Back to Journals » Biologics: Targets and Therapy » Volume 10
Pharmacoutilization of epoetins in naïve patients with hematological malignancies in an unselected Italian population under clinical practice setting: a comparative analysis between originator and biosimiliars
Authors Perrone V, Saragoni S, Buda S , Broccoli A, Degli Esposti L
Received 8 June 2016
Accepted for publication 19 September 2016
Published 1 December 2016 Volume 2016:10 Pages 157—165
DOI https://doi.org/10.2147/BTT.S114625
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Doris Benbrook
Valentina Perrone,1 Stefania Saragoni,1 Stefano Buda,1 Alessandro Broccoli,2 Luca Degli Esposti1
1CliCon S.r.l., Health, Economics & Outcomes Research, Ravenna, 2Institute of Hematology “L. e A. Seràgnoli”, University of Bologna, Bologna, Italy
Aim: The purpose of this study was to assess the prescription of epoetins and consumption of health care resources (in terms of drug treatments) in naïve patients with hematological malignancies in a real-world setting; in particular, we compared the results between reference product and biosimilar products.
Methods: An observational retrospective study based on administrative and laboratory databases of three local health units was conducted. All adults diagnosed with hematological malignancies and who had received at least one epoetin (either reference product or biosimilars) prescription for the first time between 1 January 2010 and 30 April 2012 (enrollment period) were included. The date of the first prescription of epoetin within the enrollment period was defined as index date (ID). Patients were followed up for 4 weeks after ID (follow-up period) and were investigated for the 1-year period before the ID. The difference between the last hemoglobin (Hb) measurement after ID and the one prior to ID (ΔHb) was evaluated. The drug cost analysis was conducted from the perspective of the Italian National Health System.
Results: Overall, 69 patients were included in the study; 48 of them received reference epoetin product and 21 received biosimilars as first prescription. Among reference product users, the mean ± standard deviation (SD) age was 62.5±14.7 years; this cohort of patients was slightly significantly younger than the biosimilar users (71.8±11.8 years). The mean ± SD overall Hb level prior to treatment was lower among patients who started with biosimilar products (9.6±1.1 g/dL) compared to those who started with a reference product (10.1±2.1 g/dL). No significant differences in ΔHb were observed between biosimilar and originator groups during the follow-up period. The mean ± SD cost per patient was €667.98±573.93 and €340.85±235.73 for the reference product and biosimilar users, respectively (p=0.065).
Conclusion: Our study showed that the use of biosimilar products might contribute to controlling health care costs (in terms of drug treatments) for patients with hematological malignancies being maintained by high-quality anemia therapy. Our findings also showed some discordances regarding the most appropriate therapeutic approach in daily clinical practice.
Keywords: erythropoiesis-stimulating agents, chemotherapy-induced anemia, biosimilar, real-world setting
Introduction
Anemia is a condition in which the red blood cell mass is insufficient to adequately deliver oxygen to peripheral tissues. Patients with anemia related to cancer, chronic inflammation, or chronic kidney disease (CKD) generally display a reduced response of endogenous erythropoietin (EPO) to trigger levels of hemoglobin (Hb); this aspect is worsened in those who are concomitantly receiving myelotoxic chemotherapy.1,2 Anemia may significantly impair quality of life, increase cardiovascular risk, and reduce long-term survival, when left untreated.3
Earlier, treatment options were essentially limited to blood transfusions. The introduction of recombinant human EPO and an erythropoietic stimulating agent (ESA) in 1989 resulted in a major progress in the treatment of anemia and provided a key tool for managing this condition in patients with CKD or cancer.4–7
In December 2004, the patent of epoetin-alpha (i.e. Eprex®, reference product or originator [Janssen-Cilag, Neuss, Germany]) expired and this opened the way to biosimilars. Currently, in Europe three biosimilar products have received marketing authorization from the European Medicines Agency (EMA): Binocrit® [Sandoz GmbH, Holzkirchen, Germany] (epoetin-alpha; also known as Abseamed® [Medice Arzneimittel Putter GmbH & Co., Iserlohn, Germany] and Epoetin Alfa Hexal® [Hexal Biotech Forschungs GmbH, Holzkirchen, Germany]), Retacrit® [Hospira, Maidenhead, United Kingdom] (epoetin-zeta; also called Silapo® [Stada R&D AG, Bad Vilbel, Germany]), and Eporatio® [Ratiopharm GmbH, Ulm, Germany] (epoetin- theta; also known as Biopoin® [Teva GmbH, Ulm, Germany]).8 Since 2007, biosimilars of ESAs are available on the Italian market.9,10 According to the EMA guidelines, a biosimilar is defined as a biological medicinal product that is developed to be similar to an existing biological (the “reference medicine”). A biosimilar demonstrates similarity to the reference medicinal product in terms of quality characteristics, biological activity, safety and efficacy based on a comprehensive comparability exercise.11
Although a biosimilar is approved based on its therapeutic equivalence, the interchangeability is still an open question mark;12 nevertheless, in accordance with the recent European directives and the last position paper from the Italian Medicines Agency (AIFA—Agenzia Italiana del Farmaco), biosimilar epoetins may be prescribed to naïve patients;13,14 however, the choice to treat a patient with a biological reference product or biosimilars is a clinical decision entrusted to the physician.13
Few real-life data and comparative analysis are available concerning the use of originator epoetins and biosimilar products in the Italian setting.15–19 The objective of the present study was to assess the prescription of epoetin and consumption of health care resources (in terms of drug treatments) among naïve patients with hematological malignancies in an unselected Italian population under clinical practice setting; in particular, we compared the results between reference product and biosimilar products.
Methods
Data sources
The study was conducted using administrative databases of three Italian local health units (LHUs), geographically distributed throughout the national territory, representing ~550,000 health-assisted individuals.
In particular, the following databases were used to retrieve the information: Beneficiaries, Hospital Direct Drugs Distribution Registry, Territorial Pharmacy, Hospital Discharges, Ambulatory Care Specialist, Laboratory Analysis (which records the date and result of the Hb measurements), and the Mortalities, where only death dates are reported.
The diagnosis and procedures were retrieved using codes classified according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). The information on drug prescriptions was identified through the International Anatomical Therapeutic Chemical classification system (ATC code).
To guarantee patient privacy, each subject was assigned an anonymous univocal numeric code. No identifiers related to patients were provided to the researchers. The patient code in each database permitted electronic linkage between all databases. Informed consent is not required by the LHU ethics committees for using encrypted retrospective information. In compliance with the AIFA Determination20 Guidelines for classification and conduction of observational studies on drugs and AIFA Circular Procedures for launch of observational studies on drugs, this study was notified to the local ethics committee in each participating LHU according to the Italian law regarding the conduct of observational analysis and the LHU ethics committees approved the study.
Cohort definition
This study was an observational retrospective cohort analysis. All naïve patients aged ≥18 years who received at least one dispensing of epoetin (biosimilars [epoetin-alpha biosimilars: Binocrit and Abseamed; epoetin-zeta biosimilar: Retacrit] or their corresponding reference medical product: Eprex [ATC code: B03XA01]) during the enrollment period (from 1 January 2010 to 30 April 2012) were considered eligible candidates for analysis and were enrolled. All these ESAs have been approved for the treatment of anemia induced by anticancer chemotherapy. The enrollment date was the first date on which a patient filled a prescription for one of these drugs during the enrollment period and was defined as the index date (ID); patients were followed up for 4 weeks since then (follow-up period) and were investigated for the 1-year period before the ID (characterization period). Patients without epoetin prescriptions during the 6-month period preceding the ID were defined naïve.
Only those patients diagnosed with hematological malignancies and who had at least one epoetin (either reference product or biosimilars) prescription for the first time during the enrollment period were included in the analysis.
All patients were stratified by the type of dispensed epoetin (either biosimilars or originator) at ID. Patients who were moved to other LHUs during the follow-up period were excluded from the analysis.
All enrolled patients were classified as follows: cancer patients based on the presence of at least one prescription of antineoplastic drugs (ATC code: L01) or endocrine therapy (ATC code: L02) or immunostimulant agents (ATC code: L03) or immunosuppressant agents (ATC code: L04); and/or at least one previous hospitalization with a primary or secondary diagnosis of neoplasms (ICD-9-CM codes: 140–239); and/or at least one hospitalization with a primary or secondary diagnosis of radiotherapy encounter (ICD-9-CM code: V580) or encounter for antineoplastic chemotherapy (ICD-9-CM code: V581).
Cancer patients were classified as with or without hematological malignancies based on previous hospitalization with primary or secondary diagnosis of malignant neoplasms of lymphatic and hematopoietic tissue (ICD-9-CM codes: 200–208). Only patients with hematological malignancies were recruited in the analysis.
Data on baseline characteristics, including demographics, hospital admissions, prescribed drugs, and comorbidity, were collected. Specifically, the treatments of interest were antihypertensive drugs (ATC codes: C02, C03, C07, C08, C09), oral hypoglycemic drugs and/or insulins (ATC code: A10), phosphate chelating agents (ATC code: V03AE02), lanthanum carbonate (ATC code: V03AE03), mineral supplements (calcium, ATC code: A12AA), other mineral supplements (magnesium, ATC code: A12CC), iron preparations (ATC code: B03A), sodium carbonate (ATC code: B05CB04), cardiac therapy (ATC code: C01), and vitamin D and analogs (ATC code: A11CC). Hospitalization related to diabetes was identified by ICD-9-CM code 250 (primary or accessory discharge reasons). Previous cardiovascular hospitalizations were identified by ICD-9-CM codes (primary or accessory discharge reasons); in particular, we identified myocardial infarction (ICD-9-CM codes: 410, 412); other forms of chronic ischemic heart disease (ICD-9-CM codes: 411, 413, 414), other cerebrovascular injuries (ICD-9-CM codes: 430–438), heart failure (ICD-9-CM code: 428); atherosclerosis, aneurysm, and dissection (ICD-9-CM codes: 440–442); other peripheral vascular diseases (ICD-9-CM codes: 440–443); and hypertensive diseases (ICD-9-CM codes: 401–405).
Blood transfusion requirements (defined as a transfusion occurring during the follow-up period and for 2 months after the end of therapy) among patients initiated with originator or biosimilar epoetins were evaluated. In order to assess adequate control with therapeutic target, the Hb values (levels measured) were evaluated both in the last measurement before the ID (from 2 months before the ID, value at baseline) and in the last available measurement around the end of the follow-up (up to 2 months after ID, value at follow-up). The difference between the last Hb measurement after ID (value at follow-up) and the one prior to ID (value at baseline), defined as ΔHb, was also evaluated. Likewise, the mean dose of epoetin (once weekly) according to the Hb value at baseline was also evaluated. Comorbidities were measured using the Charlson Comorbidity Index (CCI),21 and the sum of weights related to each condition (i.e. myocardial infarction, cancers, diabetes, ulcer) was identified through treatments and hospitalizations. All comorbidities during the characterization period were evaluated; the CCI score reflects a patient’s overall health status.
During the follow-up period, all epoetin prescriptions, in order to calculate the exposure to treatment, and all Hb measurements, in order to evaluate the achievement of the therapeutic targets, were evaluated.
Costs analysis
The cost of therapy was evaluated during the follow-up period. Costs are reported in euros (€). Drug costs were evaluated using the Italian National Health Service (NHS) purchase price. The cost analysis was conducted from the perspective of the NHS.
Statistical analysis
Continuous variables are presented as mean ± standard deviation (mean ± SD); categorical variables are shown as percentages and absolute numbers. Comparisons among groups were performed using analysis of variance and Pearson’s chi-square test for continuous and categorical data, respectively. Additionally, the Kruskal–Wallis test was performed for asymmetric continuous variables (skewed). The post hoc Bonferroni correction was applied to account for multiple testing. The p-values ≤0.05 were considered to be statistically significant, and all statistical analyses were conducted using STATA software, version 12.1 (StataCorp LP, College Station, TX, USA).
Results
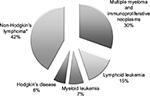
A total of 1,143 patients were identified in the database as newly prescribed for ESA therapy from 1 January 2010 to 30 April 2012. Overall, 37% of epoetin users were treated for anemia induced by anticancer chemotherapy and were eligible for analysis. Overall, 69 (16.5% of all cancer patients) patients with hematological malignancies were included in the study. Of these, 48 and 21 patients (70% and 30% of all patients with hematological malignancies) received a prescription for reference epoetin product and for biosimilars, respectively. Figure 1 shows details of the study’s inclusion and exclusion criteria. Figure 2 shows the percentage of patients stratified by hematologic cancer type at baseline. Patients’ diagnoses have been codified as follows: lymphoid leukemia (ICD-9-CM code: 204), 15%; myeloid leukemia (ICD-9-CM code: 205), 7%; Hodgkin’s disease (ICD-9-CM code: 201), 6%; multiple myeloma and immunoproliferative neoplasms (ICD-9-CM code: 203), 30%; non-Hodgkin’s lymphoma (grouped together under the diagnosis of other malignant neoplasms of lymphoid and histiocytic tissue and lymphosarcoma and reticulosarcoma and other specified malignant tumors of lymphatic tissue, ICD-9-CM codes: 200 and 202), 42%. Of all patients enrolled, ~10% were treated for off-label indications (acute lymphoblastic leukemia [ICD-9-CM code: 204.0], acute myeloid leukemia [ICD-9-CM code: 205.0], chronic myeloid leukemia [ICD-9-CM code: 205.1]).
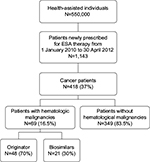  | Figure 1 Flowchart of cohort definition. Abbreviation: ESA, erythropoietic stimulating agent. |
Demographic and baseline clinical characteristics of the study population are shown in Table 1. Gender was almost equally distributed among users of original reference and biosimilar products (on average, males: 50 and 52.4%, respectively). Among patients treated with reference product, the mean age (±SD) was found to be 62.5±14.7 years. Patients in this cohort were significantly younger than the biosimilar users (71.8±11.8 years), and the difference was statistically significant. The mean doses of epoetin were 32,344 IU/week (±28,756) and 30,976 IU/week (±20,362) for the reference product and biosimilar product groups, respectively (Table 1).
Figure 3 shows the mean dose (±SD) of epoetin (once weekly) according to Hb levels at baseline. Table 2 presents the Hb values among the patients using either biosimilars or their reference product. Forty-four patients (about 64% of all enrolled patients) had data available regarding both the Hb values (at baseline and at follow-up). The mean Hb level before treatment was lower among patients who started with biosimilar products (9.5±1.1 g/dL) than those who started with a reference product of epoetin (10.0±2.2 g/dL). During the follow-up period, the mean Hb level was 10.8±1.8 g/dL in the biosimilar epoetin group and 11.3±1.9 g/dL in the reference product group. There were no significant differences between the two groups in ΔHb level. Up to 2 months after the end of therapy, blood transfusion was required by 9.5% of patients who received biosimilar epoetins and 10.4% of patients who received reference product, and the difference was not statistically significant (p=0.910; Figure 4).
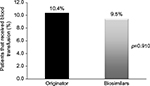  | Figure 4 Blood transfusion requirements among patients according to the different epoetin treatments (originator or biosimilar products) up to 2 months after the end of therapy. |
The distribution of cost, according to the ongoing therapeutic strategy, is reported in Figure 5. The mean cost per patient, attributable to the consumption of epoetins used in the study period, was €667.98±573.93 and €340.85±235.73 for the originator and biosimilar users, respectively. However, the difference was not statistically significant (p=0.065).
  | Figure 5 Mean (± standard deviation) cumulative cost of erythropoietic stimulating agents among patients initiated with originator and biosimilar products. Note: p=0.065 (Kruskal–Wallis test). |
Discussion
In the present observational retrospective study, we evaluated the epoetin utilization profiles and the consumption of health care resources (in terms of drug treatments) in naïve patients with hematological malignancies, in an unselected Italian population under clinical practice setting. In addition, we compared the results between reference product and biosimilar products.
During the past decade, epoetins have demonstrated a significant therapeutic role for the management of cancer-related anemia in patients undergoing chemotherapy with an increase of Hb levels and improved quality of life.5,7,22 An important limitation of ESAs and biological medicines remains the high cost, which may limit access in some countries.23,24
Since the expiration of patent protection, a number of novel biosimilar epoetins have been approved on the world market.23,25 Biosimilar medicines have significant potential to offer cost savings to health care providers, but the global value of a biosimilar is not determined entirely by its pricing.26 During the last years, the health authorities have published specific guidelines establishing regulatory requirements for the approval and use of biosimilars, which are based on efficacy and safety comparability between biosimilar and reference product.11,14,23 Indeed, in order to be commercialized, a biosimilar must be proven equivalent to the reference product in terms of quality, safety, and effectiveness.23 This comparability exercise, which is the basis of the marketing authorization, should be considered sufficiently reassuring. Regarding the use of biosimilar epoetins in Italy, the national authority responsible for drug regulation in Italy recommends prescribing biosimilars to treat naïve patients (e.g. patients never previously treated with epoetins or with previous exposure in time periods that are sufficiently distant).13
In this real-world assessment, almost 30% of all naïve patients with hematological malignancies being treated with epoetins received a prescription of biosimilar products. The national report on medicine use in Italy in 2014 showed that 55.9% of patients newly treated with epoetin-alpha (i.e. epoetin-alpha users without any prescription within the previous 6 months) were treated with biosimilars of epoetin-alpha, with an increasing trend as compared with the previous years (+54.6%).27 These data were retrieved by the Nationwide OsMed Health-DB Database and have been validated by AIFA to describe drug consumption nationwide.28
International evidence-based guidelines for the use of ESAs in cancer patients are currently being updated.5,29,30 As in other surveys,31,32 ~10% of all enrolled patients received an ESA for off-label indications. Available evidence does not identify Hb levels greater than or equal to 10 g/dL either as thresholds for starting ESA treatment or as targets for ESA therapy.30 These guidelines recommend that the clinicians should consider using ESAs for patients undergoing myelotoxic chemotherapy who have Hb threshold ≤10 g/dL to avoid the need for transfusions. Despite these recommendations, our findings showed that the mean Hb concentration was ≥10 g/dL among originator users; likewise, patients who were initiated with reference product reported a higher Hb levels than those initiated with biosimilar products. It is interesting to note that there were no significant differences between the originator and biosimilar groups with regard to clinical characteristics at baseline, except that the patients undergoing therapy with biosimilar products were older than those who started with reference product. Moreover, as observed in other studies,25,33,34 this study showed no significant differences between the two groups in ΔHb levels 2 months after the initiation of treatment.
Few studies have compared the impact of different ESA dosings or Hb targets on clinical and nonclinical outcomes;35,36 the new recommendations5,30 suggested using the lowest possible ESA dose required to reduce the need for transfusions as well as reducing the ESA dose when Hb level exceeds 100 and 110 g/dL. Our data showed that in the cohort of patients we studied, the mean administered ESA dose was higher among the originator users than among the biosimilar users.
Transfusion reduction is the primary goal of epoetin therapy in treating cancer patients with chemotherapy-induced anemia.37 In this real-world study, the number of patients who required a transfusion was low (about 10% of all included patients) and generally similar across the different epoetin treatments. These data are consistent with the results of previous observational studies.34,38 The resource use and costs associated with ESA treatments have been reported previously.19,22,34,39,40 It is well known that one large potential advantage of biosimilars over existing reference biological products is cost savings, which could improve the access for some patients to medication. Although different methodological approaches have been used to evaluate the cost of care, this study of real-world treatment patterns is in line with prior retrospective analyses.15,38 Our cost analysis suggested a lower mean drug cost in the group that received biosimilar epoetins compared with the group that received reference product, but there was no statistically significant difference between the groups; these findings require confirmation using more patients and more robust measures of cost-effectiveness.
Considering that biological medicines are among the most expensive pharmaceuticals available, the advent to market penetration of the currently available biosimilars could be an opportunity to relieve some of the financial pressure and a real strategy to improve the sustainability of NHS, especially in therapeutic areas where the demand and cost of new therapies are high.41 The authors acknowledge some limitations of the study. In general, administrative database analyses limit the interpretation of results depending on the information available. The major limitations are: small sample size, observational nature of the study design, and lack of clinical information from an administrative database. Therefore, no conclusions can be drawn concerning the possible underlying confounders such as population bias, disease severity, or other individual circumstances. The limitation relates to the use of administrative data to select and describe our patient cohort, which did not permit to explore the diagnosis of each hematological malignancy more precisely. For example, reliance on ICD-9-CM diagnostic codes to identify our cohort may have resulted in beneficiaries being included or excluded incorrectly. The limitation concerning limited data availability also did not permit to explore each reason for anemia more precisely.
Conclusion
Our data are in agreement with the relevant scientific literature and highlight how public and private payers, policy makers, and clinicians should be aware of the clinical equivalence of biosimilars, in order to improve their appropriate prescription. Besides, biosimilar products may contribute to controlling health care costs for patients with hematological malignancies and being maintained by high-quality anemia therapy. At the same time, our findings showed that in the group of patients receiving a prescription of reference product, the Hb concentration before treatment was ≥10 g/dL; evidence so far does not identify Hb levels ≥10 g/dL either as thresholds for initiating treatment or as targets for ESA therapy. As a consequence, it can be reasonably assumed that educational interventions as well as treatment strategies should be developed to improve the clinical management of these patients. Given the nature of the study (observational and based on administrative databases), further analyses on a larger sample will contribute to refine and give more context to these results.
Disclosure
The authors report no conflicts of interest in this work.
References
Locatelli F, Becker H. Update on anemia management in nephrology, including current guidelines on the use of erythropoiesis-stimulating agents and implications of the introduction of “biosimilars”. Oncologist. 2009;14(Suppl 1):16–21. | ||
Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst. 1999;91(19):1616–1634. | ||
Locatelli F, Del Vecchio L. Optimizing the management of renal anemia: challenges and new opportunities. Kidney Int Suppl. 2008;74(111):S33–S37. | ||
Locatelli F, Del Vecchio L. An expert opinion on the current treatment of anemia in patients with kidney disease. Expert Opin Pharmacother. 2012;13(4):495–503. | ||
Luo W, Nordstrom BL, Fraeman K, et al. Adherence to guidelines for use of erythropoiesis-stimulating agents in patients with chemotherapy-induced anemia: results of a retrospective study of an electronic medical-records database in the United States, 2002–2006. Clin Ther. 2008;30(12):2423–2435. | ||
Del Vecchio L, Cavalli A, Tucci B, Locatelli F. Chronic kidney disease-associated anemia: new remedies. Curr Opin Investig Drugs. 2010;11(9):1030–1038. | ||
Schrijvers D, De Samblanx H, Roila F; ESMO Guidelines Working Group. Erythropoiesis-stimulating agents in the treatment of anaemia in cancer patients: ESMO clinical practice guidelines for use. Ann Oncol. 2010;21(Suppl 5):v244–v247. | ||
Abraham I, MacDonald K. Clinical safety of biosimilar recombinant human erythropoietins. Expert Opin Drug Saf. 2012;11(5):819–840. | ||
Genazzani AA, Biggio G, Caputi AP, et al. Biosimilar drugs : concerns and opportunities. BioDrugs. 2007;21(6):351–356. | ||
Società Italiana di Farmacologia (SIF). Revisione della posizione sui farmaci biosimilari da parte della Società Italiana di Farmacologia: working paper. 2014. Available from: http://www.sifweb.org/docs/sif_position_paper_revisione_biosimilari_lug14.pdf. Accessed November 18, 2015. | ||
European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP): guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/01/WC500180219.pdf. Accessed November 18, 2015. | ||
Minghetti P, Rocco P, Schellekens H. The constrained prescription, interchangeability and substitution of biosimilars. Nat Biotechnol. 2015;33(7):688–689. | ||
Agenzia Italiana del Farmaco (AIFA). Position paper sui farmaci biosimilari (28/05/2013). Available from: http://www. agenziafarmaco.gov.it/sites/default/files/AIFA_POSITION_PAPER_ FARMACI_BIOSIMILARI.pdf. Accessed November 18, 2015. | ||
EMA. Guideline on “Similar biological medicinal products containing recombinant erythropoietins” (EMEA/CHMP/BMWP/301636/08). Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000408.jsp. Accessed November 18, 2015. | ||
Ingrasciotta Y, Giorgianni F, Bolcato J, et al. How much are biosimilars used in clinical practice? A retrospective Italian population-based study of erythropoiesis-stimulating agents in the years 2009–2013. BioDrugs. 2015;29(4):275–284. | ||
Kerkhofs L, Boschetti G, Lugini A, Stanculeanu DL, Palomo AG. Use of biosimilar epoetin to increase hemoglobin levels in patients with chemotherapy-induced anemia: real-life clinical experience. Future Oncol. 2012;8(6):751–756. | ||
D’Amore C, Da Cas R, Rossi M, Traversa G. Switching between epoetins: a practice in support of biosimilar use. BioDrugs. 2016;30(1):27–32. | ||
Giordano G, Mondello P, Tambaro R, et al. Biosimilar epoetin α is as effective as originator epoetin-α plus liposomal iron (Sideral®), vitamin B12 and folates in patients with refractory anemia: a retrospective real-life approach. Mol Clin Oncol. 2015;3(4):781–784. | ||
Loiacono C, Sgroi C, Coppolino S, et al. How much are biosimilars used in southern Italy? A retrospective analysis of epoetin utilization in the local health unit of Messina in the years 2010–2011. BioDrugs. 2012;26(2):113–120. | ||
AIFA. Guideline for the classification and conduction of the observational studies on medicines. Available from: https://www.agenziafarmaco.gov.it/ricclin/sites/default/files/files_wysiwyg/files/CIRCULARS/Circular%2031st%20May%202010.pdf. Accessed November 18, 2015. | ||
Gonnella JS, Louis DZ, Gozum MV, Callahan CA, Barnes CA. Disease Staging Clinical and Coded Criteria. Version 5.26. Ann Arbor, MI: Thomson Medstat; 2010. | ||
Shehata N, Walker I, Meyer R, Haynes AE, Imrie K; Cancer Care Ontario Hematology Disease Site Group. The use of erythropoiesis-stimulating agents in patients with non-myeloid hematological malignancies: a systematic review. Ann Hematol. 2008;87(12):961–973. | ||
Minghetti P, Rocco P, Del Vecchio L, Locatelli F. Biosimilars and regulatory authorities. Nephron Clin Pract. 2011;117(1):c1–c7. | ||
Aapro M, Cornes P, Sun D, Abraham I. Comparative cost efficiency across the European G5 countries of originators and a biosimilar erythropoiesis-stimulating agent to manage chemotherapy-induced anemia in patients with cancer. Ther Adv Med Oncol. 2012;4(3):95–105. | ||
Mikhail A, Farouk M. Epoetin biosimilars in Europe: five years on. Adv Ther. 2013;30(1):28–40. | ||
Hörbrand F, Bramlage P, Fischaleck J, Hasford J, Brunkhorst R. A population-based study comparing biosimilar versus originator erythropoiesis-stimulating agent consumption in 6,117 patients with renal anaemia. Eur J Clin Pharmacol. 2013;69(4):929–936. | ||
Agenzia Italiana del Farmaco (AIFA). L’uso dei farmaci in Italia—rapporto OsMed 2014. Available from: http://www.agenziafarmaco.gov.it/sites/default/files/Rapporto_OsMed_2014_0.pdf. Accessed November 18, 2015. | ||
Onder G, Marengoni A, Russo P, et al; Geriatrics Working Group of the Italian Medicines Agency (Agenzia Italiana del Farmaco, AIFA); Medicines Utilization Monitoring Center Health Database Network. Advanced age and medication prescription: more years, less medications? A nationwide report from the Italian Medicines Agency. J Am Med Dir Assoc. 2016;17(2):168–172. | ||
Aapro MS, Link H. September 2007 update on EORTC guidelines and anemia management with erythropoiesis-stimulating agents. Oncologist. 2008;13(Suppl 3):33–36. | ||
Rizzo JD, Brouwers M, Hurley P, et al. American Society of Clinical Oncology/American Society of Hematology Clinical Practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. J Clin Oncol. 2010;28(33):4996–5010. | ||
Papachristos A, Kani C, Litsa P, et al. Drug utilization patterns and costs of erythropoiesis-stimulating agents in an outpatient setting in Greece. Consult Pharm. 2016;31(5):271–281. | ||
Hendrick F, Davidoff AJ, Zeidan AM, Gore SD, Baer MR. Effect of erythropoiesis-stimulating agent policy decisions on off-label use in myelodysplastic syndromes. Medicare Medicaid Res Rev. 2014;4(4). | ||
Weigang-Köhler K, Vetter A, Thyroff-Friesinger U. HX575, recombinant human epoetin alfa, for the treatment of chemotherapy-associated symptomatic anaemia in patients with solid tumours. Onkologie. 2009;32(4):168–174. | ||
Michallet M, Luporsi E, Soubeyran P, et al; ORHEO study group. BiOsimilaRs in the management of anaemia secondary to chemotherapy in HaEmatology and Oncology: results of the ORHEO observational study. BMC Cancer. 2014;14(1):503. | ||
Saglimbene V, D’Alonzo D, Ruospo M, et al. [Effects of dose of erythropoiesis stimulating agents on cardiovascular outcomes, quality of life and costs of haemodialysis. the clinical evaluation of the DOSe of erythropoietins (C.E. DOSE) Trial]. G Ital Nefrol. 2013;30(2). Italian. | ||
Manns BJ, Tonelli M. The new FDA labeling for ESA – implications for patients and providers. Clin J Am Soc Nephrol. 2012;7(2):348–353. | ||
Pashos CL, Larholt K, Fraser KA, McKenzie RS, Senbetta M, Piech CT. Outcomes of erythropoiesis-stimulating agents in cancer patients with chemotherapy-induced anemia. Support Care Cancer. 2012;20(1):159–165. | ||
Rodriguez Garzotto A, Cortijo Casacajares S, Pernaut C, et al. Erythropoiesis-stimulating agents for the treatment of chemotherapy-induced anemia: comparisons from real-world clinical experience. J Blood Med. 2014;5:43–58. | ||
Vekeman F, McKenzie RS, Bookhart BK, et al. Drug utilisation and cost considerations of erythropoiesis stimulating agents in oncology patients receiving chemotherapy: observations from a large managed-care database. J Med Econ. 2009;12(1):1–8. | ||
Nikolaidi E, Hatzikou M, Geitona M. Budget impact analysis on erythropoiesis-stimulating agents use for the management of chemotherapy-induced anaemia in Greece. Cost Eff Resour Alloc. 2013;11(1):16. | ||
Farfan-Portet M-I, Gerkens S, Lepage-Nefkens I, Vinck I, Hulstaert F. Are biosimilars the next tool to guarantee cost-containment for pharmaceutical expenditures? Eur J Health Econ. 2014;15(3):223–228. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.