Back to Journals » Psychology Research and Behavior Management » Volume 11
Physical activity and sociodemographic variables related to global health, quality of life, and psychological factors in breast cancer survivors
Authors Patsou ED, Alexias GT, Anagnostopoulos FG , Karamouzis MV
Received 3 April 2018
Accepted for publication 23 May 2018
Published 6 September 2018 Volume 2018:11 Pages 371—381
DOI https://doi.org/10.2147/PRBM.S170027
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Igor Elman
Video abstract presented by Patsou et al.
Views: 559
Efrossini D Patsou,1 George T Alexias,1 Fotios G Anagnostopoulos,1 Michalis V Karamouzis2
1Department of Psychology, Panteion University of Social and Political Sciences, Athens, Greece; 2Department of Biological Chemistry, Medical School, National and Kapodistrian University of Athens, Athens, Greece
Background: Breast cancer is one of the most common cancers affecting women worldwide and depression and anxiety are disturbing side effects of cancer diagnosis and treatment. The aim of this study was to examine the associations of physical activity in global health, quality of life (QoL), and psychological factors (depressive symptoms, self-esteem, and anxiety) in breast cancer survivors after completing cancer treatment and through survivorship. Demographic variables (marital status, education, income), medical status (cancer stage), and level of physical activity (metabolic equivalent of task [MET]) were tested as predictors of depressive mood, anxiety, self-esteem, and QoL in younger and older breast cancer survivors.
Materials and methods: One hundred and seventy-one Greek breast cancer survivors, who had completed cancer treatment at least one and a half years ago, were included in this study. Demographic and medical information, self-reported and objective physical activity levels, global health, QoL, depressive symptoms, self-esteem, and anxiety were assessed in all participants.
Results: Active women had lower depressive symptoms, less anxiety, higher self-esteem, and better global health and QoL, compared to the inactive ones, even in the long term after completing treatment through survivorship. Exercise had significant positive correlations with self-esteem, global health, and QoL (physical, role, emotional, cognitive, and social aspects). Moreover, significant negative correlations with anxiety and depressive symptoms were found. Multiple regression analysis revealed that MET and covariates such as income, education, and stage of cancer were significant predictors of depressive symptoms, self-esteem, anxiety, global health, and QoL in younger survivors, while MET, income, education, stage of cancer, and marital status were significant predictors of dependent variables for the older ones.
Conclusion: It can be concluded that exercise should be recommended to cancer survivors even after treatment completion and through survivorship to achieve higher self-esteem, better QoL, and decreased anxiety and depressive symptoms.
Keywords: breast cancer, depression, self-esteem, anxiety, physical activity, quality of life
Introduction
Breast cancer is the most common cancer in women, affecting about one in ten women in the developed countries and has a presumed 5-year survival of up to 70% due to continuing improvement of cancer treatments.1 Depressive mood is a common symptom among breast cancer survivors which remains even after the completion of cancer treatment.2 It has been shown that at some point, right after diagnosis or even throughout their survivorship, all breast cancer survivors will encounter some degree of physical and psychological side effects which are related to cancer and its treatments.3 Researchers have reported that depressive mood in breast cancer survivors is mostly associated with loneliness, rather than the disease or treatment per se.4 Depressive mood is a negative psychological outcome usually reported by breast cancer survivors both during and after treatment.5 On the other hand, there is evidence that long-term survivors seem to have levels of functioning and quality of life (QoL) that are comparable to those of the general female population.6 It is well known that cancer patients after their diagnosis decrease their level of physical activity.7 However, physical activity is a non-pharmacologic, safe, feasible, and relatively low-cost alternative for depression management among women with breast cancer.8 Increased physical activity levels may reduce depressive symptoms9 and, thus, improve QoL throughout survivorship.10
QoL is a subjective and multidimensional concept with physical, social, and psychological domains. Furthermore, improving QoL after surgery and treatment is quite challenging for medical staff and patients.11 Depressive symptoms have been negatively associated with QoL. The loss of future perspectives, changes in sexual functioning, different perception of body image, and other side effects can occur due to the presence of depressive mood.12 Additionally, physical activity and exercise can help survivors to reduce specific side effects, and, at the same time, it may be an effective intervention in reducing state anxiety.13 What is more, it is noteworthy that the World Health Organization (WHO) recommends physical exercise for decreased levels of depression and anxiety in breast cancer survivors.14
Performing specific forms of regular exercise and maintaining an active lifestyle can play an important role in survivorship,15 since they help survivors to increase survival rates and decrease the risk of cancer recurrence.16 Major health organizations recommend that cancer survivors should perform at least 150 minutes of moderate or 75 minutes of high-intensity aerobic exercise combined with a minimum of two strengthening exercise sessions on a weekly basis.17
So far, few studies have examined associations regarding the several aspects of psychological side effects, namely depressive symptoms, anxiety, self-esteem, and QoL, as well as differences between active and less active breast cancer survivors in the long term after having completed treatment of any kind and through survivorship. Additionally, there is a lack of knowledge as to whether and how physical activity, income, marital status, education, and stage of cancer are related to depressive mood, anxiety, self-esteem, global health, and QoL, after treatment and through survivorship in younger and older survivors.
Understanding 1) how physical activity and exercise work even after completing any kind of treatment, as well as the influence of 2) physical activity and 3) patient characteristics on the psychological and physical functioning in younger and older survivors after treatment completion and through survivorship, is of utmost importance in order to implement strategies to reduce unpleasant and disturbing feelings and improve QoL.
Finally, there are no data about Greek breast cancer survivors regarding the advantages of exercise in reducing depressive symptoms and anxiety, and enhancing self-esteem and QoL.
The aim of this study was to investigate the associations between physical activity and global health, QoL, and psychological factors among breast cancer survivors after completion of cancer treatment and through survivorship. Demographic variables (income, education, marital status), medical status (stage of cancer), and level of physical activity (metabolic equivalent of task [MET]) were tested in light of being predictors of depressive mood, anxiety, self-esteem, and QoL in younger and older breast cancer survivors.
Our first hypothesis is that active survivors will have less depressive symptoms and anxiety and better self-esteem, global health, and QoL, compared to inactive or less active ones in the long-term period after treatment completion and through survivorship.
Our second hypothesis is that physical activity will be positively associated with self-esteem, global health, and QoL, and negatively associated with depressive symptoms and anxiety even long after having completed treatment and through survivorship.
Finally, our third hypothesis is that physical activity will be a significant predictor of less depressive symptoms and anxiety, higher self-esteem, better global health, and QoL in younger as well as in older survivors long after having completed treatment and through survivorship.
Materials and methods
Participant eligibility criteria
Women aged between 18 and 65 years, who had been diagnosed with breast cancer and completed any cancer treatment for stage I–IV cancer (including chemotherapy and/or hormone therapy and radiotherapy) at least one and a half years ago, were eligible to participate in the present study. Exclusion criteria included the presence of other diseases or oncology processes that could have been inhibiting factors for exercise (diabetes, hypertension, heart diseases).
Measures
Demographics
Sociodemographic factors such as age, marital status, income, and education were assessed using a baseline questionnaire. Cancer-related factors such as date of diagnosis, disease stage, surgical procedure, heredity, chemotherapy, radiation, hormone therapy were self-reported and later verified and complemented through doctors. Participants were also asked to declare the presence of any other diseases and comorbidities (ie, diabetes, hypertension) and the use of any medication.
Psychological mood profile
The Greek version of the Profile of Mood States (POMS)18 consists of 37, five-point adjective rating scales which are grouped into six mood domains: tension, depression, anger, vigor, fatigue, and confusion. Each of the six mood domains is defined by adjectives descriptive of the specific mood. Participants score the adjectives in response to how they feel according to the following ratings: 0= not at all, 1= a little, 2= moderately, 3= quite a bit, and 4= extremely. Scores for each of the six mood factors are obtained by summing the responses for the adjectives defining the factor. A total mood disturbance score is obtained by summing the scores across all six factors, weighing vigor negatively. Cronbach α was 0.84.
Global health and QoL
To assess global health and QoL, we used the Greek version of the European Organization for Research and Treatment of Cancer (EORTC) QOL-C3019,20 questionnaire after personal communication and authorization from the EORTC. The EORTC QOL-C30 can gather data about perceived physical, role, emotional, social, and cognitive functioning and one global health status dimension. All of the scales and single-item measures range in score from 0 to 100. A high scale score represents a higher response level. Thus, a high score for a functional scale represents a high/healthy level of functioning, a high score for the global health status/QoL represents a high QoL. Additionally, a number of symptoms such as fatigue, nausea, pain, dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties are included. Cronbach’s α was 0.87.
Anxiety
The Greek version of the State-Trait Anxiety Inventory (STAI) was used in order to measure the state anxiety of the survivors.21 The inventory has 20 items which include phrases like: “I am tense; I am worried” and “I feel calm; I feel secure.” All items are rated on a four-point scale ranging from “almost never” to “almost always.” Higher scores indicate greater anxiety. The inventory is often used in clinical settings to diagnose anxiety and to distinguish it from depressive syndromes. Cronbach α was 0.82.
Self-esteem
The Greek version of the Self-Esteem Scale (SES) is a 10-Likert type scale and is typically scored using a four-point response format (strongly agree, agree, disagree, strongly disagree) resulting in a score ranging between 10 and 40 and measuring global self-esteem. Higher scores represent more positive attitudes toward self.22 For the present study, Cronbach’s alpha coefficient for the scale was 0.85.
Self-reported physical activity
To assess the level of physical activity we used the Greek version of the International Physical Activity Questionnaire (IPAQ).23 IPAQ is a self-reported questionnaire and is composed of seven questions. Data from the IPAQ were summarized according to the physical activities recorded (eg, walking, moderate, and vigorous activities) and estimated time spent sitting per week. Vigorous activities may include aerobics, fast bicycling, or heavy lifting. Moderate physical activities may include swimming or bicycling in normal speed. Finally, in the last 7 days, data were recorded from activities that lasted at least 10 minutes. The specific types of activity that were assessed were walking, moderate-intensity activities, and vigorous-intensity activities; data on frequency (measured in days/week) and duration (time/day) were collected separately for each specific type of activity. By multiplying minutes of a given activity/week (time) by intensity (expressed in MET – units) we had intense, moderate, and walking activities. In addition, the total time spent weekly on sitting was recorded. The following classification was applied:
- insufficient activity – <600 METs per minute per week (MET·min/week);
- sufficient activity, meeting any of the following criteria:
- 20 minutes of vigorous activities each day for ≥3 days;
- 30 minutes of moderate activities or walking for ≥5 days;
- combination of vigorous and moderate activities each day for ≥5 days (at least 600 MET·min/week);
- high activity, meeting any of the following criteria:
- vigorous activities for ≥3 days (at least 1500 MET·min/week);
- combination of vigorous, moderate, or walking activities for 7 days (at least 3000 MET·min/week).23
Objective physical activity
Physical and health-related fitness was also measured with a maximal oxygen consumption (VO2 max) test in a laboratory in the presence of two sport physiologists. VO2 max is the maximum rate of oxygen consumption as measured during exercise, mostly on a treadmill and is expressed in mL/kg/min. Maximal oxygen consumption is an important determinant of an individual’s endurance capacity and reflects the level of physical fitness. Whether an individual has a good rate of oxygen uptake depends on her age and the number that results from the measure of the oxygen consumption. For example, for a woman aged between 40 and 49 years, very poor consumption is ≤21, poor is between 21 and 24, fair 24.5–28.9, good 29–32.8, excellent 32.9–36.9, and superior ≥36.9.24 Women were walking or running depending on their physical level at 0% grade for the first 2 minutes. Every 2 minutes the treadmill elevation was increased by one level until the participant could no longer continue. The treadmill speed was up to 4 miles/h. Expired air was collected for each successive minute and heart and breathing rate were continuously monitored. All participants were verbally encouraged by the physiologists to achieve their highest level.
Recruitment and data collection
The study was carried out between April 2016 and September 2017. All study procedures were approved by the university institutional review board. Research at Panteion University of Social and Political Sciences, Department of Psychology, is conducted according to the principles set out in the university research ethics policy. The policy applies to all staff and students of the university who propose to undertake research. It states that all research must be conducted according to appropriate ethical, legal and professional frameworks, obligations, and standards and that any type of research must undergo ethical scrutiny by the appropriate faculty research ethics committee. There was a cluster random sampling between the private and the public hospitals from Attica in Greece. Every participant was informed by her doctor and gave written consent for participation in the study. The patient signed the consent page in front of a witness and that document was archived in the study dossier. The patient was entitled to receive a copy. The doctor who informed the patient was aware of the ethical guidelines of the Declaration of Helsinki.
After expressing their interest to participate in the study, participants filled the demographical and medical history information form (n=320) and those meeting the inclusion criteria (n=257) were asked via email to complete the questionnaires of the study. At the same time, they were informed about their participation in the VO2 max treadmill procedure. Out of those who were classified as suitable candidates for participating and completing the questionnaires (n=220), a subgroup of 71, finally, took the treadmill test in the laboratory.
Depending on their physical activity level expressed by VO2 max and their weekly hours of exercise expressed by MET·min/week, participants were divided into two groups. The first group (n=82) consisted of women with insufficient or low levels of physical activity – low fitness group (<600 MET·min/week and very poor or poor VO2 max), while the second group (n=89) consisted of women with sufficient or moderate to high physical activity level – exercise group (between 600 and 1500 MET·min/week of moderate or vigorous activity or a combination of them and fair, good, excellent, or superior VO2 max).
Participants who reported higher levels of physical activity in the self-reported physical activity questionnaire also scored higher on VO2 max. In addition, the correlation between MET and VO2 max score (subjective and objective measure of physical activity) was calculated and found to be equal to 0.906 (p<0.01); therefore, the robustness of the fitness variable was high providing a remarkable level of content validity.
Statistical analyses
Descriptive statistics were studied regarding participants’ responses on sociodemographic and clinical factors. Independent sample t-test analyses were conducted to evaluate differences in variables between the low fitness and exercise groups. Means and standard deviations were reported for continuous variables with a normal distribution, whereas, median and range were reported for continuous variables non-normally distributed. In addition, the Kolmogorov–Smirnov normality test was applied to all variables and Pearson’s and Spearman’s correlation coefficients were calculated whenever appropriate. Correlation analyses were carried out among all study variables (MET, depression POMS, SES, STAI, global health, and QoL – physical EORTC30, role EORTC30, emotional EORTC30, cognitive EORTC30, and social EORTC30). Assumptions of normality, linearity, and homoscedasticity were checked by the corresponding residual scatter plots and suitable tests. Multiple regression analysis was employed to explore whether explanatory variables “MET,” income, education, marital status, and stage of cancer could explain a sufficient proportion of variance in dependent variables among younger and older survivors. The requirements for an independent variable to be included in the multiple regression analysis were: 1) the correlation coefficients between the dependent variable and the independent variables were significant (p<0.05); and 2) the absolute correlation coefficient values between the independent variables were <0.4.
The age groups were classified using the method of 50th percentile and the younger group was ≤51 years, while the older group was ≥52 years old. Predictors were MET, marital status (married/living together vs unmarried/divorced/single/widowed), educational status (low = primary or high school vs high = university postgraduate), income (low–moderate vs high), and stage of cancer (I–IV). For categorical variables, suitable dummy variables with the aforementioned coding were constructed and the stepwise regression method was applied. For statistical analyses, the significance level was set at p<0.05. All analyses were performed using IBM 21 SPSS software (IBM Corporation, Armonk, NY, USA).
Results
Demographic and clinical characteristics
The participants were Greek women (N=171) and their mean age at the time of data collection was 51.74±7.26 years (range, 18–65 years). In all, 48.5% of the participants were recruited from private hospitals, while 51.5% were recruited from public hospitals in Attica, Greece.
In all, 54.9% was highly educated (had at least a college degree), while the majority (64.4%) were married (69%). About two-thirds of the participants (63.7%) reported a low or moderate annual income, while the majority (79.5%) had up to two children.
Forty nine women (28.7%) had stage I, 69 women (40.4%) had stage II, and 48 women (28.1%) had stage III breast cancer. One hundred and twenty nine women (75.4%) underwent lumpectomy and the remaining 42 (24.6%) underwent mastectomy. One hundred and nine women (63.7%) received chemotherapy and radiotherapy as cancer treatment and 108 (63.2%) received hormone therapy. The most commonly reported comorbidity was endocrinological problems (38.3%), while 99 women (57.9%) had a family history of breast cancer.
The participants in the first group (insufficient level of physical activity) showed a higher body mass index (M=28.62±5.14) compared to the physical active group (M=22.21±2.09). All participants exhibited moderate to high levels of depressive symptoms (M=11.89, SD =5.24),18 moderate to low self-esteem (M=37.33, SD =6.58),22 moderate anxiety (M=38.82, SD =7.51),21 and a mild to moderate score in global health (M=62.87, SD =16.52)19,20 at baseline.
Differences in global health, QoL, and psychological factors
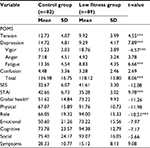
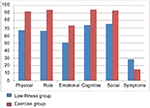
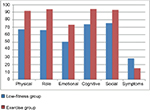
The means, standard deviations, and t-values for each of the variables included are shown in Table 1. Significant differences were found between the low fitness and exercise groups in all the variables. More specifically, the exercise group reported a better psychological mood profile in total t(169)=8.06, p≤0.001 compared to the low fitness group, with all the subscales being better as Figure 1 shows. Additionally, self-esteem in the exercise group was significantly better t(169)=12.08, p≤0.001 and anxiety significantly lower t(169)=9.78, p≤0.001 than the low fitness group. Finally, global health status was better in the exercise group compared to the low fitness group t(169)=11.26, p≤0.001, with all the functional scales being better as Figure 2 shows.
  | Figure 1 Differences in the subscales of psychological model profile. |
Correlational analysis
Correlation analysis revealed significantly positive correlations between MET and self-esteem (r=0.633, p<0.001), MET and global health (r=0.674, p<0.001), MET and QoL – physical EORTC30 (r=0.643, p<0.001), role EORTC30 (r=0.615, p<0.001), emotional EORTC30 (r=0.487, p<0.001), cognitive EORTC30 (r=0.417, p<0.001), social EORTC30 (r=0.417, p<0.001). In addition, significantly negative low to moderate correlations between MET and anxiety – STAI (r=−0.442, p<0.001), MET and depression – POMS (r=−0.552, p<0.001), and MET and age (r=−0.376, p<0.001) were observed (Table 2). Significant correlates between independent variables and age were included in the regression analysis because none was considered as causing multicollinearity (|r|<0.377).
Regression analysis
The sample was divided into two groups as regards the age of the participants and stepwise multiple regressions were conducted. More specifically, for women aged <51 years, the multiple regression analyses revealed that the independent variable MET was a significant predictor of depressive symptoms, explaining 30.5% of the total variance (F=33.858; p<0.01), whereas MET, income, and education were significant predictors of self-esteem, and when combined they explained 41.3% of the total variance (F=17.572; p<0.01). In addition, variables MET and education were significant predictors of anxiety, and when combined they explained 23.0% of the variance (F=11.363; p<0.01), while variables MET and income were significant predictors of global health, and when combined they explained 50.3% of the variance (F=38.466; p<0.01). Multiple regression analyses revealed that MET, income, and cancer stage were significant predictors of physical functioning, and when combined they explained 42.8% of the total variance (F=18.725; p<0.01), whereas MET and education were significant predictors of role, and when combined they explained 45.8% of the variance (F=21.158; p<0.01). Additionally, MET was a significant predictor of emotional status, explaining 24.5% of the variance (F=24.987; p<0.01); education and MET were significant predictors of cognitive functioning, and when combined they explained 30.7% of the variance (F=16.836; p<0.01); and income, MET, and stage of cancer were significant predictors of social functioning, and when combined they explained 34.1% of the variance (F=12.957; p<0.01) (Table 3).
For older women (age ≥52 years), multiple regression analyses revealed that the independent variables MET and marital status were significant predictors of depressive symptoms, explaining 20.0% of the total variance (F=11.106; p<0.01), whereas MET, education, and income were significant predictors of self-esteem, and when combined they explained 45.3% of the total variance (F=24.303; p<0.01). In addition, MET was a significant predictor of anxiety, explaining 15.0% of the variance (F=15.880; p<0.01), while variables MET and income were significant predictors of global health, and when combined they explained 39.8% of the variance (F=29.392; p<0.01). Multiple regression analyses also revealed that MET and education were significant predictors of physical functioning, and when combined they explained 32.2% of the total variance (F=21.152; p<0.01), whereas MET, stage of cancer, and income were significant predictors of role, and when combined they explained 28.6% of the variance (F=11.728; p<0.01). Additionally, MET was a significant predictor of emotional functioning, explaining 22.4% of the variance (F=25.933; p<0.01); also, MET and marital status were significant predictors of cognitive functioning, and when combined they explained 14.8% of the variance (F=7.712; p<0.01); stage of cancer and MET were significant predictors of social functioning, and when combined they explained 15.9% of the variance (F=8.383; p<0.01) (Table 4).
Discussion
The aim of this study was to investigate the differences and associations of physical activity in global health, QoL, and psychological factors among breast cancer survivors after completing their cancer treatment and through survivorship, and to examine whether or not physical activity and patients’ sociodemographic characteristics were predictors of depressive mood, anxiety, self-esteem, and QoL in younger and older breast cancer survivors.
According to WHO, the number of deaths from breast cancer in Greece in 2014 was 4934 and it took the first place among other cancers in women. Approximately 18% of risk factors for breast cancer was attributed to physical inactivity.25 What we already know is that physical activity can be beneficial for depression and depressed mood;2,5,13 however, we do not have enough evidence on whether there are statistically significant differences in the reported QoL and in psychological outcomes, such as anxiety, self-esteem, and depressive symptoms, long after having completed oncology treatment and through survivorship between active and less active breast cancer survivors. Although the majority of long-term, disease-free cancer survivors (≥5 years) report a QoL comparable to those with no history of cancer,26 a recent meta-analysis found that 30%–40% of cancer patients had diagnosable mood disorders through survivorship which could be improved by early detection and treatment.27,28 Our first hypothesis was confirmed as the results from this study demonstrated that significant differences were observed between the low fitness and active groups concerning depressed mood, self-esteem, anxiety, global health, and QoL even after completing any kind of treatment (chemotherapy, radiotherapy, hormone therapy) which is in line with other studies.29–33
Depressive mood is a major psychological side effect, triggered by breast cancer diagnosis and its treatment, and is associated with poor adherence to treatment plan and reduced survival rates.26–28 Due to difficulty in handling the burden of the disease, there is a possibility that women who suffer from depressive mood, either before cancer diagnosis or due to cancer diagnosis, are not willing to participate in exercise intervention programs. On the other hand, there is not much evidence concerning associations between physical activity and depressive symptoms when survivors completed treatment of any kind and are half way through the 5-year survivorship.
Results from the current study showed that women through survivorship faced moderate to high levels of depressive symptoms, which is a novel finding. On the other hand, it was also shown that physically active women through survivorship had less depressive symptoms, compared to the low fitness group, which may lead to the conclusion that physical activity can be beneficial for handling those symptoms more effectively. Exercise has been viewed as a cost-effective and noninvasive treatment alternative.6 Nevertheless, exercise can be safely recommended to women with mild or clinical levels of depressive symptoms as there are no adverse side effects resulting from participation in exercise throughout the course of cancer survivorship.34
Additionally, results from the present study showed that physical activity was positively associated with self-esteem and QoL and negatively associated with anxiety among breast cancer survivors, even in the long posttreatment period, which confirms our second hypothesis. Although there is some knowledge concerning QoL among active and inactive women through survivorship,35 there is not much evidence about the associations between physical activity, anxiety, and self-esteem in this period of time which renders the results of this research novel. The association between physical activity and several aspects of QoL during cancer treatment is evident throughout recent studies.36,37 A relevant finding from this research is that exercise seems to be beneficial in all different components of health-related QoL long after treatment.
The results from this study could be used in order to promote exercise and physical activity programs as an alternative for handling anxiety and depressive symptoms and improving QoL in breast cancer survivors through survivorship. The increasing survival rates of breast cancer patients in conjunction with lower recurrence and mortality rates necessitate that clinicians and exercise experts are enabled to recommend and offer feasible individualized and progressive programs which will aid survivors toward remaining active and lead to a healthy lifestyle throughout survivorship.
For survivors <51 years, physical activity was the most important variable related to depressive symptoms, anxiety, self-esteem, and QoL. Income, educational level, and stage of cancer were also related to QoL and self-esteem. Marital status was not related to QoL. These findings are novel. Other studies have also found that depression and anxiety were significantly worse in younger survivors38,39 and that the risk factors for depression and anxiety in the 5 years after diagnosis are related to the patient rather than to the disease or its treatment, without giving specific information about the patient characteristic or other variables such as physical activity.
For older survivors >52 years, physical activity was also significantly related to depressive symptoms, anxiety, self-esteem, and QoL. Income, education, and cancer stage were significantly related to self-esteem and QoL. Marital status seemed to be related with depressive symptoms and cognitive function in QoL. Other studies for older survivors have shown that marital status and education were predictors of better QoL and anxiety in older survivors, contrary to the stage of cancer which was not.40 For self-esteem, there is not much evidence and this finding is novel.
Our third hypothesis was also confirmed regarding the fact that physical activity was significantly related to less depressive symptoms and anxiety, higher self-esteem, and better global health and QoL in younger and older survivors long after having completed treatment and through survivorship. Moreover, there are novel findings about other factors such as income, education, marital status, and stage of cancer and their relation to psychological factors and QoL among younger and older breast cancer survivors.
To this end, it might be worth offering evidence-based information about exercise interventions and their benefits through survivorship. Starting as early as possible, after diagnosis, might help survivors deal in a better way with the psychological factors which are related to cancer and have a better QoL through survivorship. As other studies have shown, an optimal duration of ≥150 minutes of moderate or ≥75 minutes of vigorous aerobic exercise combined with resistance training is beneficial for cancer patients.41
Study limitations
The current study has several strengths such as the use of MET and VO2 max test (objective physical activity measurement). Objective measurement combined with self-reported physical activity strengthens the results of this study and reduces bias. Furthermore, the diversity of the sample, which was drawn from four hospitals in different sectors, namely public and private, is a study strength. However, there are some limitations that need to be considered, given that the current sample did not include Greek breast cancer survivors of different racial/ethnic groups, thus leading to limited generalizability of the results.
Despite these limitations, there is significant evidence that exercise is associated with beneficial outcomes in several psychological aspects during survivorship. Future studies, especially in the Greek population, should seek ways to enable survivors regardless of their willingness to participate in exercise programs.
Conclusion
Survivors should be physically active and this should be promoted by physicians and other oncology health care professionals due to biological and psychological positive effects that exercise offers during and after breast cancer treatment. To conclude, the study results emphasize that exercise and physical activity should be promoted by physicians and oncology health care professionals in breast cancer survivors, younger and older, especially after breast cancer treatment, so as to have improved QoL, higher self-esteem, and lower levels of depression and anxiety.
Author contribution
Concept/design: E Patsou; drafting and critically revising the article: G Alexias, F Anagnostopoulos, and MV Karamouzis; final approval of the submitted version: MV Karamouzis. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Doyle N. Cancer survivorship: evolutionary concept analysis. J Adv Nurs. 2008;62:499–509. | ||
Zainal NR, Nik-Jaafar NR, Baharudin A, Sabki ZA, Ng CG. Prevalence of depression in breast cancer survivors: a systematic review of observational studies. Asian Pac J Cancer Prev. 2013;14:2649–2656. | ||
Brown JC, Huedo-Medina TB, Pescatello LC, et al. The efficacy of exercise in reducing depressive symptoms among cancer survivors: a meta-analysis. PLoS One. 2012;7:30955. | ||
Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;32:57–71. | ||
Burgess C, Cornelius V, Love S, Graham J, Richards M, Ramirez A. Depression and anxiety in women with early breast cancer: five year observational cohort study. BMJ. 2005;330:702. | ||
Ganz PA, Desmond KA, Leedham B, Rowland JH, Meyerowitz BE, Belin TR. Quality of life in long-term, disease-free survivors of breast cancer: a follow-up study. J Natl Cancer Inst. 2002;94:39–49. | ||
Perna FM, Craft L, Freud KM, et al. The effect of a cognitive behavioral exercise intervention on clinical depression in a multiethnic sample of women with breast cancer: a randomized controlled trial. Int J Sport Exerc Psychol. 2010;8:36–47. | ||
Van Oers HM. Exercise effects on mood in breast cancer patients. SA J Sports Med. 2012;25:55–59. | ||
Craft LL, Vaniterson EH, Helenowski IB, Rademaker AW, Courneya KS. Exercise effects on depressive symptoms in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21:3–19. | ||
Pinto AC, de Azambuja E. Improving quality of life after breast cancer: dealing with symptoms. Maturitas. 2011;70:343–348. | ||
Brunault P, Toledano A, Aguerre C, et al. Impact de la toxicité de la radiothérapie liée au traitement tardif, de la dépression et de l’anxiété sur la qualité de vie des survivants du cancer du sein à long terme [Impact of late treatment-related radiotherapy toxicity, depression, and anxiety on quality of life in long-term breast cancer survivors]. Bull Cancer. 2012;99:589–598. French. | ||
Nock NL, Owusu C, Flocke S, et al. A community-based exercise and support group program improves quality of life in African-American breast cancer survivors: a quantitative and qualitative analysis. Int J Sports Exerc Med. 2015;1:1–17. | ||
Blanchard CM, Courneya KS, Laing D. Effects of acute exercise on state anxiety in breast cancer survivors. Oncol Nurs Forum. 2011;28:1617–1621. | ||
World Health Organization. International Statistical Classification of Diseases and Related Health Problems. Geneva: WHO; 2004. | ||
Patsou ED, Alexias GD, Anagnostopoulos FG, Karamouzis MV. Effects of physical activity on depressive symptoms during breast cancer survivorship: a meta-analysis of randomised low fitness trials. ESMO Open. 2017;2:e000271. | ||
Ganz PA. Psychological and social aspects of breast cancer. Oncology (Williston Park). 2008;22:642–646. | ||
Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62:243–274. | ||
McNair DM, Lorr M, Droppleman LF. Profile of Mood States Manual. San Diego, CA: Educational and Industrial Testing Services; 1971. | ||
Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. | ||
Michelson H, Bolund C, Nilsson B, Brandberg Y. Health-related quality of life measured by the EORTC QLQ-C30 – reference values from a large sample of Swedish population. Acta Oncol. 2000;39:477–484. | ||
Spielberger CD, Gorsuch RL, Lushene R. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. | ||
Rosenberg M. Society and Adolescent Self-Image. Princeton, NJ: Princeton University Press; 1965. | ||
Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exer. 2003;35:1381–1395. | ||
Howley ET, Bassett DR, Welch HG. Criteria for maximal oxygen uptake: review and commentary. Med Sci Sports Exer. 1995;27:1292–1301. | ||
World Health Organization. Cancer Country Profiles 2014. Available from: http://www.who.int/cancer/country-profiles/en/. Accessed April, 11 2018. | ||
Pinto BM, Dunsiger S, Waldemore M. Physical activity and psychosocial benefits among breast cancer patients. Psychooncology. 2013;22:2193–2199. | ||
Mitchell AJ, Chan M, Bhatti H, et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, hematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. Lancet Oncol. 2011;12:160–174. | ||
Naumann F, Masters EM, Philpott M, Smith C, Groff D, Battaglini C. Can counseling add value to an exercise intervention for improving quality of life in breast cancer survivors? A feasibility study. N Engl J Med. 2012;10:188–194. | ||
Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–2486. | ||
Holick CN, Newcomb PA, Trentham-Dietz A, et al. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:379–386. | ||
Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer related fatigue: the scale of the problem. Oncologist. 2007;12:4–10. | ||
Craft LL, Vaniterson EH, Helenowski IB, Rademaker AW, Courneya KS. Exercise effects on depressive symptoms in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21:3–19. | ||
Zhu G, Zhang X, Wang Y, Xiong H, Zhao Y, Sun F. Effects of exercise intervention in breast cancer survivors: a meta-analysis of 33 randomized controlled trials. Onco Targets Ther. 2016;9:2153–2168. | ||
Courneya KS, McKenzie DC, Mackey JR, et al. Effects of exercise dose and type during breast cancer chemotherapy: multicenter randomized trial. J Natl Cancer Inst. 2013;105:1821–1833. | ||
Courneya KS, Friedenreich CM. Relationship between exercise during treatment and current quality of life among survivors of breast cancer. J Psychosoc Oncol. 1998;15:35–57. | ||
Speck RM, Courneya KS, Mâsse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4:87–100. | ||
Knobf MT, Thompson AS, Fennie K, Erdos D. The effect of a community-based exercise intervention on symptoms and quality of life. Cancer Nurs. 2014;37:43–50. | ||
Gorman JR, Malcarne VL, Roesch SC, Madlensky L, Pierce JP. Depressive symptoms among young breast cancer survivors: the importance of reproductive concerns. Breast Cancer Res Treat. 2010;123:477–485. | ||
Bloom JR, Stewart SL, Chang S, Banks PJ. Then and now: quality of life of young breast cancer survivors. Psychooncology. 2004;13:147–160. | ||
Parker PA, Baile WF, Moor CD, Cohen L. Psychosocial and demographic predictors of quality of life in a large sample of cancer patients. Psychooncology. 2002;12:183–193. | ||
Runowicz CD, Leach CR, Henry NL, et al. American Cancer Society/American Society of Clinical Oncology breast cancer survivorship care guideline. CA Cancer J Clin. 2016;66:43–73. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.