells
PI3K activity in B cells is regulated by different pathways as shown in Figure 1. These pathways are detailed by Tuveson et al as they describe how transmembrane proteins control the PI3K activity in B cells, both in BCR dependent and independent pathways.21 PI3K signaling is initiated downstream of the B cell receptor (BCR), as one of the activating signals coming from CD19, which is a B-lymphocyte specific transmembrane protein. Other enhancing signals include cytosolic adapter proteins B cell adaptor for PI3K (BCAP) and TC21. The interaction between PI3K-mediated signals and CD19 signals is active in both ways. The dysregulation of the PI3K pathway, leading to its overactivation, has been frequently observed in B and T-cell lymphoid malignancies and therefore has been an important target for development of selective anticancer therapies.22
As Abdelrasoul et al describe in their paper, class IA PI3K-mediated signals also induce the expression of the transcription factor Pax5, which leads to the commitment and differentiation of B cells. Decreased PI3K activity in pre-B cells results in reduced Pax5 expression and lineage plasticity.23 Depletion of PI3K causes the decrease in peripheral B cells and the decrease of B cell maturation. While PI3K depleted B cells fail to proliferate in vitro in response to B cell receptor or CD40 activating signals, BCR deficient mature B cells can be rescued by activation of the PI3K signaling pathway.24,25
Furthermore, the increase of PI3K activity has been implicated in different malignancies.26 Genetic evidence supports the role of PI3K mutations in the pathogenesis of B cell lymphoid malignancies and respiratory infections.27 Analysis of protein microarray of follicular lymphoma patients showed an increase in Akt phosphorylation,28 as part of the phosphoinositide 3-kinase (PI3K), protein kinase B (PKB/AKT), mammalian target of rapamycin (mTOR) PI3K/Akt/mTOR pathway, which is also observed in diffuse large B cell lymphoma patients.29 Finally, increased PI3K activity has been observed in chronic lymphocytic leukemia, and other B cell malignancies such as Burkitt lymphoma and mantle cell lymphoma,30 possibly by a BCR-mediated downstream activation of NF-κB,31 or by exerting a compensatory role in survival signaling.32 In view of its pivotal role in both cancer, metabolism and immunity, the PI3K signaling pathway has become the major focus of substantial drug discovery and development efforts in the past two decades, with four compounds approved in B-cell malignancies.33
Landmark Studies of PI3K Inhibitors in B cell malignancies
Recently, the class of PI3K inhibitors came under scrutiny, when an FDA expert panel voted on April 21, 2022, to recommend that future FDA approvals of PI3K inhibitors be supported by randomized data, rather than single-arm data only.34
This decision came after randomized trials showed PI3K inhibitors had higher fatal events and other serious adverse events than shown in single-arm trials.35 As we further review the reasons and the context of the recent withdrawals, we must first examine the landmark studies that lead to the approval of PI3K inhibitors in R/R CLL/SLL and iNHL.
Since 2014, four PI3K inhibitors used for the treatment of R/R CLL/SLL and iNHL have received full or accelerated FDA approvals based mainly on single-arm studies: Idelalisib (PI3K-δ inhibitor),36 copanlisib (dual PI3K-α and PI3K-δ inhibitor),37 duvelisib (dual PI3K-γ and PI3K-δ inhibitor),38 and recently, umbralisib (dual PI3Kδ and casein kinase 1 epsilon (CK1ε) inhibitor).39
For the treatment of patients with R/R CLL, regular approvals were granted for idelalisib with rituximab and duvelisib on the grounds of the demonstrated improvement in progression-free survival (PFS) observed in RCTs. Alternatively, accelerated approvals in iNHL were granted to the four aforementioned PI3K inhibitors, based on durable ORR (overall response rates), observed in single-arm trials with the requirement of demonstrating clinical benefit by showing improvement in PFS in post-marketing RCTs. Of note, a major criticism for the initial registration studies for BCRi has been the use of a weak comparator arm. The landmark studies that led to drug approval or withdrawal are summarized in Table 1. Despite this, there are several active trials of PI3Ki being developed in lymphoid malignancies as summarized in Table 2.
 |
Table 1 FDA-Approved PI3K Inhibitors for Hematological Malignancies |
 |
Table 2 Examples of Recruiting Studies Using PI3Ki in Lymphoid Malignancies Posted on ClinicalTrials.gov |
Idelalisib
Idelalisib (GS-1101, CAL-101, Zydelig) is the first-in-class PI3K-δ inhibitor that inhibits cellular proliferation and interferes with homing capabilities of CLL cells.36 Idelalisib-treated cells showed decrease in AKT, mitogen-activated protein kinase (MAPK), B cell activating factor (BAFF), TNFα, CLL3, CCL4, CXCL13, and inhibition of CXCR4 and CXCR5 signaling. A double-blind, Phase 3 randomized placebo-controlled RCT (NCT01539512), compared Idelalisib + rituximab (I+R) to placebo + rituximab in patients with R/R CLL. It was stopped prematurely due to the superiority of the idelalisib arm with an improved PFS in the I+R arm [20.3 vs 6.5 months] and an improved OS [40.6 months vs 34.6 months], compared to the placebo + rituximab arm. This lead to the 2014 FDA-approval of idelalisib and rituximab for patients with R/R CLL.40 In a phase II single-arm, open-label trial, of 125 rituximab-refractory iNHL, single-agent Idelalisib showed promising antitumor activity as monotherapy41: FL (ORR 54%; Duration of response (DoR) median not reached) and in SLL (ORR 58%; DoR median= 11.9 months), with an acceptable safety profile, resulting in accelerated approvals of idelalisib for R/R FL and SLL patients who have received at least two prior systemic therapies (Table 1).
Copanlisib
Copanlisib (BAY 80–6946, Aliqopa) is a pan-class I PI3K inhibitor with a specific activity mainly against the α and δ isoforms.37 In CHRONOS-1, a single-arm, open-label phase II trial in patients with R/R Indolent or Aggressive NHL, copanlisib showed an ORR 59%, CR 12%, median DOR 22.6 months, median PFS of 11.2 months in patients with iNHL leading to the accelerated FDA-approval in 2017 of copanlisib for patients with relapsed FL after 2 or more systemic lines of therapy.42 In the post-approval CHRONOS-3, a randomized, placebo-controlled phase 3 trial testing the combination of copanlisib + rituximab vs placebo + rituximab in patients with R/R iNHL, the combination showed a significant improvement in PFS versus placebo plus rituximab (PFS, hazard ratio [HR] = 0.52 [95% CI 0.39–0.69] without improvement in the interim OS HR 1.07 [95% CI 0.63–1.82]. This led the company Bayer to withdraw the FDA application for sNDA for copanlisib and rituximab in December 2021.
Duvelisib
Duvelisib (IPI-145, INK1197, Copiktra), is a first-in-class, small-molecule, selective dual inhibitor of the δ and γ isoforms of PI3K.38 In animal models, targeting PI3Kδ,γ with duvelisib has demonstrated superior efficacy in comparison to blocking either isoform alone.43 In the single-arm open-label phase II trial DYNAMO (NCT01476657), duvelisib demonstrated an ORR of 42% in patients with R/R iNHL, including FL (42.2%), MZL (38.9%) and SLL (67.9%).44 Median DOR was 10 months, and median PFS was 9.5 months. This led to the 2018 FDA-accelerated approval of single-agent duvelisib in patients with R/R FL after 2 or more systemic therapies. However, in December 2021, the company Secura Bio announced the voluntary US withdrawal of duvelisib for relapsed or refractory follicular lymphoma indication, and the strategic focus on T-cell lymphomas. On April 13, 2022, the FDA withdrew the approval of duvelisib in R/R FL.
In the randomized, open-label, multicenter, phase III DUO trial (NCT02004522), duvelisib showed improved efficacy in comparison with ofatumumab for patients with R/R CLL/SLL with median PFS 13.3 vs 9.9 months, [HR] = 0.52; P <0.0001), ORR (74% vs 45%; P<0.001).45 This led to the 2018 FDA-approval of single-agent duvelisib for patients with R/R CLL/SLL after 2 or more line of therapies. However, the final analysis showed a median OS 43.9 months with duvelisib vs 46.8 months with ofatumumab at five years, with HR 1.11 (95% CI 0.80–1.53). On September 2022, the oncology drug advisory committee voted (8–4) against duvelisib’s use in patients with R/R CLL/SLL after at least 2 prior therapies, with experts citing unfavorable risk/benefit profile.46
Umbralisib
Umbralisib (Ukoniq) is an oral, next-generation first-in-class dual PI3Kδ and casein kinase 1 epsilon (CK1ε) inhibitor.39 It has different chemical structure and safety profile compared to other PI3Kδ inhibitors, due to CK1ε inhibition capacity for blocking the effect of PI3K inhibition on Treg, therefore reducing the incidence of autoimmune complications.
In the phase II UNITY NHL trial, umbralisib monotherapy was studied in 208 patients with R/R MZL, FL, and SLL. At a median follow-up of 27.7 months, umbralisib showed an ORR 47.1%, (MZL 49.3%, FL 45.3%, SLL 50%), mDOR not reached for MZL, 11.1 months for FL, and 18.3 months for SLL, with mPFS not reached for MZL, 10.6 months for FL, and 20.9 months for SLL.47 The safety profile was manageable, with low incidence of immune-mediated adverse events, which led to the initial FDA accelerated approval of single-agent umbralisib for patients with R/R FL after 3 or more systemic therapies and for R/R MZL after 1 or more anti-CD20-based therapy.
In the randomized phase III trial (UNITY-CLL), the combination of umbralisib and the novel anti-CD20 antibody ublituximab (U2) showed a significant improvement in PFS ([HR] 0.546, 95%, P<0.0001) versus obinutuzumab plus chlorambucil (O+Chl) in patients with treatment-naïve and R/R CLL.48 However, interim OS showed an OS HR 1.23 in favor of the control arm. This led the company to the voluntary withdrawal of BLA/sNDA for U2 in CLL/SLL on April 15, 2022, as well as the voluntary withdrawal of umbralisib for MZL and FL. This was followed by the FDA withdrawal of umbralisib approval for MZL and FL on June 1, 2022.
Safety Profile for PI3K Inhibitors in B cell malignancies
Despite their remarkable and demonstrated efficacy, many adverse events (AEs) have been observed with PI3K inhibitors. Targeting PI3Kδ isoform has been associated with on-target class-specific side effects, best characterized with the toxicity profile of idelalisib, the first-in-class PI3Kδ inhibitor, that include infectious and autoimmune toxicities such as colitis, pneumonitis, and rash.49,50 The auto-immune pattern of these side effects has been supported by the decrease in peripheral blood of the Treg as well as the increase in the lymphocytic infiltrate in liver biopsies for patients experiencing these side effects. Furthermore, targeting PI3Kα isoform leads to on target hyperglycemia and hypertension, a distinguishing side effect of copanlisib compared to the other previously approved PI3Ki. These AEs led to the termination or disruption of many trials: for example, in the Phase 2 studies of idelalisib, copanlisib, and duvelisib in patients with R/R iNHL, AEs led to discontinuation of study drug in 20–31% of the patients, dose reduction in 19–34% of the patients, and dose delays/interruptions in 47–68% of the patients.41,42,44 Conversely, the majority of treatment discontinuations of PI3K inhibitors – mostly in CLL trials – was due to AEs, rather than disease progression, which highlights the need for successful strategies to decrease toxicities while maintaining efficacy.51 In this regard, Ma et al have shown that for patients who experienced AEs, Idelalisib interruptions and dose reductions were associated with enhanced clinical outcomes.52 Interestingly, immune-related AEs (irAEs) may further boost the potential of PI3Kδ inhibition and are associated with improved clinical efficacy.53
In March 2016, the FDA halted six RCTs in iNHL or CLL exploring idelalisib in combination studies due to increased rates of adverse events, including death. While those trials used progression-free survival as their primary efficacy endpoint to show improved efficacy, the increased toxicity affected OS.49 In this regard, three double-blind, placebo-controlled RCTs evaluating idelalisib combination regimens were discontinued early because of increased deaths (5–8% in Idelalisib arm compared to 1–6% in placebo arm) and increased serious toxicity in the Idelalisib arms.54 The other global trials were not resumed, and their results never published.
The increase in death rates was caused by an increase in fatal infections and autoimmune toxicities such as colitis and pneumonitis in the Idelalisib arm.55
A similar pattern has been observed when using PI3Ki in combination with anti-CD20 m Abs. As previously mentioned in CHRONOS-3, while copanlisib plus rituximab showed improvement in PFS (HR 0.52; 95% CI 0.39–0.69) compared to rituximab + placebo, there was tendency for decrease in OS (HR 1.07; 95% CI 0.63–1.82) for patients randomized to the copanlisib arm.56 This led Bayer to the voluntary withdrawal of NDA of copanlisib in combination with rituximab. However, single-agent copanlisib is still available but based on the accelerated approval status.
Davids et al performed an integrated toxicity data analysis from four open-label, Phase I and II studies involving umbralisib that included 371 adult patients. AEs leading to discontinuation of umbralisib were seen in 13% of the patients, and treatment-emergent serious AEs occurred in 95 of 371 patients (25.6%), with limited AEs of special interest highlighting a favorable long-term tolerability profile.57 However, a safety alert was issued by the FDA58 about an increased risk of mortality in patients taking umbralisib, upon analyzing data from the U2 combination in UNITY-CLL. Interim overall survival outcomes tended to favor the control arm (chlorambucil + obinutuzumab) (HR 1.10; 95% CI: 0.75, 1.59) over the trial arm (umbralisib + ublituximab). Therefore, the company voluntarily withdrew the BLA/sNDA for U2 in patients with R/R CLL/SLL.
Of note, the inferior outcome observed with PI3Ki when directly or indirectly compared with BTKi is due to the fact that most patients while on PI3Ki are not able to stay on the drug due to a worse side effects profile, but on the other hand, long-term remissions with PI3Ki have been associated with toxicity-related discontinuations unlike what has been observed with BTKi.
As Richardson et al suggested, these trials showed an increase in efficacy in the setting of a potential decrease in overall survival, highlighting the issue of toxicity affecting OS, the ultimate safety endpoint.34 The concerning trends in decrease in overall survival and the high rates of severe adverse events and discontinuation have prompted the FDA to discontinue almost all the PI3K inhibitors in hematologic malignancies, and to review the mechanisms of accelerated approvals for this class of drugs that were based on surrogate endpoints using ORR/PFS in single-arm trials.
In this context, an assessment of drug approval process in this class of drugs might also lead to more stringent procedures concerning the development of other cancer drugs. As the path forward might require randomized control trial as a standard for initial approval, these trials would also necessitate the inclusion of existing relevant standard of care therapies in their placebo/non-intervention arm. However, withdrawal of several approvals should not mean a death sentence for all PI3Ki, as we have learned from the long and windy road for approval of gemtuzumab ozogamicin in acute myelogenous leukemia and gefitinib in EGFR mutated non-small cell lung cancer. In this regard, while acknowledging the low number of patients, recent data from the novel PI3Kδ inhibitor, leniolisib are showing encouraging safety profile up to 5 years in patients with activated PI3Kδ syndrome (APDS) with only one discontinuation thought not to be drug-related.59
Strategies in Decreasing Toxicities
It has been theorized that a possible cause of the increase in mortality and AEs from the PI3Kδ inhibitors, was the use of continuous dosing in the trials, as on-target inhibition of normal B lymphocytes and T regulatory cells (Tregs) leads to increase infection and auto-immune side effects. For example, in the UNITY-CLL trial, a regimen of an oral continuous dose of the PI3Kδ inhibitor umbralisib in combination with the anti-CD20 inhibitor ublituximab administered IV was used until progressive disease or unacceptable toxicity.48 This led to severe B-cell depletion, a major risk factor for poor outcome from COVID-19 infection.
Furthermore, Dreyling et al suggested that an intermittent intravenous dosing with weekly IV copanlisib administered 3 weeks on 1 week off was associated with a lower incidence of severe hepatotoxicity and diarrhea/colitis, compared with the continuous oral idelalisib treatment.42
This dosing approach is being further investigated in trials such as the COASTAL trial.60 Recent results from that trial showed that an intermittent use of Zandelisib (a novel PI3Kδ inhibitor) was safe, with an established reduction of grade 3 or worse adverse effects in patients with relapsed/refractory B-cell malignancy.61 Furthermore, intermittent dosing of duvelisib using 2 weeks on, 2 weeks off in the TEMPO trial (NCT04038359), a phase 2 study in patients with r/r iNHL, seems to support this approach as a successful strategy for maintaining efficacy while decreasing toxicity.
Conclusion
As scientists discovered the BCR signaling pathway, they furthermore uncovered the important role that the PI3K signaling pathway plays in cell proliferation, growth, survival, metabolism, and malignancy. These discoveries opened the door wide open for the pharmaceutical companies from around the world racing to develop novel inhibitors for this critical pathway.
Preclinical data provide overwhelming evidence of the efficacy of PI3K inhibitors in vitro and in-vivo for targeting the BCR signaling.33 Moreover, many investigational PI3K inhibitors have shown encouraging efficacy profiles in the clinical setting. Of these agents, we discussed four different PI3K inhibitors that demonstrated robust antitumor activity in R/R iNHL and/or CLL clinical trials. In fact, preliminary results from many single-arm phase II trials involving these agents showed promising clinical efficacy, an improvement in overall response rates, progression-free survival, and relatively manageable safety profiles.
Unfortunately, the clinical advantage of PI3K inhibitors (used in hematologic malignancies) was recently overshadowed by safety concerns substantiated by an increase in adverse effects, leading to a trend for decrease in overall survival observed in double-blind randomized clinical trials involving some of these investigational PI3K inhibitors, during a challenging COVID-19 pandemic. These concerns have led the FDA to reconsider this promising group of drugs, to halt clinical trials involving many PI3K inhibitors and change its policy with regards to accelerated approvals requiring further trials for optimal dose selection, and the use of randomized trials as regulatory strategies.
As the preclinical and clinical evidence of significant efficacy persists, strategies to reduce toxicity and increase patient adherence should be developed to address the safety concerns. We reviewed possible ways that could benefit the successful development of this class of drugs: one strategy that is gaining momentum is the adoption of intermittent dosing instead of the continuous dosing. In summary, considering their clinical efficacy, further investigations are needed to fully potentialize the benefit of PI3K inhibitors by improving their safety profiles in order to meet the needs of patients with iNHL and CLL.
Disclosure
Dr Victor Yazbeck reports grants, personal fees from Seagen, grants, personal fees from AstraZeneca, personal fees from ADC Therapeutics, personal fees from Verastem, grants, personal fees from Gilead, personal fees from TG Therapeutics, grants, personal fees from Beigene, grants, personal fees from Genmab, personal fees from MorphoSys, outside the submitted work. The authors report no conflicts of interest in this work.
References
1. Rodon J, Dienstmann R, Serra V, Tabernero J. Development of PI3K inhibitors: lessons learned from early clinical trials. Nat Rev Clin Oncol. 2013;10(3):143–153. doi:10.1038/nrclinonc.2013.10
2. Schatz JH. Targeting the PI3K/AKT/mTOR pathway in non-Hodgkin’s lymphoma: results, biology, and development strategies. Curr Oncol Rep. 2011;13(5):398. doi:10.1007/s11912-011-0187-7
3. Kikushige Y. Pathogenesis of chronic lymphocytic leukemia and the development of novel therapeutic strategies. J Clin Exp Hematop. 2020;60(4):146–158. doi:10.3960/jslrt.20036
4. Hallek M, Cheson BD, Catovsky D, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131(25):2745–2760. doi:10.1182/blood-2017-09-806398
5. Burger JA. Treatment of chronic lymphocytic leukemia. N Engl J Med. 2020;383(5):460–473. doi:10.1056/NEJMra1908213
6. Hallek M, Shanafelt TD, Eichhorst B. Chronic lymphocytic leukaemia. The Lancet. 2018;391(10129):1524–1537. doi:10.1016/S0140-6736(18)30422-7
7. Barnett AK, Waddell JA, Solimando DA. Idelalisib and rituximab regimen. Hosp Pharm. 2017;52(3):187–190. doi:10.1310/hpj5203-187
8. Dreger P, Schetelig J, Andersen N, et al. Managing high-risk CLL during transition to a new treatment era: stem cell transplantation or novel agents? Blood. 2014;124(26):3841–3849. doi:10.1182/blood-2014-07-586826
9. Ghia P, Pluta A, Wach M, et al. ASCEND: phase III, randomized trial of acalabrutinib versus idelalisib plus rituximab or bendamustine plus rituximab in relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2020;38(25):2849–2861. doi:10.1200/JCO.19.03355
10. Armitage JO, Gascoyne RD, Lunning MA, Cavalli F. Non-Hodgkin lymphoma. Lancet. 2017;390(10091):298–310. doi:10.1016/S0140-6736(16)32407-2
11. Al Juhaishi T, Yazbeck V. Choosing the right pharmacotherapy for non-Hodgkin’s lymphoma: does one size fit all? Expert Opin Pharmacother. 2019;20(7):773–775. doi:10.1080/14656566.2019.1582643
12. Jiang M, Bennani NN, Feldman AL. Lymphoma classification update: B-cell non-Hodgkin lymphomas. Expert Rev Hematol. 2017;10(5):405–415. doi:10.1080/17474086.2017.1318053
13. Freedman A, Jacobsen E. Follicular lymphoma: 2020 update on diagnosis and management. Am J Hematol. 2020;95(3):316–327. doi:10.1002/ajh.25696
14. Casulo C, Dixon JG, Le-Rademacher J, et al. Validation of POD24 as a robust early clinical end point of poor survival in FL from 5225 patients on 13 clinical trials. Blood. 2022;139(11):1684–1693. doi:10.1182/blood.2020010263
15. Sindel A, Al-Juhaishi T, Yazbeck V. Marginal zone lymphoma: state-of-the-art treatment. Curr Treat Options Oncol. 2019;20(12):90. doi:10.1007/s11864-019-0687-5
16. Luminari S, Merli M, Rattotti S, et al. Early progression as a predictor of survival in marginal zone lymphomas: an analysis from the FIL-NF10 study. Blood. 2019;134(10):798–801. doi:10.1182/blood.2019001088
17. Leslie LA, Skarbnik AP, Bejot C, Stives S, Feldman TA, Goy AH. Targeting indolent non-Hodgkin lymphoma. Expert Rev Hematol. 2017;10(4):299–313. doi:10.1080/17474086.2017.1303374
18. Ramadani F, Bolland DJ, Garcon F, et al. The PI3K Isoforms p110α and p110δ are essential for pre–B cell receptor signaling and B cell development. Sci Signal. 2010;3(134):ra60–ra60. doi:10.1126/scisignal.2001104
19. Okkenhaug K, Bilancio A, Farjot G, et al. Impaired B and T cell antigen receptor signaling in p110δ PI 3-kinase mutant mice. Science. 2002;297(5583):1031–1034. doi:10.1126/science.1073560
20. Hus I, Puła B and Robak T. (2022). PI3K Inhibitors for the Treatment of Chronic Lymphocytic Leukemia: Current Status and Future Perspectives. Cancers, 14(6), 1571 10.3390/cancers14061571
21. Tuveson David A, Carter Robert H, Soltoff Stephen P, Fearon douglas T. CD19 of B cells as a surrogate kinase insert region to bind phosphatidylinositol 3-kinase. Science. 1993;260(5110):986–989. doi:10.1126/science.7684160
22. Kong D, Yamori T. Phosphatidylinositol 3-kinase inhibitors: promising drug candidates for cancer therapy. Cancer Sci. 2008. doi:10.1111/j.1349-7006.2008.00891.x
23. Abdelrasoul H, Werner M, Setz CS, Okkenhaug K, Jumaa H. PI3K induces B-cell development and regulates B cell identity. Sci Rep. 2018;8(1):1327. doi:10.1038/s41598-018-19460-5
24. Vanhaesebroeck B, Ali K, Bilancio A, Geering B, Foukas LC. Signalling by PI3K isoforms: insights from gene-targeted mice. Trends Biochem Sci. 2005;30(4):194–204. doi:10.1016/j.tibs.2005.02.008
25. Srinivasan L, Sasaki Y, Calado DP, et al. PI3 kinase signals BCR-dependent mature B cell survival. Cell. 2009;139(3):573–586. doi:10.1016/j.cell.2009.08.041
26. Westin JR. Status of PI3K/Akt/mTOR pathway inhibitors in lymphoma. Clin Lymphoma Myeloma Leuk. 2014;14(5):335–342. doi:10.1016/j.clml.2014.01.007
27. Crank MC, Grossman JK, Moir S, et al. Mutations in PIK3CD can cause hyper IgM syndrome (HIGM) associated with increased cancer susceptibility. J Clin Immunol. 2014;34(3):272–276. doi:10.1007/s10875-014-0012-9
28. Gulmann C, Espina V, Petricoin E, et al. Proteomic analysis of apoptotic pathways reveals prognostic factors in follicular lymphoma. Clin Cancer Res. 2005;11(16):5847–5855. doi:10.1158/1078-0432.CCR-05-0637
29. Hasselblom S, Hansson U, Olsson M, et al. High immunohistochemical expression of p-AKT predicts inferior survival in patients with diffuse large B-cell lymphoma treated with immunochemotherapy: p-AKT Expression in DLBCL. Br J Haematol. 2010;149(4):560–568. doi:10.1111/j.1365-2141.2010.08123.x
30. Fang X, Zhou X, Wang X. Clinical development of phosphatidylinositol 3-kinase inhibitors for non-Hodgkin lymphoma. Biomark Res. 2013;1(1):30. doi:10.1186/2050-7771-1-30
31. Lampson BL, Brown JR. PI3Kδ-selective and PI3Kα/δ-combinatorial inhibitors in clinical development for B-cell non-Hodgkin lymphoma. Expert Opin Investig Drugs. 2017;26(11):1267–1279. doi:10.1080/13543784.2017.1384815
32. Fruman DA. Targeting PI3K-gamma in non-Hodgkin lymphoma. J Clin Oncol. 2019;37(11):932–934. doi:10.1200/JCO.19.00215
33. Vanhaesebroeck B, Perry MWD, Brown JR, André F, Okkenhaug K. PI3K inhibitors are finally coming of age. Nat Rev Drug Discov. 2021;20(10):741–769. doi:10.1038/s41573-021-00209-1
34. Richardson NC, Kasamon Y, Pazdur R, Gormley N. The saga of PI3K inhibitors in haematological malignancies: survival is the ultimate safety endpoint. Lancet Oncol. 2022;23(5):563–566. doi:10.1016/S1470-2045(22)00200-5
35. FDA. Research C for DE and. FDA investigating possible increased risk of death with lymphoma medicine Ukoniq (umbralisib). FDA; 2022. Availabe from: https://www.fda.gov/drugs/development-approval-process-drugs/fda-investigating-possible-increased-risk-death-lymphoma-medicine-ukoniq-umbralisib.
36. Markham A. Idelalisib: first global approval. Drugs. 2014;74(14):1701–1707. doi:10.1007/s40265-014-0285-6
37. Markham A. Copanlisib: first global approval. Drugs. 2017;77(18):2057–2062. doi:10.1007/s40265-017-0838-6
38. Blair HA. Duvelisib: first global approval. Drugs. 2018;78(17):1847–1853. doi:10.1007/s40265-018-1013-4
39. Dhillon S, Keam SJ. Umbralisib: first Approval. Drugs. 2021;81(7):857–866. doi:10.1007/s40265-021-01504-2
40. Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370(11):997–1007. doi:10.1056/NEJMoa1315226
41. Gopal AK, Kahl BS, de Vos S, et al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370(11):1008–1018. doi:10.1056/NEJMoa1314583
42. Dreyling M, Santoro A, Mollica L, et al. Phosphatidylinositol 3-kinase inhibition by copanlisib in relapsed or refractory indolent lymphoma. J Clin Oncol. 2017;35(35):3898–3905. doi:10.1200/JCO.2017.75.4648
43. Dong S, Guinn D, Dubovsky JA, et al. IPI-145 antagonizes intrinsic and extrinsic survival signals in chronic lymphocytic leukemia cells. Blood. 2014;124(24):3583–3586. doi:10.1182/blood-2014-07-587279
44. Flinn IW, Miller CB, Ardeshna KM, et al. DYNAMO: a phase II study of duvelisib (IPI-145) in patients with refractory indolent non-Hodgkin lymphoma. J Clin Oncol. 2019;37(11):912–922. doi:10.1200/JCO.18.00915
45. Flinn IW, Hillmen P, Montillo M, et al. The phase 3 DUO trial: duvelisib vs ofatumumab in relapsed and refractory CLL/SLL. Blood. 2018;132(23):2446–2455. doi:10.1182/blood-2018-05-850461
46. Meeting of the Oncologic Drugs Advisory Committee (ODAC) - Day 2; 2022. Available from: https://www.youtube.com/watch?v=cjkgIpaxHlI.
47. Fowler NH, Samaniego F, Jurczak W, et al. Umbralisib, a Dual PI3Kδ/CK1ε inhibitor in patients with relapsed or refractory indolent lymphoma. J Clin Oncol. 2021;39(15):1609–1618. doi:10.1200/JCO.20.03433
48. Gribben JG, Jurczak W, Jacobs RW, et al. Umbralisib plus ublituximab (U2) is superior to obinutuzumab plus chlorambucil (O+Chl) in Patients with Treatment Naïve (TN) and Relapsed/Refractory (R/R) Chronic Lymphocytic Leukemia (CLL): results from the Phase 3 Unity-CLL Study. Blood. 2020;136(1):37–39. doi:10.1182/blood-2020-134783
49. Phillips TJ, Michot JM, Ribrag V. Can next-generation PI3K inhibitors unlock the full potential of the class in patients with B-cell lymphoma? Clin Lymphoma Myeloma Leuk. 2021;21(1):8–20.e3. doi:10.1016/j.clml.2020.08.022
50. Greenwell IB, Flowers CR, Blum KA, Cohen JB. Clinical use of PI3K inhibitors in B-cell lymphoid malignancies: today and tomorrow. Expert Rev Anticancer Ther. 2017;17(3):271–279. doi:10.1080/14737140.2017.1285702
51. Shah A, Barrientos J. Oral PI3K-δ,γ Inhibitor for the Management of People with Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma: a Narrative Review on Duvelisib. OncoTargets Ther. 2021;14:2109–2119. doi:10.2147/OTT.S189032
52. Ma S, Chan RJ, Gu L, et al. Retrospective analysis of the impact of adverse event–triggered idelalisib interruption and dose reduction on clinical outcomes in patients with relapsed/refractory B-cell malignancies. Clin Lymphoma Myeloma Leuk. 2021;21(5):e432–e448. doi:10.1016/j.clml.2020.12.016
53. Wagner-Johnston ND, Sharman J, Furman RR, et al. Idelalisib immune-related toxicity is associated with improved treatment response. Leuk Lymphoma. 2021;62(12):2915–2920. doi:10.1080/10428194.2021.1948038
54. Zelenetz AD, Barrientos JC, Brown JR, et al. Idelalisib or placebo in combination with bendamustine and rituximab in patients with relapsed or refractory chronic lymphocytic leukaemia: interim results from a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2017;18(3):297–311. doi:10.1016/S1470-2045(16)30671-4
55. Sharman JP, Coutre SE, Furman RR, et al. Final results of a randomized, phase III study of rituximab with or without idelalisib followed by open-label idelalisib in patients with relapsed chronic lymphocytic leukemia. J Clin Oncol. 2019;37(16):1391–1402. doi:10.1200/JCO.18.01460
56. Matasar MJ, Capra M, Özcan M, et al. Copanlisib plus rituximab versus placebo plus rituximab in patients with relapsed indolent non-Hodgkin lymphoma (CHRONOS-3): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22(5):678–689. doi:10.1016/S1470-2045(21)00145-5
57. Davids MS, O’Connor OA, Jurczak W, et al. Integrated safety analysis of umbralisib, a dual PI3Kδ/CK1ε inhibitor, in relapsed/refractory lymphoid malignancies. Blood Adv. 2021;5(23):5332–5343. doi:10.1182/bloodadvances.2021005132
58. FDA. Commissioner O of the. Ukoniq (Umbralisib): drug safety communication - FDA investigating possible increased risk of death with lymphoma. FDA; 2022. Available from: https://www.fda.gov/safety/medical-product-safety-information/ukoniq-umbralisib-drug-safety-communication-fda-investigating-possible-increased-risk-death-lymphoma.
59. Rao VK, Webster S, Sediva A, et al. Interim analysis of safety and hematological parameters of an ongoing long-term open-label extension study of investigational PI3Kδ inhibitor leniolisib for patients with activated PI3K Delta Syndrome (APDS) through December 2021. Blood. 2022;140(1):1643–1645. doi:10.1182/blood-2022-160334
60. Zelenetz AD, Jagadeesh D, Kenkre VP, et al. The pi3kδ inhibitor me-401 ± rituximab in relapsed/refractory (R/r) follicular lymphoma (FL), chronic lymphocytic leukemia (Cll), and small lymphocytic lymphoma(Sll). Hematol Oncol. 2019;37:176–177. doi:10.1002/hon.133_2629
61. Pagel JM, Soumerai JD, Reddy N, et al. Zandelisib with continuous or intermittent dosing as monotherapy or in combination with rituximab in patients with relapsed or refractory B-cell malignancy: a multicentre, first-in-patient, dose-escalation and dose-expansion, phase 1b trial. Lancet Oncol. 2022;23(8):1021–1030. doi:10.1016/S1470-2045(22)00333-3
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
