Back to Journals » International Journal of Nanomedicine » Volume 17
Photothermal Nanoheaters-Modified Spores for Safe and Controllable Antitumor Therapy
Authors Zhang X, Zhang Y, Wang N, Shen Y, Chen Q, Han L, Hu B
Received 11 August 2022
Accepted for publication 1 December 2022
Published 15 December 2022 Volume 2022:17 Pages 6399—6412
DOI https://doi.org/10.2147/IJN.S385269
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Farooq A. Shiekh
Xin Zhang,1,* Yang Zhang,2,* Ning Wang,1 Yetong Shen,1 Qing Chen,2 Lu Han,1 Bo Hu1
1Department of Biochemistry and Molecular Biology, School of Life Sciences, China Medical University, Shenyang, 110122, People’s Republic of China; 2School of Pharmacy, Shenyang Medical College, Shenyang, 110034, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Bo Hu, Email [email protected]; [email protected]
Introduction: To present a safer tumor therapy based on bacteria and identify in detail how the activation and infection behavior of spores can be controlled remotely by near-infrared light (NIR-irradiation) based on nanoheaters’ modification.
Methods: Spores bring a better tolerance to surface modification. Transitive gold-nanorods-allied-nanoclusters-modified spores (Spore@NRs/NCs) were constructed by covalent glutaraldehyde crosslink. The photothermal properties of nanoheaters before and after attachment to spores were studied by recording temperature–irradiation time curves. The controlled viability and infection behavior of Spore@NRs/NCs were investigated by NIR-irradiation.
Results: In this work, a controllable sterilizing effect to activated vegetative bacteria was obtained obviously. When met with a suitable growth-environment, Spore@NRs/NCs could germinate, activate into vegetative bacteria and continue to reproduce. Without NIR-irradiation, nanoheaters could not affect the activity of both spores and vegetative bacterial cells. However, with NIR-irradiation after incubating in growth medium, nanoheaters on spores could control the spores’ germination and affect the growth curve as well as the viability of the vegetative bacterial cells. For Spore@NRs/NCs (Spore:NCs:NRs=1:1:4, 67.5 μg mL− 1), a ~98% killing rate of vegetative bacterial cells was obtained with NIR-irradiation (2.8 W cm− 2, 20 min) after 2 h-incubation. In addition, these nanoheaters modified on spores could be taken not only to the vegetative bacteria cells, but also to the first-generation bacteria cells with their excellent photothermal and bactericidal performance, as well as synergetic anticancer effect. NIR-irradiation after 2 h-incubation could also trigger Spore@NRs/NCs (1:1:4, 6 μL) to synergistically reduce the viability of HCT116 cells to 15.63± 2.90%.
Conclusion: By using NIR-irradiation, the “transitive” nanoheaters can remotely control the activity of both bacteria (germinated from spore) and cancer cells. This discovery provides basis and a feasible plan for controllable safer treatment of bacteria therapy, especially anaerobes with spores in hypoxic areas of the malignant solid tumors.
Keywords: transitive nanoheaters, modified spores, NIR-remotely triggered, controlled sterilization, hyperthermia, adjustable activity behavior
Graphical Abstract:
Introduction
Malignant tumor is one of the global public health problems endangering human health, which has become a leading cause of death every year.1 Although conventional therapy methods, including radiotherapy, chemotherapy and surgery, have achieved good therapeutic effects, it is still lack of effective treatment methods for nearly half of the patients with advanced cancer due to their intolerance to surgery, insensitivity to radiotherapy/chemotherapy drugs, or even the frequent recurrence and metastasis after recovery.2 So an increasing number of experts have begun to study the causes of tumor formation and the developmental characteristics of tumors, in order to find out new strategies to realize highly targeted, more efficient and minimal or non-invasive therapy on tumor.3
Tumor has specific microenvironment characteristics that are different from normal tissues, such as typical inflammation, hypoxia, high content of reducing substances and mildly acidity (low pH), that provide targets for tumor therapy.4 It has been demonstrated that the disordered/irregular vascular distribution and vascular defects endow tumor tissue a hypoxia in the inner site,5 which hinders the effective transportation of drugs and greatly reduces the efficacy of radiotherapy/chemotherapy.6–8 However, this hypoxia in tumor provides a suitable environment for anaerobic bacteria growth. Therefore, targeted reproduction of anaerobic bacteria in hypoxia of tumor tissue9,10 can be used for tumor targeting therapy with better biocompatibility and more efficient motion compared with synthetic materials.11 Bacteriotherapy had been already proposed for tumor treatment for more than 200 years.12 Since then, many bacteria have been applied in oncolytic treatments, such as Salmonella, Escherichia coli, Listeria, Bifidobacterium, etc.13,14 In this regard, bacteria can act as tumor therapeutic agent directly with their own substances (bacteriocin, peptides, toxin, enzymes and antibiotics)15 as immunotherapeutic agents that can cause immune response, as a vehicle for target delivery.16–18
Even though anaerobe19 or anaerobe combined with chemotherapy,20,21 radiotherapy22 and immunotherapy23 present a remarkable targeting and efficient therapeutic effect to tumors,24,25 there is still a risk of infection raising safety concerns.26–28 In order to get a safer use of anaerobe on tumor therapy, some genetically engineered bacteria have been constructed to reduce cytotoxicity.29,30 Additionally, some functional antibacterial or tumor-targeted nanomaterials have also been introduced and modified on bacteria surface by some works. Tumor-targeted aptamer,31 tumor-specific antigen,32 or tumor cell-derived membrane33 are modified on bacteria to improve the homologous-targeting tumor localization, reduce inflammatory reaction, and slow down bodily elimination. Functional antibacterials are also used to control the activity of bacteria, or even simultaneously act as tumor agents to bring a better synergistic treatment effect by combining with photothermal therapy (PTT),34–36 hydroxyl radicals generation,37 tumor metabolism coupling,38 DNA39/drug40,41/inhibitor42 loading, or other mechanisms.43,44
However, how the antibacterial nanomaterials that modified on bacteria control the bioactivity of bacteria is still lack of investigation and description in detail. Moreover, modification process with specific antibacterial materials might also affect the activity of bacteria, especially anaerobe. Spore is a self-protective mechanism of bacteria under malignant conditions such as high temperature or low nutrients.15 Spores are highly tolerant and stable to adverse situations. They are the dormant period of bacteria, which can germinate into vegetative bacterial cells when the environment suitable for bacterial growth is encountered.45 Therefore, spore is a wonderful candidate for bacteriotherapy due to its better resistance compared to vegetative bacterial cells, especially for nanomaterial modification.
Hereby, we chose Bacillus subtilis (B. subtilis 10275), a kind of nonpathogenic and spore-forming bacterial strain, to act as the living bacterial model to establish gold nanoheaters-modified spore, Spore@NRs/NCs. It is expected that the nanoheaters-modified Spore@NRs/NCs could target tumor tissues and revert to vegetative bacterial cell when meet their suitable growth environment, then the vegetative bacteria may colonize and compete for nutrients to inhibit the growth of tumor cells. Furthermore, the viability of nanoheaters-modified spores and their infection behavior to tumor cells can be remotely controlled with NIR irradiation by hyperthermia based on the nanoheaters attached to the spores. A synergistic killing of tumor cells could also be observed that being provided by the hyperthermia of nanoheaters. It is expected that the use of nanoheaters-modified Spore@NRs/NCs provides a better resistance to biological microenvironment and a highly tumor-targeted property and avoids the risk of bacteriotherapy.
Materials and Methods
Major chemical reagents: chloroauric acid tetrahiydrate (HAuCl3·HCl·4H2O, AR), L(+)-ascorbic acid (AA, AR), glutaraldehyde 25% aqueous solution (BR) were obtained from Sinopharm Chemical Reagent Co. Ltd, China. Hexadecyl trimethyl ammonium bromide (CTAB, AR) was received from Aladdin Reagent Co. Ltd, China. Sodium tetrahydroborate (NaBH4, 96%) and hydrochloric acid were purchased from Tianjin Damao Chemical Reagent Co. Ltd, China. Silver nitrate (AgNO3, AR), bovine serum albumin (BSA, 96% purity), poly(sodium 4-styrenesulfonate) (PSS, Mw ~70,000) and poly(allylamine hydrochloride) (PAH, Mw ~15,000) were purchased from Sigma-Aldrich LLC, USA. Ultra-pure water was used throughout the experiment.
Bacterial nutrient culture medium (pH 7.0) contained 0.3 g beef power (Aobox, BR), 0.5 g tryptone (Oxoid, BR), 0.5 g sodium chloride (Sinopharm, AR), 10 μL of manganese sulfate solution (MnSO4∙H2O, 0.5% w/v, Sinopharm) and 200 μL of sodium hydroxide solution (NaOH, 1.0 mol L−1, Sinopharm) in every 100 mL of ultra-pure water. Agar power (Aobox, BR, 1.5 g/100 mL) was additionally added to obtain solid growth medium. Bacterial mediums were aseptic before use.
Phosphate buffer solution (PBS, pH 5.5, 6.0, 7.0) for nanomaterial-preparation and spore modification was obtained by mixing a certain volume of Na2HPO4 (0.2 mol L−1) and NaH2PO4 (0.2 mol L−1) solutions. The pH value was adjusted and controlled by a Delta 320 pH meter (Mettler Toledo, Switzerland).
The Characterization of Nanoheaters and Nanoheaters-Modified Spores
Absorption spectra were recorded by a Varian Cary 100 UV-visible spectrophotometer (Agilent Technologies, USA) with a 1 cm quartz cell and a data interval setting of 1 nm with a scan speed of 600 nm min−1 within 200–900 nm. Fluorescence and excitation-emission-matrix spectra were recorded by a Nicolet-6700 FT-IR spectrometer (Thermo Ltd, USA) within 300–700 nm with a scan speed of 2400 nm min−1. Transmission electron microscopy (TEM) images were obtained by using an H7560 microscope (Hitachi Ltd, Japan) operated at 80 kV and a Tecnai G2 20 microscope (Philips, Holland) operated at 200 kV. The surface charges of materials were acquired with a Malvern Zetasizer Nano ZS-ZEN3690 (Malvern, England). A Near-Infrared Ray (NIR) laser of 808 nm (Changchun New Industries Optoelectronics Technology Ltd, China) was used as a light source with adjustable power.
The Preparation of Spore
Bacteria culture. The bacterial model B. subtilis 10275 was purchased from China Center of Industrial Culture Collection (CICC) China. A fresh B. subtilis 10275 solution was obtained by inoculating 50 μL of the second-generation bacteria into a 50-mL nutrient culture medium solution containing Mn2+ (pH 7.0), and growing in the shaker (200 rpm) for 19 h at 37°C, then stored at 4°C for counting and subsequent use.
Spore preparation. Three hundred microlitres of fresh B. subtilis 10275 solution was inoculated on the growth solid medium (15 cm-culture dish) with Mn2+ (pH 7.0) and incubated in the incubator at 37°C for 7 days. After the incubation, the spore of B. subtilis 10275 was scraped and collected in a 15-mL centrifuge tube, after washing twice with PBS, the spore was re-dispersed in 10 mL PBS. To further break down and remove the active B. subtilis bacterial cell from spores’ solution, the spore was boiled with 80°C bathing for 30 min followed by washing thrice with water and then re-dispersed in PBS (pH 7.4), stored at 4°C for counting and further use. The prepared spore needed to be washed with PBS every week and could be used for 3 months. During the spore preparation process, all the centrifuge tubes and solutions were aseptic. Spore production rate is 85.3%, which can be calculated by dividing the number of the obtained spores by the number of bacteria used for spore production. The number of B. subtilis 10275 vegetative bacteria cell and spore were obtained by plate count method.
The Preparation of Nanoheaters-Modified Spores
Nanoheaters preparation. Nanoheaters were prepared by previous method that are briefly described as follows: Au nanorods. Au nanorods were synthesized by the seed-mediated and silver (I)-assisted growth method.46 The seed solution was prepared by rapidly adding a freshly prepared ice-cold NaBH4 solution (0.01 mol L−1, 0.6 mL) into a 10 mL CTAB solution (0.1 mol L−1) containing HAuCl4 (0.1 mol L−1, 0.25 mL), followed by strong stirring for 3 min then kept at 30°C for 1.5 h. After that, 0.04 mL seed solution was added into 86.94 mL CTAB growth solution with HAuCl4 (0.1 mol L−1, 3.5 mL), AgNO3 (0.01 mol L−1, 0.8 mL), HCl (1.0 mol L−1, 1.5 mL), AA (0.1 mol L−1, 0.64 mL) in it, and then incubated at 27°C overnight. The obtained Au nanorods were centrifuged (9000 rpm, 10 min) to remove redundant CTAB twice, then re-dispersed in water and the absorbance of the re-dispersed solution was adjusted to 4.0 following incubation with PSS (20 mg mL−1) overnight. The mixture was centrifuged (7000 rpm, 10 min) twice, re-dispersed in water and continually incubated with PAH (20 mg mL−1) overnight. Polymer-modified Au nanorods (AuNRs-PSS-PAH) were obtained after centrifugation (7000 rpm, 10 min) twice and re-dispersed in water.
Au nanoclusters. Ten milliliters of HAuCl4 (0.01 mol L−1) was slowly added into 10.0 mL of BSA solution (50 mg mL−1) with strong stirring for 2 min. Then, 0.5 mL NaOH (1.0 mol L−1) was added in it and stirred at 37°C overnight. The obtained orange supernatant was centrifuged (12,000 rpm, 10 min) and then dialyzed in a dialysis bag (Mw 8000–14,000).47 The BSA-coated Au nanoclusters (BSA-AuNCs) were obtained.
Nanoheaters-modified spores. The spore (1 × 1010 cfu mL−1) of B. subtilis 10275 solution (1.0 mL) was diluted with 4.0 mL of PBS (pH 5.5) and then activated by 1.0 mL of 5% w/v glutaraldehyde solution for 1 h. After that, the mixture was washed thrice with PBS (pH 5.5) buffer (4500 rpm, 30 min) and re-dispersed in 5.0 mL PBS (pH 5.5) buffer. Later, a series volume of prepared AuNRs-PSS-PAH solution was added in the above diluted spore solution. An hour later, 1.0 mL of prepared BSA-AuNCs solution was added into the mixture and incubated overnight in dark. The nanoheater modified spore (Spore@NRs/NCs) was obtained after centrifugation (4000 rpm, 20 min), washed twice and re-dispersed in 5.0 mL sterile ultra-pure water. The obtained Spore@NRs/NCs were stored at 4°C for further use.
The Photothermal Conversion Property of Nanoheaters Before and After Modified on Spores
Photothermal effect. A specific device (Figure S1) was used to investigate the photothermal conversion property of nanoheaters before and after modification onto spore. One millilter of nanoheaters-modified spores (Spore@NRs/NCs) with different Spore:NCs:NRs ratio (1:1:0.5, 1:1:1, 1:1:2, 1:1:4) were irradiated by 808 nm NIR laser at different power (1.9, 2.2, 2.5, 2.8 W cm−2) within 20 min. The temperature changes were recorded. Then, 1.0 mL of water, corresponding equivalent AuNRs-PSS-PAH and Spore@NRs/NCs (1:1:4, 2.7 μg μL−1) were irradiated by NIR laser (808 nm, 2.8 W cm−2). The temperature changes were recorded.
Photothermal stability. The photothermal stability of nanoheaters-modified spores (Spore@NRs/NCs, 1:1:4, 2.7 μg μL−1) was also evaluated by recording the temperature changes during and after NIR irradiation (808 nm, 2.8 W cm−2, 15 min).
The Controllable Activity of Spores After Being Modified with Nanoheaters
Growth curve. Five microliters of B. subtilis 10275 spore, Spore@NRs/NCs (1:1:4, 13.5 μg) before and after 20 min-NIR irradiation (2.8 W cm−2) were respectively added into the prepared sterilized glass test tube with 5.0 mL of bacterial nutrient culture medium. Totally 60 tubes after inoculation were incubated in the shaker (200 rpm) for 40 h at 37°C. The optical densities at 600 nm (OD600) were recorded every 2 h for each sample and plotted to get the growth curve.
Controlled bactericidal performance. Five microliters of Spore@NRs/NCs (1:1:4, 13.5 μg) were cultured in 5.0 mL of nutrient culture medium at 37°C, and irradiated by an 808 nm laser (2.8 W cm−2, 20 min) after different incubation time (0, 1, 2, 3, 4, 5 h). Spore@NRs/NCs were inoculated onto solid medium for counting after irradiation.
Five microliters of Spore@NRs/NCs with different Spore:NCs:NRs ratio (1:1:0.5, 1:1:1, 1:1:2, 1:1:4) were cultured in 5.0 mL of nutrient culture medium at 37°C, respectively. Then, the cultured Spore@NRs/NCs were irradiated by NIR laser with different power density (1.9, 2.2, 2.5, 2.8 W cm−2). After irradiation, the Spore@NRs/NCs were inoculated onto solid medium for counting.
The Controlled Safety and Antitumor Effect of Nanoheaters-Modified Spores
Cell culture. Human colon cancer cell (HCT116) was chosen as cancer model cell and purchased from Shanghai Genechem Co., Ltd with standard survey report. HCT116 cells were cultivated and maintained in McCoy’s 5A medium (Procell Life Science&Technology Co., Ltd, China) containing 10% w/v fetal bovine serum (FBS, Gibco Sera, Life Technologies, Australia) without penicillin-streptomycin in incubator at 37°C under 5% CO2, then the standard Cell Counting Kit-8 (CCK-8, ApexBio Technology LLC, USA) assay was performed to detect the cell viability.
Controlled antitumor properties of Spore@NRs/NCs. HCT116 (4×104 cells) were cultured and then seeded in a 96-well plate and incubated in 37°C, 5% CO2 for 24 h. After that, the cell culture medium was removed and the cells were washed twice with PBS buffer (pH 7.4). One hundred microliters of serum-free McCoy’s 5A medium containing a corresponding number of spores, Spore@NRs/NCs were added into the prepared HCT116 cells and incubated in 37°C, 5% CO2 for some time, then irradiated by 808-nm laser (2.8 W cm−2, 20 min). After being cultured in incubator for 4 h, 10 μL of CCK-8 was added into all the groups, the results were tested by automatic microplate reader at 450 nm.
The cell viability (or bacteria viability) under the conditions of different dosage of Spore@NRs/NCs (2.7 μg or 13.5 μg) with different Spore:NCs:NRs ratios (1:1:2 and 1:1:4) and different incubation time (2 or 4 h) were investigated following the upper process. The corresponding concentration of spore was chosen as a control group to remove the interference of CCK-8 signal produced by activated vegetative bacterial cells. Each group had three parallel samples. Serum-free McCoy’s 5A medium was used as a blank.
Results and Discussion
Design and Characterization of Nanoheaters-Modified Spores
Anaerobic bacteria can target the hypoxic areas within solid tumors, which is expected to address the negative impact of hypoxia on tumor prognosis, but there is still a risk of bacterial infection. Even though some works have already been done by using nanomaterials to reduce the risks, the stable modification (such as a covalent binding) of nanomaterials on bacteria, especially anaerobic bacteria, is still a complex process, which has challenges and needs to be improved. In this context, a well-tolerated spore was chosen and acted as a model of bacteria, to simplify, regularize and stabilize the modification of bacteria with nanomaterials.
In the present work, a kind of nanoheaters-modified spores were constructed to make the cancer therapy based on bacteria more simple, safer and more controllable. It should be noted that in order to simple the modification and the bacterial anticancer process, we chose a nontoxic probiotic, aerobic bacterium that produce spores, Bacillus subtilis as bacteria model, a classic photothermal agent gold-based nanomaterials as nanoheaters, to systematically and in detail study the bactericidal behavior of nanoheaters after attached on spore, as well as the additional anticancer property. Meanwhile, unfortunately, because of aerobic bacteria selection, solid tumor experiments in vivo could not be carried out. However, it could still provide a detailed data for the antitumor-study in vivo with spore-producing anaerobic bacteria in the future.
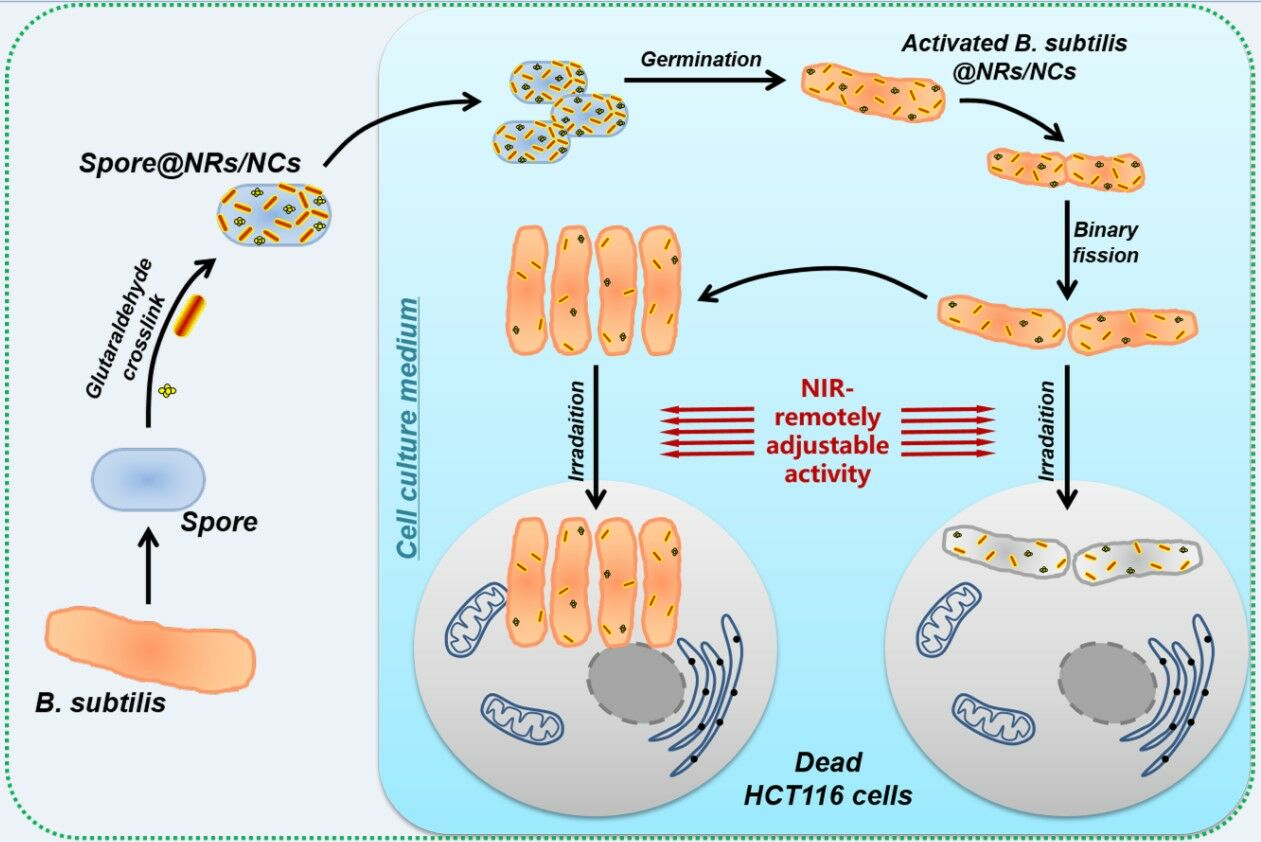
Scheme 1 illustrates the composed process of Spore@NRs/NCs and their controlled anticancer treatment process. Firstly, a nutritional deficiency approach was adopted to get the spore of B. subtilis, and the typical nanoheaters (polymers modified gold nanorods and nanotracer gold nanoclusters) were chosen and synthesized according to the published literature. Afterwards, a simple and convenient method, glutaraldehyde crosslink, was used to attach the amino-modified nanoheaters (NRs/NCs) onto the well-tolerated spore of B. subtilis to form nanoheaters stable modified spore, Spore@NRs/NCs. When the Spore@NRs/NCs are in a suitable growth environment, they can take the nanoheaters and germinate to vegetative bacteria cell and continue to reproduce. The controlled bactericidal and antitumor properties of the obtained Spore@NRs/NCs were investigated by using human colon cancer cell (HCT116) as cancer model cells.
 |
Scheme 1 Schematic illustration of the transitive nanoheaters-modified spores and their NIR-remotely adjustable activity in antitumor mechanism. |
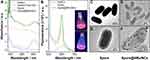
Firstly, the nanoheaters-modified spores, Spore@NRs/NCs, were synthesized and characterized. After preparation, AuNRs with uniform size were obtained and continue to be modified with (‒)/(+)-charged polymers. The zeta potential of polymers-modified AuNRs (AuNRs-PSS-PAH) decreased from 28.7±1.3 mV to −35.2±1.0 mV and then increased to 39.3±1.8 mV, which indicated the successful modification of negatively charged polymer PSS and positively charged polymer PAH (Figure S2A). Simultaneously, BSA-stabilized AuNCs (BSA-AuNCs) were synthesized with a red fluorescence at ~610 nm wavelength (Figure S2B). In the present work, the use of well-tolerated spore made the modification conditions of nanoheaters to bacteria not so harsh. A simple and stable glutaraldehyde crosslinking method was used to link the nanoheaters and nanotracers onto the spore. On the one hand, glutaraldehyde can help to kill the living vegetative bacterial cells and further to purify the spore. On the other hand, it can provide covalent and tight attachment of nanoheaters NRs/NCs onto spores’ surface, which is equivalent to putting a “time bomb” on bacteria and is the basis of controlled sterilization. It was observed that, after crosslinking, the absorption spectra of Spore@NRs/NCs had the specific longitudinal absorption of NRs at 814 nm (Figure 1A) and the fluorescence emission of NCs (Figure 1B). The surface morphological features of spores before and after nanoheaters’ modification had been observed by transmission electron microscopy (TEM). The TEM images showed clearly that the native spores of B. subtilis had a smooth surface (Figure 1C and D). While after modification, the nanoheaters obviously appeared to the surface of the spores, which verified that the nanoheaters had adhered to the spores’ surface uniformly (Figure 1E and F). All the above data demonstrated that the nanoheaters-modified spores were successfully synthesized.
The Transitive Property of Nanoheaters That Modified on Spores
The usage of spore in bacterial therapy for cancer can effectively simplify the process of spore modification with bactericidal nanomaterials and improve the modification efficiency, but there are still two tricky problems needed to be investigated and solved. One question is whether the nanomaterials can be passed on to progeny bacteria; the other is a suitable timing of NIR irradiation in the triggered sterilization.
Theoretically, the PTT effect produced by the gold-based nanoheaters attached to the spores could not affect and inhibit the activity of the spores in Spore@NRs/NCs due to the spores’ strong heat resistance. While only when the spores are placed in a suitable vegetative environment and germinate into vegetative bacterial cells, their activity can be controlled by nanoheaters. If the spores belong to the anaerobic bacteria, then this growth conditions-triggered spore germination can well target the hypoxic area inside the tumor. Anyhow, this requires that the nanoheaters are still left on the bacteria surface during the spore germination, and also can be transitive and passed to the activated vegetable bacterial cells, and still can control the activity of the vegetable bacterial cells by PTT effect with NIR irradiation.
To prove the assertion of “transitive” property of the nanoheaters on spores’ surface, three stages of Spore@NRs/NCs (Figure 2A), activated vegetative bacterial cell@NRs/NCs (Figure 2B and C) and first generation of vegetative bacterial cells (Figure 2D) have been analyzed with TEM. TEM images demonstrated that after the spores germinated into vegetative bacterial cells, the surfaces of activated vegetable bacterial cells were still covered by many nanoheaters (Figure 2B and C). Surprisingly, the next generation (first) of the activated vegetable bacterial cells was also covered by nanoheaters although the number of the nanoheaters was less than that on the parental activated vegetable bacterial cells (Figure 2D). Compared with previous work, it provided clear evidence that proved and confirmed the “transitive” behavior of nanoheaters attached to spore. Due to the special di-fission propagation mode of bacteria, the nanoheaters on spores can be transitive and passed to the activated vegetative bacterial cells or even to the next generations. Therefore, the bactericidal performance of nanoheaters based on PTT under NIR irradiation can also be transitive.
 |
Figure 2 Transitive nanoheaters. TEM images of Spore@NRs/NCs (A), activated vegetative bacterial cell@NRs/NCs (B and C) and first generation of vegetative bacterial cell@NRs/NCs (D). |
The Photothermal Conversion Property of Nanoheaters Before and After Modified on Spores
Photothermal effect produced by nanoheaters is the main force for sterilization, and gold-based nanomaterials, especially AuNRs, are ideal photothermal agents. Later on, the photothermal conversion property of nanoheaters on Spore@NRs/NCs with different Spore:NCs:NRs ratios (nanoheaters’ amount) was investigated in detail (Figure 3A).
It was demonstrated that the larger amount of nanoheaters adhesion and higher laser density applied, the better photothermal conversion efficiency would be provided by Spore@NRs/NCs. The temperature of Spore@NRs/NCs with Spore:NCs:NRs ratio of 1:1:4 raised rapidly to ca. 48°C within a short laser irradiation time of 20 min by using 2.8 W cm−2 power intensity (Figure 3B). The photothermal conversion efficiency of nanoheaters was not significantly affected by covalent modification. Even modified on spore, the nanoheaters could still exhibit a high photothermal conversion efficiency and gave a temperature raising to 47°C only under 15 min-NIR irradiation of 2.8 W cm−2, which was a little bit lower than the temperature of nanoheaters before modification (Figure 3C). It should be noted that 47°C was the temperature of the Spore@NRs/NCs solution, while the AuNRs that attached to the spore could produce hyperthermia to destroy the bacterial wall/membrane to kill bacteria, which had already been demonstrated by previous work.48 In additional, 47°C is soft and suitable for cancer treatment in vivo instead of higher temperature above 50°C.49 All the data in this part indicated that the PTT effect of Spore@NRs/NCs had a potential of bacterial and cancer treatment. Notably, the nanoheaters after being attached to spores still retained not only a good photothermal conversion efficiency but also a favorable photothermal stability. Figure 3D illustrated that, even after five heating and cooling cycles, the temperature produced by PTT based on nanoheaters AuNRs-PSS-PAH on spore barely had any downward trend.
The Controllable Activity of Spores After Being Modified with Nanoheaters
It is mentioned before that in order to control and inhibit the activity of spore, the timing of NIR irradiation is very important. This is because as bacteria proliferate through di-division, the nanoheaters attached to spore or passed to the activated bacterial cells will become less and less, may even be too few to effectively inhibit bacterial activity. So the controlled activity of Spore@NRs/NCs by PTT based on nanoheaters NRs/NCs was investigated by studying the bacteria viability under different NIR irradiation conditions at a specific timing.
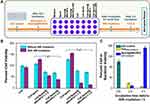
Figure 4A shows the growth curves of spore before and after nanoheaters modification, as well as the NIR irradiation. It was demonstrated that nanoheaters attachment alone did not affect the spore germination and proliferation of B. subtilis. However, NIR irradiation inhibited the activity of Spore@NRs/NCs and shortened their logarithmic phase. Within the first 6 h, the spore or activated B. subtilis cells grew slowly, and then they went into logarithmic growth, which indicated that the time point for NIR irradiation that could control the Spore@NRs/NCs activity by the attached nanoheaters might lie in this beginning incubation time range.
In order to find out the exact time point of NIR irradiation for controlling the activity of Spore@NRs/NCs, the bacteria viabilities under NIR irradiation were checked out at every 1 h of the beginning 5 h incubation time. The data showed that, in the growth culture medium, without NIR irradiation, the Spore@NRs/NCs germinated to vegetative bacterial cell (B. subtilis@NRs/NCs) and then proliferated, thus the bacteria viability increased regularly (Figure 4B, Spore@NRs/NCs group). However, by applying NIR irradiation after different incubation time, the bacteria viability showed a different trend. Applying NIR irradiation before incubation, there was no significant effect on Spore@NRs/NCs viability. While applying NIR irradiation after 1 h, 2 h and 3 h of incubation, good bactericidal abilities of nanoheaters on spore were obtained. But as continued to increase the incubation time before NIR irradiation in Spore@NRs/NCs-NIR group, the bacterial survivability significantly increased again and was not very different from that of the non-NIR irradiation group (Figure 4B, Spore@NRs/NCs-NIR group).
This maybe because that at the beginning of incubation, the PTT effect produced by nanoheaters on spores could not kill the spores because of their strong tolerance. However, along the incubation time increase, parts of the nanoheaters-modified spores (Spore@NRs/NCs) converted to the vegetable bacterial cells (B. subtilis@NRs/NCs), and the activated vegetable bacterial cells could be killed by the PTT effect of nanoheaters under NIR irradiation. But as the B. subtilis@NRs/NCs continued to proliferate, there were too much more bacterial cells in the system and the nanoheaters on bacterial cells get fewer and not enough to inhibit bacterial activity. Of these, NIR irradiation after 2 h incubation exhibited the best controlled bactericidal property and provided only a small bacterium viability of 6.74±1.61% (Figure 4B and C). From this, it can be considered that after 2 h incubation in growth culture medium, most of Spore@NRs/NCs were germinated to B. subtilis@NRs/NCs but had not yet to produce the next generation. And the nanoheaters attached on the activated bacterial cells could inhibit bacterial activity by PTT effect. Therefore, it could be concluded that the best timing of NIR irradiation for Spore@NRs/NCs to control their own activity is after 2 h-incubation in culture.
Further studies derived how the dosage of nanoheaters that attached to spore and power intensity affect the controllable bactericidal property of Spore@NRs/NCs. When NIR irradiation after 2 h incubation, both the Spore@NRs/NCs with ratios of 1:1:2 and 1:1:4 showed good controllable bactericidal effect (Figure 5A, Figure S3), which was consistent with the photothermal conversion efficiency. The controllable bactericidal efficiency became better along with the increase in the amount of nanoheaters and the power of NIR irradiation. Similar bacterial killing rates of Spore@NRs/NCs with ratios of 1:1:2 (78.25±1.56%) under 20 min NIR irradiation and 1:1:4 (78.12±1.56%) under 10 min NIR irradiation were produced. The nanoheaters on the Spore@NRs/NCs with ratio of 1:1:4 could control the bacteria viability low to 2.06±1.24% under 20 min NIR irradiation (2.8 W cm−2) (Figure 5B and C). Compared with the free NRs,46 the nanoheaters modified on spores still exhibited a wonderful bactericidal efficiency. This demonstrated the excellent ability triggered by NIR of Spore@NRs/NCs to control their own activity.
The Controlled Safety and Antitumor Effect of Nanoheaters-Modified Spores
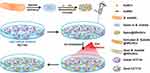
Human colon cancer cell (HCT116) was chosen as a cancer cell model to evaluate the controlling infection behavior and antitumor property of nanoheaters-modified spores under NIR irradiation. Figure 6A shows the treatment process of Spore@NRs/NCs to colon cancer HCT116 cells. In order to remove the interference of the active bacterial cells, the corresponding concentration of spores was also chosen as control. The cell viability of each sample was detected by CCK-8 assay. Because different inoculation amounts of Spore@NRs/NCs would affect the growth cycle of spore and bacteria, the dosage of Spore@NRs/NCs and the needed incubation time with HCT116 cells were optimized and compared in a small range based on the data of controlled bactericidal property we had got before.
It was demonstrated that after being added into the HCT116 cells, the Spore@NRs/NCs could be germinated and activated into vegetative B. subtilis cells taking with nanoheaters together by the nutrient-rich cell culture medium firstly and then reproduced. As extracellular bacteria and a kind of probiotics without cytotoxicity but not pathogenic bacteria, B. subtilis@NRs/NCs grow outside the cell (Figure S4), and can only affect the viability of cancer cells by competing for the nutrition. The germination and growth of the added Spore@NRs/NCs caused the lack of nutrition and inhibited the proliferation and viability of HCT116 cells. It could be predicted that without the controlling of NIR irradiation, the activated vegetative B. subtilis cells would continue to grow by consuming the nutrition in the cell culture medium, and finally result in the death of HCT116 cells. While with the NIR irradiation, the activity of B. subtilis@NRs/NCs could be controlled by nanoheaters, which had been already verified in Figure 4.
It further showed that NIR irradiation triggered PTT effect could not only control the activity of bacterial cells but also present a synergistic effect of cancer cell treatment. Spore@NRs/NCs with Spore:NCs:NRs of 1:1:4 presented a much better photothermal effect, antibacterial performance and synergistic antitumor effect. And, 20 min-NIR irradiation (2.8 W cm−2) after 2 h co-incubation with HCT116 cells could trigger the nanoheaters on Spore@NRs/NCs (1:1:4, 6 μL) effectively to synergistically affect the viability of HCT116 cells, kept the cancer cell viability low to 15.63±2.90%. The more dosage of Spore@NRs/NCs and the more nanoheaters adhered on the spores, the better antitumor efficiency of Spore@NRs/NCs exhibited under NIR irradiation (Figure 6B). By continuously investigating the viability of HCT116 and B. subtilis@NRs/NCs, both good bactericidal efficiency (9.08±1.59%) and antitumor efficiency (18.26±2.27%) under NIR irradiation after 2 h co-incubation were obtained. However, with NIR irradiation after 4 h co-incubation, only the viability of HCT116 could be limited to 5.69±1.59%, while the B. subtilis@NRs/NCs grew out of control (Figure 6C). That meant with co-incubation more than 4 h, the “transitive” nanoheaters on the bacterial cells could not effectively play their bactericidal performance and control bacterial activity as well as infection behavior because of the weak PTT effect provided by the less adhesion amount on the breeding bacterial cells. It indicated that it was important to select the timing of NIR irradiation for safer treatment of tumor based on nanoheaters-modified bacteria. In addition, the further decrease in cancer cell viability also suggested that the Spore@NRs/NCs (or B. subtilis@NRs/NCs) could inhibit cancer cell growth through nutrient competition. By comparing with the growth curve of Spore@NRs/NCs, it was demonstrated that the nanoheaters modified on spore could control the activity of activated vegetative bacterial cells as well as the first generation of vegetative bacterial cells and had a good synergistic therapeutic effect on cancer cells. It indeed provided a safer strategy for bacterial therapy on tumor by attaching the bactericidal nanoheaters on spore as controllable elements.
Conclusion
In conclusion, we construct a nanoheaters-modified spore (Spore@NRs/NCs) acting as a model of bacterial therapy to show the controlled behavior of spore/bacteria for presenting a much safer tumor therapy based on bacteria. By glutaraldehyde crosslink, the nanoheaters can not only be successfully covalently attached to spores’ surface but also be “transitive” from the spores to the activated vegetative bacterial cells or even to the first generation of the activated bacterial cells. NIR irradiation at a specific time point can trigger the photothermal conversion of nanoheaters on spores, remotely control the bioactivity of spore/bacteria. Meanwhile, the nanoheaters-modified spores also present a wonderful synergistic antitumor effect controlled remotely by NIR irradiation. Our current work establishes a much safer and easier modified candidate, spore, for the bacteria therapy used in tumor treatment. The combination of spores and glutaraldehyde crosslinking method proposed in this paper can be widely used in the stable modification of bacteria by different kinds of functional nanomaterials, further improve and broaden the application of bacteria therapy. These discoveries provide a new strategy and detail evidence for controllable and much safer cancer treatment of bacteria therapy, which can be further applied to targeted treatment for hypoxic areas of the malignant solid tumors by anaerobes with spores.
Acknowledgments
Special thanks are due to Professor Ting Yang at department of chemistry, College of Sciences, Northeastern University for providing support and advice. This present work was financially supported by the National Natural Science Foundation of China (No. 21605161, 21804093), Shenyang Innovative Talents Supporting Program (RC180167) and Natural Science Foundation Liaoning Province (2021-BS-105).
Supplementary Materials
Supplementary materials associated with the present work can be found in the online version.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Theys J, Lambin P. Clostridium to treat cancer: dream or reality? Ann Transl Med. 2015;3:S21. doi:10.3978/j.issn.2305-5839
3. Cai Y, Liang P, Tang Q, et al. Diketopyrrolopyrrole-triphenylamine organic nanoparticles as multifunctional reagents for photoacoustic imaging-guided photodynamic/photothermal synergistic tumor therapy. ACS Nano. 2017;11(1):1054–1063. doi:10.1021/acsnano.6b07927
4. Attia MF, Anton N, Wallyn J, Omran Z, Vandamme TF. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J Pharm Pharmacol. 2019;71(8):1185–1198. doi:10.1111/jphp.13098
5. Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11(6):393–410. doi:10.1038/nrc3064
6. Pollheimer MJ, Kornprat P, Lindtner RA, et al. Tumor necrosis is a new promising prognostic factor in colorectal cancer. Hum Pathol. 2010;41(12):1749–1757. doi:10.1016/j.humpath.2010.04.018
7. Torchilin V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv Drug Deliv Rev. 2011;63(3):131–135. doi:10.1016/j.addr.2010.03.011
8. Beck B, Blanpain C. Unravelling cancer stem cell potential. Nat Rev Cancer. 2013;13(10):727–738. doi:10.1038/nrc3597
9. Pettersen EO, Ebbesen P, Gieling RG, et al. Targeting tumour hypoxia to prevent cancer metastasis. From biology, biosensing and technology to drug development: the METOXIA consortium. J Enzyme Inhib Med Chem. 2015;30(5):689–721. doi:10.3109/14756366.2014.966704
10. Minton NP. Clostridia in cancer therapy. Nat Rev Microbiol. 2003;1(1):237–242. doi:10.1038/nrmicro777
11. Sonntag L, Simmchen J, Magdanz V. Nano-and micromotors designed for cancer therapy. Molecules. 2019;24(18):3410. doi:10.3390/molecules24183410
12. Mengesha A, Dubois L, Chiu RK, et al. Potential and limitations of bacterial-mediated cancer therapy. Front Biosci. 2007;12:3880–3891. doi:10.2741/2357
13. Forbes NS. Engineering the perfect (bacterial) cancer therapy. Nat Rev Cancer. 2010;10(11):785–794. doi:10.1038/nrc2934
14. Li S, Jiang W, Zheng C, et al. Oral delivery of bacteria: basic principles and biomedical applications. J Control Release. 2020;327:801–833. doi:10.1016/j.jconrel.2020.09.011
15. Yaghoubi A, Khazaei M, Jalili S, et al. Bacteria as a double-action sword in cancer. BBA-Rev Cancer. 2020;1874(1):188338. doi:10.1016/j.bbcan.2020.188388
16. Luo Y, Xu D, Gao X, et al. Nanoparticles conjugated with bacteria targeting tumors for precision imaging and therapy. Biochem Bioph Res Co. 2019;514(4):1147–1153. doi:10.1016/j.bbrc.2019.05.074
17. Cao Z, Liu J. Bacteria and bacterial derivatives as drug carriers for cancer therapy. J Control Release. 2020;326:396–407. doi:10.1016/j.jconrel.2020.07.009
18. Luo CH, Huang CT, Su CH, Yeh CS. Bacteria-mediated hypoxia-specific delivery of nanoparticles for tumors imaging and therapy. Nano Lett. 2016;16(6):3493–3499. doi:10.1021/acs.nanolett.6b00262
19. Dang LH, Bettegowda C, Huso DL, Kinzler KW, Vogelstein B. Combination bacteriolytic therapy for the treatment of experimental tumors. PNAS. 2001;98(26):15155–15160. doi:10.1073/pnas.251543698
20. Dang LH, Bettegowda H, Agrawal N, et al. Targeting vascular and avascular compartments of tumors with C. novyi-NT and anti-microtubule agents. Cancer Boil Ther. 2004;3(3):326–337. doi:10.4161/cbt.3.3.704
21. Smith AB, Freeze BS, LaMarche MJ, Sager J, Kinzler KW, Vogelstein B. Discodermolide analogues as the chemical component of combination bacteriolytic therapy. Bioorg Med Chem Lett. 2005;15(15):3623–3626. doi:10.1016/j.bmcl.2005.05.068
22. Bettegowda C, Dang LH, Abrams R, et al. Overcoming the hypoxic barrier to radiation therapy with anaerobic bacteria. PNAS. 2003;100(25):15083–15088. doi:10.1073/pnas.2036598100
23. Agrawal N, Bettegowda C, Cheong I, et al. Bacteriolytic therapy can generate a potent immune response against experimental tumors. PNAS. 2004;101(42):15172–15177. doi:10.1073/pnas.0406242101
24. Maletzki C, Gock M, Klier U, Klar E, Linnebacher M. Bacteriolytic therapy of experimental pancreatic carcinoma. World J Gastroenterom. 2010;16(28):3546–3552. doi:10.3748/wjg.v16.i28.3546
25. Staedtke V, Bai RY, Sun WY, et al. Clostridium novyi-NT can cause regression of orthotopically implanted glioblastomas in rats. Oncotarget. 2015;6(8):5536–5546. doi:10.18632/oncotarget.3627
26. Krick EL, Sorenmo KU, Rankin SC, et al. Evaluation of Clostridium novyi-NT spores in dogs with naturally occurring tumors. Am J Vet Res. 2012;73(1):112–118. doi:10.2460/ajvr.73.1.112
27. Roberts NJ, Zhang L, Janku F, et al. Intratumoral injection of Clostridium novyi-NT spores induces antitumor responses. Sci Transl Med. 2014;6(249):249ra111. doi:10.1126/scitranslmed.3008982
28. Pinato DJ, Gramenitskaya D, Altmann DM, et al. Antibiotic therapy and outcome from immune-checkpoint inhibitors. J Immunother Cancer. 2019;7(1):287. doi:10.1186/s40425-019-0775-x
29. Sedighi M, Bialvaei AZ, Hamblin MR, et al. Therapeutic bacteria to combat cancer; current advances, challenges, and opportunities. Cancer Med. 2019;8(6):3167–3181. doi:10.1002/cam4.2148
30. Mi Z, Feng ZC, Li C, Yang X, Ma MT, Rong PT. Salmonella-mediated cancer therapy: an innovative therapeutic strategy. J Cancer. 2019;10(20):4765–4776. doi:10.7150/jca.32650
31. Geng ZM, Cao ZP, Liu R, Liu K, Liu JY, Tan WH. Aptamer-assisted tumor localization of bacteria for enhanced biotherapy. Nat Commun. 2021;12:6584. doi:10.1038/s41467-021-26956-8
32. Li JJ, Xia Q, Guo HY, et al. Decorating bacteria with triple immune nanoactivators generates tumor-resident living immunotherapeutics. Angew Chem Int Edit. 2022;61(27):e202202409. doi:10.1002/anie.202202409
33. Liu R, Cao ZP, Wang L, et al. Multimodal oncolytic bacteria by coating with tumor cell derived nanoshells. Nano Today. 2022;45:101537. doi:10.1016/j.nantod.2022.101537
34. Kuo WS, Wu CM, Yang ZS, et al. Biocompatible bacteria@Au composites for application in the photothermal destruction of cancer cells. Chem Commun. 2008;37:4430–4432. doi:10.1039/b808871c
35. Chen W, Wang Y, Qin M, et al. Bacteria-driven hypoxia targeting for combined biotherapy and photothermal therapy. ACS Nano. 2018;12(6):5995–6005. doi:10.1021/acsnano.8b02235
36. Fan JX, Li ZH, Liu XH, Zheng DW, Chen Y, Zhang XZ. Bacteria-mediated tumor therapy utilizing photothermally controlled TNF-# expression via oral administration. Nano Lett. 2018;18(4):2373–2380. doi:10.1021/acs.nanolett.7b05323
37. Fan JX, Peng MY, Wang H, et al. Engineered bacterial bioreactor for tumor therapy via Fenton-like reaction with localized H2O2 generation. Adv Mater. 2019;31(16):1808278. doi:10.1002/adma.201808278
38. Chen QW, Wang JW, Wang XN, et al. Inhibition of tumor progression through the coupling of bacteria respiration with tumor metabolism. Angew Chem Int Edit. 2020;59(48):21562–21570. doi:10.1002/anie.202002649
39. Hu Q, Wu M, Fang C, et al. Engineering nanoparticle-coated bacteria as oral DNA vaccines for cancer immunotherapy. Nano Lett. 2015;15(4):2732–2739. doi:10.1021/acs.nanolett.5b00570
40. Kazmierczak R, Choe E, Sinclair J, Eisenstark A. Direct attachment of nanoparticle cargo to Salmonella typhimurium membranes designed for combination bacteriotherapy against tumors. Methods Mol Biol. 2015;1225:151–163. doi:10.1007/978-1-4939-1625-2_11
41. Suh SB, Jo A, Traore MA, et al. Nanoscale bacteria-enabled autonomous drug delivery system (NanoBEADS) enhances intratumoral transport of nanomedicine. Adv Sci. 2018;6(3):1801309. doi:10.1002/advs.201801309
42. Wang L, Cao ZP, Zhang MM, Lin SS, Liu JY. Spatiotemporally controllable distribution of combination therapeutics in solid tumors by dually modified bacteria. Adv Mater. 2022;34:2106669. doi:10.1002/adma.202106669
43. Uthaman S, Zheng S, Han J, et al. Preparation of engineered Salmonella typhimurium-driven hyaluronic-acid-based microbeads with both chemotactic and biological targeting towards breast cancer cells for enhanced anticancer therapy. Adv Healthcare Mater. 2016;5(2):288–295. doi:10.1002/adhm.201500556
44. Zheng DW, Chen Y, Li ZH, et al. Optically-controlled bacterial metabolite for cancer therapy. Nat Commun. 2018;9(1):1680. doi:10.1038/s41467-018-03233-9
45. Chang WW, Lee CH. Salmonella as an innovative therapeutic antitumor agent. Int J Mol Sci. 2014;15(8):14546–14554. doi:10.3390/ijms150814546
46. Hu B, Wang N, Han L, Chen ML, Wang JH. Core-shell-shell nanorods for controlled release of silver that can serve as a nanoheater for photothermal treatment on bacteria. Acta Biomateria. 2015;11:511–519. doi:10.1016/j.actbio.2014.09.005
47. Zhang J, Tu L, Zhao S, et al. Fluorescent gold nanoclusters based photoelectrochemical sensors for detection of H2O2 and glucose. Biosens Bioelectron. 2015;67:296–302. doi:10.1016/j.bios.2014.08.037
48. Hu B, Zhang LP, Chen XW, Wang JH. Gold nanorod-covered kanamycin-loaded hollow SiO2 (HSKAurod) nanocapsules for drug delivery and photothermal therapy on bacteria. Nanoscale. 2013;5:246–252. doi:10.1039/c2nr32457a
49. Huang HC, Rege K, Heys JJ. Spatiotemporal temperature distribution and cancer cell death in response to extracellular hyperthermia induced by gold nanorods. ACS Nano. 2010;4(5):2892–2900. doi:10.1021/nn901884d
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.