Back to Journals » Infection and Drug Resistance » Volume 11
Phenotypic and molecular characteristics of methicillin-resistant and methicillin-susceptible Staphylococcus aureus isolated from pigs: implication for livestock-association markers and vaccine strategies
Authors Guo D, Liu Y, Han C, Chen Z, Ye X
Received 9 May 2018
Accepted for publication 17 June 2018
Published 23 August 2018 Volume 2018:11 Pages 1299—1307
DOI https://doi.org/10.2147/IDR.S173624
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eric Nulens
Dan Guo, Yangqun Liu, Changlin Han, Zhiyao Chen, Xiaohua Ye
Laboratory of Molecular Epidemiology, School of Public Health, Guangdong Pharmaceutical University, Guangzhou, China
Background: Routine non-therapeutic antimicrobial use and overcrowding in animal farming may facilitate the propagation of methicillin-resistant Staphylococcus aureus (MRSA). This study aimed to examine the carriage prevalence and phenotype–genotype characteristics of MRSA and methicillin-susceptible S. aureus isolated from pigs.
Methods: Nasal swabs were collected from 1,458 pigs in 9 pig farms and 3 slaughterhouses. All strains were tested for antimicrobial susceptibility, resistance genes, and virulence genes, and characterized by multilocus sequence typing. The correspondence analysis was conducted to explore the relationships between multiple phenotypic and molecular characteristics of S. aureus isolates.
Results: In the 1,458 pigs, the carriage prevalence was 9.5% for S. aureus, 3.3% for MRSA, and 9.3% for multidrug-resistant S. aureus. Notably, 97.1% S. aureus isolates were multidrug resistant, and the predominant resistance pattern was non-susceptible to clindamycin, tetracycline, and erythromycin. The predominant genotype was CC9 (ST9) for S. aureus and MRSA isolates. Importantly, all S. aureus isolates were negative for the scn gene and resistant to tetracycline. Notably, all 9 linezolid-resistant isolates were classified as multidrug resistance, including 1 expressing the cfr gene and 6 expressing the optrA gene. The correspondence analysis showed a significant relationship between clonal complexes and resistance pattern or virulence genes. For example, CC9 was associated with extensive drug-resistance and co-carrying chp, sak, and hlb, and CC1 was associated with multidrug resistance and co-carrying sak and hlb.
Conclusion: The significant correspondence relationship between multiple characteristics provides some implication for vaccine strategies and new ideas for monitoring new epidemiologic clones.
Keywords: livestock, animals, Staphylococcus aureus, antimicrobial susceptibility, multidrug resistance, molecular characterization
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) is an important cause of nosocomial infections worldwide.1 Human-related MRSA is usually divided into 2 types according to epidemiological and molecular characteristics, including community-associated and hospital-associated MRSA. However, latest evidence has revealed that the epidemiological trend has changed with the increasing appearance of livestock-associated MRSA (LA-MRSA). In Asia, the most common lineage of LA-MRSA is ST9; while in Europe and North America, ST398 isolates are most prevalent.2 Notably, there is increasing emergence of outbreaks of LA-MRSA in hospitals and invasive LA-MRSA infections in humans.3 Therefore, LA-MRSA has become an important public health issue that warrants close monitoring.
More and more evidence revealed that non-therapeutic antimicrobial use and overcrowding in animal farming may facilitate the spread of LA-MRSA.4,5 Additionally, recent reports found that LA-MRSA could colonize in multiple animals and related workers.2,6–9 Notably, MRSA epidemiology in livestock is increasing and the resulting food products may become contaminated through the MRSA-positive livestock, polluted environment, and related workers.10–12 Foodstuffs contaminated by MRSA have been found recently, suggesting the possibility of spread to humans through consumption and meat handling.13–15 Therefore, there is a growing concern that MRSA of animal origin may be transmitted by the food chain or contact with colonized animals. It is noteworthy that specific markers for S. aureus species association are still uncertain. Although some studies have identified LA-MRSA from farm and slaughterhouse animals in Asia,2 there is still little evidence of potential genetic markers for livestock association of MRSA isolates, and not much is known about potential relationships between molecular typing and phenotype–genotype characteristics of MRSA isolates using statistical testing methods. Therefore, we undertook a cross-sectional study in pig farms and slaughterhouses to sample pigs for S. aureus analysis. This study aimed to examine the carriage prevalence, antimicrobial susceptibility, virulence genes, and molecular typing of S. aureus isolates. Additionally, this study builds on previous research to explore the high-dimensional relationships between molecular typing and phenotype–genotype characteristics of S. aureus isolates using correspondence analysis.
Methods
Study design and sample collection
This cross-sectional study was conducted from May to July 2015 in Jiangmen, China. The target population was pigs, which were sampled by the method of multistage sampling process. First, 3 county-level cities were randomly sampled from 7 county-level city in Jiangmen city. Second, in each county-level city, 3 pig farms and 1 slaughterhouse were selected to reach a sample size of about 140 farm pigs and 350 slaughterhouse pigs. In all, 1,458 pigs were sampled in this survey, including 411 pigs from 9 pig farms and 1,047 pigs from 3 slaughterhouses. Of these, 194 were piglets (<3 months) and 1,264 were grower pigs (≥3 months). Non-duplicate swabs were obtained from both nares of each pig.
Bacterial isolation and identification
Swabs were soaked into 5-mL enrichment broth (0.25% yeast extract, 1% mannitol, 1% tryptone, and 7.5% NaCl) at 4°C during transportation, and incubated at 35°C ± 1°C for 24 hours. Then a loopful of the broth was plated onto mannitol salt agar and incubated at 37°C for 24–48 hours. Suspected colonies were selected and subcultured to 5% sheep blood agar plates and incubated at 35°C ± 1°C overnight. S. aureus or MRSA isolates were confirmed by a combination of morphology, Gram staining, catalase test, tube coagulase test, DNase test, and polymerase chain reaction (PCR) assays for the carriage of the staphylococci 16S rRNA, nuc and mecA (or mecC) genes.16,17 All S. aureus isolates carried the 16S rRNA and nuc genes, and all MRSA isolates carried the 16S rRNA, nuc and mecA (or mecC) genes.
Antimicrobial susceptibility testing and resistant genes
Antimicrobial susceptibility testing was performed by the disk diffusion method according to the recommendations of the Clinical and Laboratory Standards Institute (CLSI, 2015).18 Susceptibility was tested to the following antibiotic disks: cefoxitin (30 µg), erythromycin (15 µg), clindamycin (2 µg), tetracycline (30 µg), trimethoprim-sulfamethoxazole (25 µg), rifampin (5 µg), chloramphenicol (30 µg), ciprofloxacin (5 µg), gentamicin (10 µg), quinupristin-dalfopristin (15 µg), and linezolid (30 µg). According to the CLSI guidelines, S. aureus isolates were classified as susceptible, intermediate, or resistant to each antibiotic. Multidrug-resistant (MDR) S. aureus (MDRSA) was defined as being non-susceptible to ≥1 agent in ≥3 antimicrobial categories, extensively drug-resistant (XDR) S. aureus was defined as being non-susceptible to ≥1 agent in all but ≤2 categories, and pan-drug-resistant (PDR) S. aureus was defined as being non-susceptible to all antimicrobial agents listed.19 For all S. aureus isolates, the presence of erythromycin-resistant genes (erm[A] and erm[C]) and tetracycline-resistant genes (tet[M] and tet[K]) was determined by PCR tests described previously.20 For linezolid-resistant isolates, the presence of resistant genes (cfr and optrA) was determined by PCR tests.21
Molecular characterization
For all S. aureus isolates, multilocus sequence typing (MLST) was performed as previously described.22 Alleles and sequence types (STs) were assigned by submitting the DNA sequences to the MLST database (http://saureus.mlst.net). BURST analysis was conducted to analyze clonal complexes (CCs) using eBURST V3 software program (Department of Infectious Disease Epidemiology, Imperial College London, London, UK; http://eburst.mlst.net). We conducted specific PCR tests for the presence of the Panton–Valentine leucocidin (pvl) toxin gene, the immune evasion cluster (IEC) genes (scn, chp, sea, sak, and sep), the beta-hemolysin gene (hlb) and the staphylococcal cassette chromosome mec element (SCCmec) type.23–25
Data analysis
The differences in S. aureus (including MRSA and MDRSA) carriage between groups were determined using the Pearson’s chi-squared test or Fisher’s exact test when appropriate. Since correspondence analysis provides a useful graphic and statistical method for exploring the internal relationship between categorical variables, we used the correspondence analysis to explore potential relationships between CC and phenotype–genotype characteristics of S. aureus isolates. These analyses were performed using STATA version 14.0 (StataCorp LP, College Station, TX, USA), and a 2-sided P value of <0.05 was defined as being of statistical significance.
Ethics statement
Ethics approval for the study was obtained from the Ethics Committee of Guangdong Pharmaceutical University, Guangzhou, China.
Results
S. aureus, MRSA, and MDRSA detection in pigs
Of 1,458 pigs sampled in this survey (Table 1), 139 (9.5%) carried S. aureus, including 135 (9.3%) MDRSA and 48 (3.3%) MRSA isolates. All MRSA isolates carried the mecA gene, but all these isolates were absent of the mecC (the novel mecA homolog). When comparing the carriage rates between farm and slaughterhouse pigs, there were statistically significant differences in the carriage of S. aureus (6.8% vs 10.6%; P=0.027) and MDRSA (6.8% vs 10.2%; P=0.043). When comparing the carriage rates between piglets and grower pigs, there were statistically significant differences in the carriage of S. aureus (4.1% vs 10.4%; P=0.006).
Antibiotic susceptibility and resistance genes
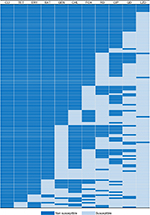
Among 139 S. aureus isolates, most of isolates were resistant to clindamycin (97.1%), tetracycline (96.4%), erythromycin (92.8%), and trimethoprim-sulfamethoxazole (82.7%) (Table 2). Notably, 87 isolates were resistant to cefoxitin, including 48 MRSA and 39 non-MRSA isolates. Additionally, 135 (97.1%) isolates were classified as MDRSA, with the most common resistance pattern being non-susceptible to clindamycin, tetracycline, and erythromycin (Figure 1). In terms of the macrolide-resistant genes (Table 2), the most predominant gene was erm(C) (79.1%), followed by erm(A) (5.0%). Additionally, there were 6 (4.3%) isolates co-expressing erm(C) and erm(A). In terms of the tetracycline-resistant genes, 87 (62.6%) carried the tet(K) and 81 (58.3%) carried the tet(M). In addition, 51 (36.7%) isolates co-expressed the tet(K) and tet(M). Notably, all linezolid-resistant isolates (9 isolates) were classified as MDRSA, including 1 expressing the cfr gene and 6 expressing the optrA gene.
  | Table 2 Antibiotic susceptibility and resistance genes of 139 Staphylococcus aureus isolates |
Molecular characteristics
Among 139 S. aureus isolates (Table 3), we observed 40 unique STs belonging to 11 CCs. The most common S. aureus CCs were CC9 (73 isolates), CC1 (17 isolates), and CC5 (15 isolates), with the predominant MRSA being CC9 (28 isolates) and CC1 (10 isolates). The most common S. aureus STs were ST9 (33 isolates), ST2931 (13 isolates), ST920 (12 isolates), ST2454 (11 isolates), and ST1 (8 isolates). Among 48 MRSA isolates, the predominant STs were ST9 (19 isolates, including 12 isolates for SCCmec IV, 5 for non-types I–VII, 1 for SCCmec III, and 1 for SCCmec V) and ST1 (5 isolates, including 2 isolates for SCCmec IV, 2 for non-types I–VII, and 1 for SCCmec V). As to the IEC genes, 112 (80.6%) isolates expressed the sak, followed by the chp (60.4%), sea (15.8%), and sep (8.6%). Notably, scn gene was absent in all the S. aureus isolates. Additionally, hlb was present in 82 (59.0%) isolates, but pvl was only found in 1 isolate.
Relationships between predominant CCs and phenotype–genotype characteristics
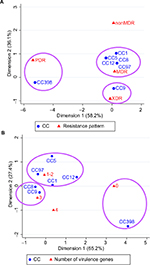
The correspondence analysis showed good corresponding relationships between CC typing and resistance pattern of S. aureus isolates (chi-squared=54.98, P<0.001; Figure 2A). For example, CC398 was associated with PDR, CC9 with XDR, and CC1/CC5/CC8/CC12/CC97 isolates were associated with MDR. As shown in Table 3, CC398 was associated with nonsusceptibility to almost all these antibiotics; CC9 (including ST9, ST2931, and ST2454) was associated with nonsusceptibility to 9 antibiotics; CC1 (including ST1) and CC5 (including ST920) were associated with nonsusceptibility to 7 antibiotics; and CC8/CC12/CC97 isolates were nonsusceptible to 4 or 5 antibiotics.
Another correspondence analysis revealed good corresponding relationships between CC typing and the number of virulence genes (including scn, chp, sea, sak, sep, and hlb) of S. aureus isolates (chi-squared=38.03, P=0.004; Figure 2B). For example, CC9 and CC8 were associated with carrying 3 virulence genes, CC1/CC5/CC12/CC97 were associated with carrying 1 or 2 virulence genes, and CC398 was associated with absence of these virulence genes. As shown in Table 3, CC9 (including ST9, ST2931, and ST2454) and CC8 were associated with carrying chp, sak, and hlb; CC1 was associated with carrying sak and hlb; and CC5 (including ST920), CC12 and CC97 were associated with carrying chp and sak.
Discussion
This study of pigs indicated that the carriage rate was 9.5% for S. aureus and 3.3% for MRSA, which is similar to results from previous studies (4.6%–15.9% for S. aureus and 0.9%–7.7% for MRSA).9,26–31 However, imparity results also have been reported. For example, S. aureus carriage rate was significant high in India (71.4%) and Ireland (26%–73%),32,33 and MRSA carriage rate was significant high in Germany (49%–70.8%) and Spain (46%).34,35 There may be several reasons for this difference, such as geographical location, sampling methods, bacterial isolation and identification methods (conventional biochemical methods or PCR test; enrichment or no enrichment), livestock density, and antibiotic usage. In this study, S. aureus (including MDRSA) prevalence rate in slaughterhouse pigs was significantly higher than that observed in farm pigs. Similarly, a particularly high MRSA carriage rate was reported in slaughterhouse pigs in Shanghai and Spanish,36,37 which may be explained by co-transportation with MRSA-colonized animals.38 Additionally, we observed that the prevalence of S. aureus carriage in grower pigs was higher than piglets, which is consistent with the report from Trinidad.27 This situation may be due to the abuse of antimicrobial agents, and grower pigs may have been exposed to antibiotics for much longer than piglets.
The antimicrobial resistance of animal S. aureus and MRSA has become an important public health issue. We observed high rates of resistance to erythromycin, tetracycline, and clindamycin among S. aureus isolates, which may be due to the abuse of antimicrobial agents in animals in China.39 Similarly, recent reports on animal-related MRSA in China, Portugal, and Japan showed that most isolates were resistant to clindamycin (88%–100%), tetracycline (47%–100%), and erythromycin (96%–100%).29,40,41 Notably, in this study, about 97.1% isolates were MDR. A study in Hongkong demonstrated that almost all of livestock S. aureus were MDR, suggesting that antimicrobial abuse and overcrowding in animal farming may facilitate the spread of MDRSA isolates.42 The most predominant resistance pattern observed in this study was co-nonsusceptible to clindamycin, tetracycline, and erythromycin, which is similar to the findings from China and USA.8,28 Notably, we observed that all linezolid-resistant isolates were classified as MDRSA, including 1 expressing the multi-resistance cfr gene. Similarly, the latest studies in China and Germany reported the emergence of cfr-mediated multi-resistance in staphylococci from animals and livestock-related humans.43,44 In contrast to cfr, a novel optrA gene confers cross-resistance only to oxazolidinones and phenicols. Presence of the optrA gene has been detected in Staphylococcus sciuri and Enterococcus of human and animal origin,21,45 and we also found 6 linezolid-resistant isolates expressing the optrA gene. Therefore, both linezolid-resistant and MDR isolates must be monitored in prospective surveillance programs.
LA-MRSA has been reported worldwide. In Asia, of particular interest is livestock-associated CC9. The present study revealed that the predominant genotype of S. aureus and MRSA isolates was CC9, including ST9, ST2931, and ST2454. MRSA ST9 was first detected from pig finishing holdings in Italy46 and has also been reported to predominate in pigs in Asian countries.9,30,47,48 Additionally, LA-MRSA CC9 can colonize in retail meat and other host species (including cows, sheep, and poultry).2,29,49,50 Notably, previous studies revealed that pig workers carried LA-MRSA ST9 and patients without contact with animals were also tested with MRSA ST9, suggesting the potential LA-MRSA transmission from animals to humans by direct and indirect animal exposure.8,51 Notably, LA-MRSA CC9 isolates express different types of SCCmec, including type IX for Thailand, type V for Malaysia, types IVb/V for Hong Kong, and type III for China.2 However, MRSA ST9 in this study mainly carried SCCmec IV, followed by non-types I–VII. These findings show some regional characteristics in LA-MRSA ST9.
Another important LA-MRSA is CC398, which prevails in animals and related workers in North America and European countries.6,52 In our study, no MRSA CC398 was found, but 2 MSSA CC398 isolates were isolated from pigs. Although CC398 isolates are rare in Asia, the study from South Korea found a high rate of ST398 isolated from breeding pigs since these pigs were imported from other countries.2 Besides, CC5 and CC97 have been identified in this study, which were also reported as LA-MRSA in Germany and Senegal.11,26 Therefore, MRSA-carrying animals pose a potential threat to human health. Surprisingly, ST1 and ST59 as the typical human-associated MRSA clones were detected in pigs in this study, which is consistent with the reports in Africa and China.29,53 These findings may add to the evidence of inter-species MRSA transmission between humans and animals.
It should be noted that a few studies have attempted to explore the specific markers for S. aureus species association. Recent evidence of European–American CC398 has revealed that absence of the bacteriophage-encoded IEC genes may be associated with animal specificity and presence of IEC genes may be related with human specificity.54,55 This animal-specificity relation for Asian CCs is still uncertain. The present study first revealed that all S. aureus were absence of scn gene, suggesting that absence of scn may be associated with pig specificity. Additionally, susceptibility to tetracycline was observed only in isolates from workers without animal contact, but tetracycline resistance was only found in CC9 from animals and animal-related workers.8 Similarly, we observed that all pig S. aureus (including MRSA) isolates were resistant to tetracycline, indicating that phenotypic resistance to tetracycline may be associated with pig specificity.
The potential relationship between CCs and phenotype–genotype characteristics of S. aureus is still unclear. Recent evidence is limited to use the statistical description to explore potential relations between molecular characteristics. For example, the latest studies of LA-MRSA indicated that all MRSA CC97 isolates were MDR and about 90% of MRSA ST9 isolates were MDR.56,57 These findings suggest that there may be differences in resistance patterns between different clones. This study adds to existing literature to reveal a significant correspondence between CC and resistance pattern of S. aureus isolates, indicating that LA CC9 and CC398 were associated with severe XDR or PDR. Notably, latest studies found that absence of IEC genes may be associated with animal specificity.8,58 For example, all MDRSA CC9 and MRSA CC9 carried by pig-related workers were absent of IEC genes.8 This study first revealed a significant correspondence between CCs and the number of virulence genes of S. aureus isolates; CC9/CC8 isolates were associated with carrying 3 virulence genes (chp, sak, and hlb) and CC5/CC12/CC97 isolates with carrying 2 virulence genes (chp and sak). These findings provide new ideas for monitoring new epidemiologic trends and provide implication for facilitating vaccine developments.
This study is a new attempt to reveal potential relationships between CC typing and phenotype–genotype characteristics of S. aureus isolates using the correspondence analysis. However, the current study has some limitations. First, the research design was cross-sectional, so it could not determine whether the nasal colonization was persistent or transient. Future longitudinal study may reveal more information about colonization dynamics of S. aureus. Second, the present study observed 39 isolates resistant to cefoxitin but absent of the mecA, suggesting that the MRSA definition based on mecA in this study may underestimate the true prevalence. Third, no nasal samples were obtained from livestock-related workers, so it could not determine whether exists the risk of LA-MRSA transmission from animals to livestock-related workers.
Conclusion
All pig-related S. aureus isolates were negative for scn gene and resistant to tetracycline, suggesting that absence of scn and presence of tetracycline resistance may be associated with pig specificity. Additionally, the correspondence analysis found good corresponding relationships between CC typing and phenotype–genotype characteristics, which provides some implication for vaccine strategies and new ideas for monitoring new epidemiologic trends. Notably, the proportion of MDRSA was high in 97.1%, supporting growing concern about the potential hazards of non-therapeutic antibiotic use.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81602901) and the Science and Technology Planning Project of Guangdong province (No. 2014A020212306). The funders have no role in the study design, data collection and analysis, or preparation of the manuscript.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Woodford N, Livermore DM. Infections caused by Gram-positive bacteria: a review of the global challenge. J Infect. 2009;59(Suppl 1):S4–16. | ||
Chuang YY, Huang YC. Livestock-associated meticillin-resistant Staphylococcus aureus in Asia: an emerging issue? Int J Antimicrob Agents. 2015;45(4):334–340. | ||
Lewis HC, Mølbak K, Reese C, et al. Pigs as source of methicillin-resistant Staphylococcus aureus CC398 infections in humans, Denmark. Emerg Infect Dis. 2008;14(9):1383–1389. | ||
Hollis A, Ahmed Z. Preserving antibiotics, rationally. N Engl J Med. 2013;369(26):2474–2476. | ||
Nadimpalli M, Rinsky JL, Wing S, et al. Persistence of livestock-associated antibiotic-resistant Staphylococcus aureus among industrial hog operation workers in North Carolina over 14 days. Occup Environ Med. 2015;72(2):90–99. | ||
Smith TC, Male MJ, Harper AL, et al. Methicillin-resistant Staphylococcus aureus (MRSA) strain ST398 is present in midwestern U.S. swine and swine workers. PLoS One. 2009;4(1):e4258. | ||
Khanna T, Friendship R, Dewey C, Weese JS. Methicillin resistant Staphylococcus aureus colonization in pigs and pig farmers. Vet Microbiol. 2008;128(3-4):298–303. | ||
Ye X, Fan Y, Wang X, et al. Livestock-associated methicillin and multidrug resistant S. aureus in humans is associated with occupational pig contact, not pet contact. Sci Rep. 2016;6:19184. | ||
Neela V, Mohd Zafrul A, Mariana NS, van Belkum A, Liew YK, Rad EG. Prevalence of ST9 methicillin-resistant Staphylococcus aureus among pigs and pig handlers in Malaysia. J Clin Microbiol. 2009;47(12):4138–4140. | ||
de Neeling AJ, van den Broek MJ, Spalburg EC, et al. High prevalence of methicillin resistant Staphylococcus aureus in pigs. Vet Microbiol. 2007;122(3–4):366–372. | ||
Köck R, Schaumburg F, Mellmann A, et al. Livestock-associated methicillin-resistant Staphylococcus aureus (MRSA) as causes of human infection and colonization in Germany. PLoS One. 2013;8(2):e55040. | ||
Vandendriessche S, Vanderhaeghen W, Soares FV, et al. Prevalence, risk factors and genetic diversity of methicillin-resistant Staphylococcus aureus carried by humans and animals across livestock production sectors. J Antimicrob Chemother. 2013;68(7):1510–1516. | ||
Agersø Y, Hasman H, Cavaco LM, Pedersen K, Aarestrup FM. Study of methicillin resistant Staphylococcus aureus (MRSA) in Danish pigs at slaughter and in imported retail meat reveals a novel MRSA type in slaughter pigs. Vet Microbiol. 2012;157(1–2):246–250. | ||
de Boer E, Zwartkruis-Nahuis JT, Wit B, et al. Prevalence of methicillin-resistant Staphylococcus aureus in meat. Int J Food Microbiol. 2009;134(1–2):52–56. | ||
Virgin JE, van Slyke TM, Lombard JE, Zadoks RN. Short communication: methicillin-resistant Staphylococcus aureus detection in US bulk tank milk. J Dairy Sci. 2009;92(10):4988–4991. | ||
Zhang K, Sparling J, Chow BL, et al. New quadriplex PCR assay for detection of methicillin and mupirocin resistance and simultaneous discrimination of Staphylococcus aureus from coagulase-negative staphylococci. J Clin Microbiol. 2004;42(11):4947–4955. | ||
García-Álvarez L, Holden MT, Lindsay H, et al. Meticillin-resistant Staphylococcus aureus with a novel mecA homologue in human and bovine populations in the UK and Denmark: a descriptive study. Lancet Infect Dis. 2011;11(8):595–603. | ||
CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fifth Informational Supplement (M100-S25): Clinical and Laboratory Standards Institute; 2015. | ||
Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. | ||
Strommenger B, Kettlitz C, Werner G, Witte W. Multiplex PCR assay for simultaneous detection of nine clinically relevant antibiotic resistance genes in Staphylococcus aureus. J Clin Microbiol. 2003;41(9):4089–4094. | ||
Wang Y, Lv Y, Cai J, et al. A novel gene, optrA, that confers transferable resistance to oxazolidinones and phenicols and its presence in Enterococcus faecalis and Enterococcus faecium of human and animal origin. J Antimicrob Chemother. 2015;70(8):2182–2190. | ||
Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol. 2000;38(3):1008–1015. | ||
van Wamel WJ, Rooijakkers SH, Ruyken M, van Kessel KP, van Strijp JA. The innate immune modulators staphylococcal complement inhibitor and chemotaxis inhibitory protein of Staphylococcus aureus are located on beta-hemolysin-converting bacteriophages. J Bacteriol. 2006;188(4):1310–1315. | ||
Mcclure JA, Conly JM, Lau V, et al. Novel multiplex PCR assay for detection of the staphylococcal virulence marker Panton–Valentine leukocidin genes and simultaneous discrimination of methicillin-susceptible from -resistant staphylococci. J Clin Microbiol. 2006;44(3):1141–1144. | ||
Zhang K, Mcclure JA, Elsayed S, Louie T, Conly JM. Novel multiplex PCR assay for characterization and concomitant subtyping of staphylococcal cassette chromosome mec types I to V in methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 2005;43(10):5026–5033. | ||
Fall C, Seck A, Richard V, et al. Epidemiology of Staphylococcus aureus in pigs and farmers in the largest farm in Dakar, Senegal. Foodborne Pathog Dis. 2012;9(10):962–965. | ||
Gordon A, Marshall J, Ramdass K, Stewart-Johnson A, Adesiyun A. Frequency of resistance to methicillin and other antimicrobial agents among Staphylococcus aureus strains isolated from pigs and their human handlers in Trinidad. Infect Ecol Epidemiol. 2014;4(1):22736. | ||
Dressler AE, Scheibel RP, Wardyn S, et al. Prevalence, antibiotic resistance and molecular characterisation of Staphylococcus aureus in pigs at agricultural fairs in the USA. Vet Rec. 2012;170(19):495. | ||
Wang W, Liu F, Baloch Z, et al. GenGenotypic characterization of methicillin-resistant Staphylococcus aureus isolated from pigs and retail foods in China. Biomed Environ Sci. 2017;30(8):570–580. | ||
Baba K, Ishihara K, Ozawa M, Tamura Y, Asai T. Isolation of meticillin-resistant Staphylococcus aureus (MRSA) from swine in Japan. Int J Antimicrob Agents. 2010;36(4):352–354. | ||
Kraemer JG, Pires J, Kueffer M, et al. Prevalence of extended-spectrum β-lactamase-producing Enterobacteriaceae and methicillin-resistant Staphylococcus aureus in pig farms in Switzerland. Sci Total Environ. 2017;603-604:401–405. | ||
Zehra A, Singh R, Kaur S, Gill JPS. Molecular characterization of antibiotic-resistant Staphylococcus aureus from livestock (bovine and swine). Vet World. 2017;10(6):598–604. | ||
Burns A, Shore AC, Brennan GI, et al. A longitudinal study of Staphylococcus aureus colonization in pigs in Ireland. Vet Microbiol. 2014;174(3-4):504–513. | ||
Tenhagen BA, Fetsch A, Stührenberg B, et al. Prevalence of MRSA types in slaughter pigs in different German abattoirs. Vet Rec. 2009;165(20):589–593. | ||
Reynaga E, Navarro M, Vilamala A, et al. Prevalence of colonization by methicillin-resistant Staphylococcus aureus ST398 in pigs and pig farm workers in an area of Catalonia, Spain. BMC Infect Dis. 2016; 16(1):716. | ||
Li J, Jiang N, Ke Y, et al. Characterization of pig-associated methicillin-resistant Staphylococcus aureus. Vet Microbiol. 2017;201:183–187. | ||
Gómez-Sanz E, Torres C, Lozano C, et al. Detection, molecular characterization, and clonal diversity of methicillin-resistant Staphylococcus aureus CC398 and CC97 in Spanish slaughter pigs of different age groups. Foodborne Pathog Dis. 2010;7(10):1269–1277. | ||
Bangerter PD, Sidler X, Perreten V, Overesch G. Longitudinal study on the colonisation and transmission of methicillin-resistant Staphylococcus aureus in pig farms. Vet Microbiol. 2016;183:125–134. | ||
Wang X, Li G, Xia X, Yang B, Xi M, Meng J. Antimicrobial susceptibility and molecular typing of methicillin-resistant Staphylococcus aureus in retail foods in Shaanxi, China. Foodborne Pathog Dis. 2014;11(4):281–286. | ||
Conceição T, de Lencastre H, Aires-de-Sousa M. Frequent isolation of methicillin resistant Staphylococcus aureus (MRSA) ST398 among healthy pigs in Portugal. PLoS One. 2017;12(4):e0175340. | ||
Sato T, Usui M, Motoya T, Sugiyama T, Tamura Y. Characterisation of meticillin-resistant Staphylococcus aureus ST97 and ST5 isolated from pigs in Japan. J Glob Antimicrob Resist. 2015;3(4):283–285. | ||
Ho PL, Chow KH, Lai EL, et al. Clonality and antimicrobial susceptibility of Staphylococcus aureus and methicillin-resistant S. aureus isolates from food animals and other animals. J Clin Microbiol. 2012;50(11):3735–3737. | ||
Cuny C, Arnold P, Hermes J, et al. Occurrence of cfr-mediated multiresistance in staphylococci from veal calves and pigs, from humans at the corresponding farms, and from veterinarians and their family members. Vet Microbiol. 2017;200:88–94. | ||
Wang Y, He T, Schwarz S, et al. Multidrug resistance gene cfr in methicillin-resistant coagulase-negative staphylococci from chickens, ducks, and pigs in China. Int J Med Microbiol. 2013;303(2):84–87. | ||
Fan R, Li D, Wang Y, et al. Presence of the optrA gene in methicillin-resistant Staphylococcus sciuri of porcine origin. Antimicrob Agents Chemother. 2016;60(12):7200–7205. | ||
Battisti A, Franco A, Merialdi G, et al. Heterogeneity among methicillin-resistant Staphylococcus aureus from Italian pig finishing holdings. Vet Microbiol. 2010;142(3–4):361–366. | ||
Cui S, Li J, Hu C, et al. Isolation and characterization of methicillin-resistant Staphylococcus aureus from swine and workers in China. J Antimicrob Chemother. 2009;64(4):680–683. | ||
Larsen J, Imanishi M, Hinjoy S, et al. Methicillin-resistant Staphylococcus aureus ST9 in pigs in Thailand. PLoS One. 2012;7(2):e31245. | ||
Hanson BM, Dressler AE, Harper AL, et al. Prevalence of Staphylococcus aureus and methicillin-resistant Staphylococcus aureus (MRSA) on retail meat in Iowa. J Infect Public Health. 2011;4(4):169–174. | ||
Dhup V, Kearns AM, Pichon B, Foster HA. First report of identification of livestock-associated MRSA ST9 in retail meat in England. Epidemiol Infect. 2015;143(14):2989–2992. | ||
Yu F, Lu C, Liu Y, et al. Emergence of quinupristin/dalfopristin resistance among livestock-associated Staphylococcus aureus ST9 clinical isolates. Int J Antimicrob Agents. 2014;44(5):416–419. | ||
Goerge T, Lorenz MB, van Alen S, Hübner NO, Becker K, Köck R. MRSA colonization and infection among persons with occupational livestock exposure in Europe: prevalence, preventive options and evidence. Vet Microbiol. 2017;200:6–12. | ||
Schaumburg F, Pauly M, Anoh E, et al. Staphylococcus aureus complex from animals and humans in three remote African regions. Clin Microbiol Infect. 2015;21(4):345.e1–8. | ||
Sung JM, Lloyd DH, Lindsay JA. Staphylococcus aureus host specificity: comparative genomics of human versus animal isolates by multi-strain microarray. Microbiology. 2008;154(Pt 7):1949–1959. | ||
Verkaik NJ, Benard M, Boelens HA, et al. Immune evasion cluster-positive bacteriophages are highly prevalent among human Staphylococcus aureus strains, but they are not essential in the first stages of nasal colonization. Clin Microbiol Infect. 2011;17(3):343–348. | ||
He W, Liu Y, Qi J, et al. Food-animal related Staphylococcus aureus multidrug-resistant ST9 strains with toxin genes. Foodborne Pathog Dis. 2013;10(9):782–788. | ||
Feltrin F, Alba P, Kraushaar B, et al. A livestock-associated, multidrug-resistant, methicillin-resistant Staphylococcus aureus clonal complex 97 lineage spreading in dairy cattle and pigs in Italy. Appl Environ Microbiol. 2016;82(3):816–821. | ||
Rinsky JL, Nadimpalli M, Wing S, et al. Livestock-associated methicillin and multidrug resistant Staphylococcus aureus is present among industrial, not antibiotic-free livestock operation workers in North Carolina. PLoS One. 2013;8(7):e67641. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.