Back to Journals » Journal of Pain Research » Volume 12
Pharmacotherapeutic outcomes in atypical odontalgia: determinants of pain relief
Authors Tu TTH , Miura A , Shinohara Y , Mikuzuki L , Kawasaki K , Sugawara S , Suga T , Watanabe T , Aota Y, Umezaki Y, Takenoshita M, Toyofuku A
Received 21 September 2018
Accepted for publication 4 January 2019
Published 27 February 2019 Volume 2019:12 Pages 831—839
DOI https://doi.org/10.2147/JPR.S188362
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Katherine Hanlon
Trang TH Tu,1 Anna Miura,1 Yukiko Shinohara,1 Lou Mikuzuki,1 Kaoru Kawasaki,1 Shiori Sugawara,1 Takayuki Suga,1 Takeshi Watanabe,1 Yuma Aota,1 Yojiro Umezaki,2 Miho Takenoshita,1 Akira Toyofuku1
1Department of Psychosomatic Dentistry, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University, Tokyo, Japan; 2Department of Geriatric Dentistry, Fukuoka Dental College, Fukuoka, Japan
Objectives: There has been considerable research which has focused on clarifying the origin of pain in patients with atypical odontalgia (AO), also known as “idiopathic toothache”, and on identifying effective treatment, but there has been limited success so far. In this study, we assessed the outcomes of treatment and attempted to identify factors that could account for pain remission in patients with AO.
Patients and methods: Data for 165 patients diagnosed with AO from June 2015 to August 2017 were retrospectively reviewed. The patients’ sex, age, duration of pain, and psychiatric history were collected, along with information on pain intensity, depressive status, and catastrophizing scores. Responses at 4 and 16 weeks from the start of treatment were observed. The associations between potentially associated factors and outcome were investigated using Bayesian model averaging.
Results: A 30% reduction in pain was reported by 38 patients (46.3%) at 4 weeks and by 54 patients (65.9%) at 16 weeks. The pain intensity decreased as the depression and catastrophizing score improved; all of the changes were statistically significant (P<0.001). Four elements, that is, patient sex, depression score at baseline, pain score at 4 weeks, and change in the catastrophizing score, explained 52.5% of the variation in final outcome between individual patients.
Conclusion: Our findings confirm the efficacy of tricyclic antidepressants (TCAs) as a treatment for AO and indicate that other medications, especially aripiprazole used in combination with a TCA, may be useful. A considerable number of patients, especially women, those with lower levels of depression at baseline, and those who responded to 4 weeks of treatment, achieved pain relief.
Keywords: atypical odontalgia, orofacial chronic pain, depression, pain catastrophizing, tricyclic antidepressant, atypical antipsychotic
Introduction
Atypical odontalgia (AO), also known as “phantom tooth pain”, “idiopathic toothache”, and more recently, “persistent dentoalveolar pain disorder”, is characterized by continuous pain in a tooth or tooth socket after extraction in the absence of any major pathology.1–3 The origin of pain in AO is unclear, but there is good evidence to suggest an interplay between a neuropathic pain condition and traumatic, psychosocial, and biological risk factors. The prevalence of AO after endodontic treatment reportedly ranges from 2.1% to 6%.4,5 However, AO could occur in association with other dental procedures, as well as spontaneously, or for no identifiable reason. In a previous study, we found that 43.3% of 383 patients developed AO that was not related to dental treatment,6 suggesting that the actual prevalence of AO might be higher than reported. Although AO is not life-threatening, it is a chronic painful condition that has a negative impact on daily life.
Much effort has been made over decades to clarify the pathology, diagnostic criteria, and origin of the pain in AO, as well as to find an effective treatment protocol, but with limited success so far.7–11 AO is a rare condition that dentists and clinicians could expect to encounter only a few times in their working lifetimes. Moreover, it is very difficult to diagnose and hard to persuade patients to accept the diagnosis.7,12 AO is often misdiagnosed, so affected patients are likely to undergo multiple unnecessary invasive procedures before being referred to an appropriate specialist. The pain tends to become worse over time, and sufferers become generally tired of seeking care and resign themselves to a hopeless situation.7,13,14
Several studies and case series have attempted to identify the pathophysiologic mechanism of AO.15–19 However, there is limited comparative research on the effectiveness of treatment. Moreover, interpretation of the significance of the findings and clinical implications of the small amount of research conducted to date has been hampered by small sample sizes and data collection difficulties.10,11,17 Most reports recommend tricyclic antidepressants (TCAs) as the first-line agents for the management of AO.1,13,20–22 However, TCAs have not been studied in either randomized controlled trials or open-label studies in patient populations with AO. The aims of this study were to 1) gain an overview of the outcomes of treatment in patients with AO, 2) identify factors that could account for remission of pain, and 3) assess whether information available at baseline and patient self-reports can predict a good pharmacotherapeutic response.
Patients and methods
Study design and treatment setting
The study had a retrospective design and analyzed routinely collected real-world data obtained from the medical records of patients with a diagnosis of AO who first visited the Psychosomatic Dentistry Clinic at Tokyo Medical and Dental University in Tokyo, Japan, between June 2015 and August 2017. All patients had been screened thoroughly by a structured step-by-step examination and diagnosed by an expert in psychosomatic dentistry (AT) to have AO if their symptoms fitted the criteria for the persistent idiopathic facial pain subclassification.3 These criteria are as follows: 1) continuous pain in one or more teeth or in a tooth socket after extraction; 2) pain lasting for >2 hours daily for at least 3 months; 3) a normal clinical neurologic examination; and 4) a dental cause is excluded by appropriate investigations. Patients with conditions that could affect the reliability of their self-reports (eg, dementia, objective cognitive impairment, or difficulty reading, understanding, and/or answering questions) were excluded, as well as those who had been referred to another specialist (eg, a psychiatrist, internal medicine physician, or general practitioner), those who declined pharmacotherapy at the first visit, those who were lost to follow-up within the first 16 weeks for any reason, and those who had not continued their most recent prescription for at least 8 weeks by week 16. After application of the eligibility criteria, data for 82 patients were available for analysis (Figure 1).
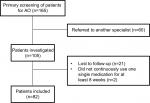  | Figure 1 Flowchart showing the selection of patients with AO for inclusion in this study. Abbreviation: AO, atypical odontalgia. |
The first-line treatment was amitriptyline (a TCA) at a dose of 5–10 mg/day. If a TCA was contraindicated or the patient was concerned about side effects, aripiprazole, a partial dopamine agonist (DPA; 0.3–0.5 mg/day) was prescribed. During the study period, drug prescriptions were changed, doses were increased or decreased, and drugs were used in combination according to the response and side effects, in an effort to find the most effective and best-tolerated medication. However, the main medication used was selected for recording purposes, and was defined as the agent that had been prescribed and taken continuously for at least 8 weeks by 16 weeks.
Measurements and data collection
The patient data were retrieved using the electronic medical chart system in our Psychosomatic Dentistry Clinic. Sociodemographic information was collected, including patient age and sex. At the initial visit, all patients had been asked about their duration of pain and psychiatric history, as well as the details of any psychiatric diagnoses.
Pain intensity was self-reported using a VAS, a continuous horizontal scale that ranges from 0 (no pain) to 100 (worst imaginable pain). Information on each patient’s thoughts and feelings when they were experiencing their pain was collected using the Pain Catastrophizing Scale (PCS),23 which includes 13 items, each of which is rated on a 5-point Likert scale (from 0 “not at all” to 4 “all the time”). A 20-item assessment tool known as the Zung’s Self-Rating Depression Scale (SDS)24 was used to evaluate the symptoms of depression. The response to each item on the SDS is ranked from 1 (“a little of the time”) to 4 (“most of the time”). Patients are classified according to their total score on the SDS as follows: normal (25–49), mildly depressed (50–59), moderately depressed (60–69), or severely depressed (>70). Patients’ responses to these questionnaires are collected as part of routine patient assessment at our institution.
Outcomes and associated factors
The primary outcome was the VAS pain score and the secondary outcomes were the global scores on the SDS and PCS at 16 weeks from the start of treatment. If a patient did not attend an appointment at the exact time (112 days from the first day of the prescription), the data for the closest point (no >14 days on either side of the exact date) were recorded. Data indicating the early response (the VAS pain score at 4 weeks or no >7 days on either side) were also collected. Moderate and significant responses were respectively defined as ≥30% and≥80% improvement in the VAS pain score. Based on the findings of previous research,9,10 we identified the following ten factors to be potentially associated with the main outcome: baseline characteristics (sex, age, duration of pain, psychiatric history, and VAS, SDS, and PCS scores at the first visit), the VAS pain score at 4 weeks, and changes in the SDS and PCS scores between baseline and 16 weeks.
Data protection and analysis
The study protocol was approved by the Ethical Committee of Tokyo Medical and Dental University (D2013-005). All patients had been informed about the possibility of their data being used for study purposes at their first visit and had provided written informed consent. The investigator who collected, cleaned, analyzed, and interpreted the data (TTHT) was not directly involved in treatment and was blinded to the identity of individual patients.
The statistical analysis was performed using R version 3.4.2 for Mac OS (R Foundation for Statistical Computing, Vienna, Austria). A P-value <0.05 was considered statistically significant. Comparisons were carried out using the simple t-test, Wilcoxon signed rank/bootstrap test, and chi-squared test (for sex differences), the paired t-test (for baseline and outcomes data), and the chi-squared and Kruskal–Wallis post hoc tests (for medications). The associations between ten potentially associated factors and the main outcome were investigated by simple linear regression analysis followed by Bayesian model averaging (BMA Package, R Foundation for Statistical Computing) to identify the best multiple linear model.
Results
Baseline characteristics
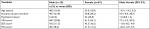
One hundred and sixty-five patient charts were reviewed. Sixty patients (32.5%) were referred to another specialist because of specific treatment needs or at their own request, 21 (13.9%) were lost to follow-up during the study period (because of a change in the clinic attended [n=5], side effects of medication [n=4], lack of satisfaction with the results of treatment [n=3], spontaneous recovery without medication [n=1], disagreement with the attending doctor [n=1], weakness [n=1], or for an unknown reason [n=6]), and 2 (1.2%) did not use a single prescription for at least 8 weeks (Figure 1), leaving data for 82 patients available for the analysis. The mean patient age was 52.1±13.5 years. Sixty-seven patients (82%) were female. About one-third of the patients (n=27) had a psychiatric history. The mean (± SD) duration of pain and VAS, SDS, and PCS scores at baseline were 32.6±38.2 months and 62.6±23.5, 45.0±10.6, and 31.2±10.7, respectively. Using the criterion of a total SDS score >49, 28 patients (34%) were classified as being mildly to severely depressed. There was a positive correlation between the PCS and SDS scores (r=0.44, P<0.0001), but a weak relationship between the PCS and VAS pain scores (r=0.20, P=0.07).
Unexpectedly, there was no significant association between the VAS pain score and SDS score at baseline (r=0.09, P=0.44). The mean duration of pain was 10 months longer in men (n=15, 18%) than in women. None of the sex-related differences at baseline were statistically significant (Table 1).
Medications
Thirty-eight patients were prescribed a TCA (amitriptyline in 37, imipramine in 1). The usual starting dose was 5–10 mg/day at bedtime and could be increased up to 50 mg/day in two or three divided doses. The average daily dose was 24.12±10.02 mg. The most common side effects were drowsiness (n=10), dry mouth (n=9), and constipation (n=9).
Aripiprazole was prescribed for 16 patients at a starting dose of 0.3–0.5 mg and titrated up to 1.5–2.0 mg. The average daily dose was 1.01±0.47 mg. The most common side effects were drowsiness (n=3) and constipation (n=2).
Twenty patients received a TCA combined with aripiprazole (a DPA). The mean daily doses were 24.28±10.86 mg for the TCA and 0.99±0.51 mg for the DPA. The most common side effects were constipation (n=7), drowsiness (n=4), and light-headedness on standing (n=3).
Eight patients who could not be treated with a TCA or DPA alone because of contraindications, side effects, or inadequate response received additional medications, comprising mirtazapine (a noradrenergic and specific serotonergic antidepressant; n=1), sodium valproate (n=2), sodium valproate combined with a TCA (n=1), and sodium valproate combined with a DPA (n=4). Two of these patients reported dry mouth, drowsiness, and constipation.
Forty-nine (60%) of the 82 patients did not report any side effects during 16 weeks of treatment. Importantly, there were no significant differences in the baseline characteristics (proportion of women, age, duration of pain, presence of a psychiatric history, and VAS, SDS, or PCS scores at the first visit) or treatment outcomes (VAS score at week 4 and VAS, SDS, and PCS scores at week 16) according to the type of medication prescribed (Table 2).
Treatment outcomes
An average pain reduction of 30% was reported by 38 patients (46.3%) and 54 patients (65.9%) at 4 and 16 weeks, respectively. At week 16, 15 patients (18.3%) reported significant (≥80%) remission of pain. Table 3 shows the changes in self-reported VAS, SDS, and PCS scores between baseline and 16 weeks, all of which were statistically significant (P<0.001). At 16 weeks, 16 patients (20%) were classified as having mild to severe depression in comparison with 28 (34%) at baseline. The pain intensity decreased as the depression and PCS score improved (Figure 2).
Figure 2 shows the sex-related differences in response to treatment. At 16 weeks, there were no significant differences in the SDS and PCS scores between men and women (P=0.63, 95% CI: −4.5, –7.4 and P=0.96, 95% CI: −7.1, –6.8, respectively). However, the mean VAS pain score was significantly higher in men than in women (51.8±18.6 vs 30.8±21.8, 95% CI: 9.7–32.4, P<0.0001).
Factors associated with pain relief
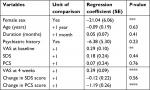
Simple linear regression analysis revealed four independent associations between the main outcome (VAS pain score at week 16) and the factors potentially related to pain relief (Table 4). Female sex was associated with a greater likelihood of response (regression coefficient –21.4, P<0.001), as was a lower VAS pain score at baseline (r=0.29, P<0.01), a better early response, that is, a lower VAS pain score at 4 weeks (r=0.39, P<0.0001), and a greater change in PCS score (r=–1.19, P<0.0001).
Using Bayesian model averaging, we determined that the multivariate regression model included only four elements, that is, patient sex, SDS score at baseline, VAS pain score at 4 weeks, and change in the PCS score (Table 5). After adjusting for the SDS score at baseline, the VAS pain score at 4 weeks, and the change in PCS score, women had a VAS pain score at 16 weeks that was on average about 23 units lower than that in men (95% CI: 18.2–27.6). In both sexes, each 10-unit change in the SDS score at baseline, VAS pain score at 4 weeks, and PCS score was associated with respective unit increases of 4.1 (95% CI: 0.6–7.6), 3.2 (95% CI: 1.6–4.8), and 8.9 (95% CI: 4.6–13.2) in the VAS pain score at 16 weeks. Collectively, these four factors explained 52.5% of the variation in the final pain score between individual patients.
Discussion
AO is a chronic unremitting type of pain that causes intense suffering and negatively impacts quality of life. Since the first description of the concept of AO by Marbach in 1978,1 several research groups have proposed hypotheses to explain the underlying mechanism,6,7,9,13,15,16,19 but there has been little research on treatment outcomes.5,10,11,17,18 Our real-world study, which includes the largest sample to date, provides outcome data that should help to bridge some of the gaps on AO in the literature. Our main findings are as follows: approximately two-thirds of the patients achieved a moderate reduction in pain after 16 weeks of pharmacotherapy; administration of low-dose aripiprazole may be an alternative therapy; improvement of pain was associated with a decrease in depressive symptoms and was less catastrophizing; and 52.5% of the pain intensity at 16 weeks could be accounted for by sex, severity of pain and depressive symptoms at baseline, extent of catastrophizing, and the response to treatment at 4 weeks.
Overall outcomes and medications
We found a high response rate (65.9%) in patients with AO at 16 weeks. In two studies with smaller sample sizes, one of which was reported by Vickers et al11 (n=50) in 1998 and the other by Pigg et al10 (n=37) in 2013, ~63% and 51% of patients, respectively, were reported to have “… a positive and frequently substantial response” after 13 months and 7 years of follow-up. These studies used the same definition of pain relief as that used in our study (ie, at least a 30% decrease in the VAS pain score or Characteristic Pain Intensity scale score, which is similar to the VAS). However, these studies differed in their sample size, duration of follow-up, and particularly in the treatment strategies used, which might explain the differences in response rates.
Our findings confirm the efficacy of TCAs as a treatment for AO and indicate that other medications (aripiprazole [a DPA], combination of a TCA and DPA, sodium valproate, and a noradrenergic and specific serotonergic antidepressant) may be useful. We found no significant differences in the extent of pain reduction achieved using these agents and when using a TCA alone. This finding is consistent with the increasing evidence of the effectiveness of second-generation antipsychotics in the management of chronic pain syndromes.25–27 Theoretically, high tonic dopamine activity is associated with increased pain via a reduction in the release of μ-opioids.28 Therefore, it is not surprising that dopamine stabilizing agents such as aripiprazole could play a role in reduction of pain intensity in patients with AO. We also found that using DPA, and in particular, a combination of a TCA and a DPA, resulted in fewer side effects, which is one of the main reasons that patients declined or ceased treatment with a TCA. Our present findings may provide a good basis for a prospective study of DPA used alone or in combination with a TCA as a therapeutic option in the management of AO.
Pain, depression, and catastrophizing
Depression and pain-related catastrophizing are common in patients with chronic pain.29,30 Previous researchers have mentioned catastrophizing as a key factor determining the individual’s experience of pain, especially in the development of pain and increases in its severity.31,32 In our study, we found that the correlation of pain catastrophizing with depression at baseline was stronger than that of catastrophizing with pain intensity, which is consistent with a previous report by Keefe et al.33 However, no significant correlation was found between pain intensity and depression when measured as a global score, which is similar to the observation made by Estlander et al.34
It has been suggested recently that chronic pain, which includes AO, is a more complex and personal problematic condition than acute pain, and that reducing the intensity of chronic pain in isolation is inadequate.35 In this study, the improvement in VAS, SDS, and PCS scores at 16 weeks demonstrates the effectiveness of medication in patients with AO in decreasing not only the pain intensity but also the emotional distress, negative cognition, and erroneous beliefs that are directly associated with the experience of pain. It is difficult to determine exactly if one component is alleviated first and then leads to improvement in the other components because they share a common pathway in brain activity via the same neurotransmitters.28,31,36 Furthermore, the pharmacological actions of antidepressant and antipsychotic agents include both analgesic and stabilization effects.37 Hence, we suggest that a three-way interaction and simultaneous improvement in the VAS, SDS, and PCS scores might be a reasonable hypothesis in this circumstance.
Determinants of pain relief
There is some evidence suggesting that there are sex-related differences in response to TCAs and atypical antipsychotics in patients with major depressive disorder or schizophrenia.38–40 However, these differences were not found in a study of patients with AO by Pigg et al.10 This discrepancy may reflect the problems associated with small sample sizes and the fact that AO is more common in women than in men. In our study, although there was no sex-related difference in the distribution of baseline characteristics, the pain response in women during follow-up was significantly better. Considering all potential explanations and our finding that only the VAS pain score at week 16 was significantly different between men and women, we suggest that a pharmacokinetic hypothesis (differences in body weight, plasma protein levels, drug transport, and clearance rate) might be more reasonable than explanations involving female reproductive hormone levels, given that almost patients were menopausal, or psychosocial factors, given that there were no differences in the PCS scores.41
There has been a report of an early response to topical capsaicin in patients with AO. In that study, 19 (63.3%) of 30 patients reported a reduction in VAS pain scores by 10%–100% at 4 weeks, but with no significant change in these scores at 13 weeks.10 In contrast, our results suggest that the VAS pain score at 4 weeks is a predictor of the score at 16 weeks. However, use of topical analgesia might involve a pain relief mechanism that is different from that of systemic medication, so the difference is understandable. Our finding is consistent with that of a study in geriatric patients with depression by Mulsant et al, who reported that patients who showed early improvement after 4 weeks of antidepressant therapy tended to achieve full remission at a later date.42 These findings have an important clinical implication, that is, poor responders in need of more attention to improve their chances of long-term remission can be identified after the first 4 weeks of treatment.
Another interesting finding in our study was that the primary outcome was associated with a change in the PCS score, but not the PCS score at baseline. This was unlikely to have been a chance finding because both simple and multiple linear regression analyses yielded the same statistically significant results. Therefore, female sex, an early response, positive coping strategies, and shedding of erroneous beliefs and negative thinking that affect the patients’ mindset have a strong relationship with the final degree of pain relief, regardless of the perceived pain intensity at the first visit. Theoretically, “the ones with high PCS are likely to be vulnerable to the negative impacts of pain and treatment.”43 Therefore, patients with more severe pain are likely to be more prone to catastrophizing and depression, which cannot be treated by focusing only on reduction of pain intensity. Multiple approaches, including acceptance of pain and rehabilitation therapy, both of which can have a beneficial effect on the patient’s comprehension of their pain, hold promise in the management of AO.
Limitations
This study has some limitations in that it was an uncontrolled retrospective analysis that was not blinded and had a limited follow-up duration. Medications were prescribed empirically by a number of clinicians, and there was no washout period when switching from one medication to another, so it is not possible to draw definitive conclusions regarding the effectiveness of pharmacologic therapy. Furthermore, the adverse effects of medication were not recorded using a validated questionnaire. Nonetheless, we believe that our findings contribute significantly to the scant literature on AO, in which application of a strictly defined treatment regimen in data sets may reduce bias from a retrospective origin. One particular strength of our study is that it included the largest sample size of the relevant trials published thus far.
Conclusion
A considerable number of patients with AO, especially women, derived benefit from 16 weeks of treatment with a TCA, an atypical antipsychotic, or a combination of these agents. Regardless of the initial pain intensity or propensity to catastrophize, patients who experience lower levels of depression at baseline and have a better early response after 4 weeks of treatment might subsequently achieve more pain relief than patients who are more depressed at baseline and do not respond to treatment by 4 weeks. For many patients, especially those with strongly negative coping strategies, additional therapy that improves their mindset is as important as analgesic factors in terms of achieving a positive outcome. Further well-controlled prospective studies are needed to evaluate the effectiveness of TCAs, atypical antipsychotics, or a combination of these agents in patients with AO and develop a useful predictive model for their clinical management.
Ethics approval and consent to participate
The study protocol was approved by the Ethical Committee of Tokyo Medical and Dental University (D2013-005). All patients were informed about the possibility of their data being used for study purposes at their first visit and provided written informed consent.
Acknowledgment
This study was supported in part by the Japan Society for the Promotion of Science KAKENHI (grant number 16K11881).
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors have no competing interests to report concerning this study and report no conflicts of interest in this work.
References
Marbach JJ. Phantom tooth pain. J Endod. 1978;4(12):362–372. | ||
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013. Available from: https://dsm.psychiatryonline.org/doi/book/10.1176/appi.books.97808904255966. Accessed September 24, 2018. | ||
Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629–808. | ||
Marbach JJ, Hulbrock J, Hohn C, Segal AG. Incidence of phantom tooth pain: an atypical facial neuralgia. Oral Surg Oral Med Oral Pathol. 1982;53(2):190–193. | ||
Ram S, Teruel A, Kumar SK, Clark G. Clinical characteristics and diagnosis of atypical odontalgia: implications for dentists. J Am Dent Assoc. 2009;140(2):223–228. | ||
Miura A, Tu TTH, Shinohara Y, et al. Psychiatric comorbidities in patients with atypical Odontalgia. J Psychosom Res. 2018;104:35–40. | ||
Baad-Hansen L. Atypical odontalgia – pathophysiology and clinical management. J Oral Rehabil. 2008;35(1):1–11. | ||
Graff-Radford SB, Solberg WK. Is atypical odontalgia a psychological problem? Oral Surg Oral Med Oral Pathol. 1993;75(5):579–582. | ||
Marbach JJ. Is phantom tooth pain a deafferentation (neuropathic) syndrome? Part I: evidence derived from pathophysiology and treatment. Oral Surg Oral Med Oral Pathol. 1993;75:95–105. | ||
Pigg M, Svensson P, Drangsholt M, List T. Seven-year follow-up of patients diagnosed with atypical odontalgia: a prospective study. J Orofac Pain. 2013;27(2):151–164. | ||
Vickers ER, Cousins MJ, Walker S, Chisholm K. Analysis of 50 patients with atypical odontalgia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85(1):24–32. | ||
Toyofuku A. Psychosomatic problems in dentistry. Biopsychosoc Med. 2016;10(1):14. | ||
Lilly JP, Law AS. Atypical odontalgia misdiagnosed as odontogenic pain: a case report and discussion of treatment. J Endod. 1997;23(5):337–339. | ||
Melis M, Lobo SL, Ceneviz C, et al. Atypical odontalgia: a review of the literature. Headache. 2003;43(10):1060–1074. | ||
Brooke RI. Atypical odontalgia. A report of twenty-two cases. Oral Surg Oral Med Oral Pathol. 1980;49:196–199. | ||
Ciaramella A, Paroli M, Lonia L, Bosco M, Poli P. Biopsychosocial aspects of atypical Odontalgia. ISRN Neurosci. 2013;2013(1):1–10. | ||
List T, Leijon G, Helkimo M, Oster A, Dworkin SF, Svensson P. Clinical findings and psychosocial factors in patients with atypical odontalgia: a case–control study. J Orofac Pain. 2007;21(2):89–98. | ||
Takenoshita M, Miura A, Shinohara Y, et al. Clinical features of atypical odontalgia; three cases and literature reviews. Biopsychosoc Med. 2017;11(1):1–5. | ||
Woda A, Pionchon P. A unified concept of idiopathic orofacial pain: clinical features. J Orofac Pain. 1999;13(3):172–195. | ||
Melis M, Secci S. Diagnosis and treatment of atypical odontalgia: a review of the literature and two case reports. J Contemp Dent Pract. 2007;8(3):81–89. | ||
Pertes RA, Bailey DR, Milone AS. Atypical odontalgia – a nondental toothache. J N J Dent Assoc. 1995;66(1):29–31. | ||
Tarce M, Barbieri C, Sardella A. Atypical odontalgia: an up-to-date view. Minerva Stomatol. 2013;62(5):163–181. | ||
Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess. 1995;7(4):524–532. | ||
Zung WW. A self-rating depression scale. Arch Gen Psychiatry. 1965;12(1):63–70. | ||
Calandre EP, Rico-Villademoros F. The role of antipsychotics in the management of fibromyalgia. CNS Drugs. 2012;26(2):135–153. | ||
Kasahara S, Kunii Y, Mashiko H, Otani K, Konno S, Niwa S. Four cases of chronic pain that improved dramatically following low-dose aripiprazole administration. Prim Care Companion CNS Disord. 2011;13(2). | ||
Umezaki Y, Takenoshita M, Toyofuku A. Low-dose aripiprazole for refractory burning mouth syndrome. Neuropsychiatr Dis Treat. 2016;12:1229–1231. | ||
Leknes S, Tracey I. A common neurobiology for pain and pleasure. Nat Rev Neurosci. 2008;9(4):314–320. | ||
Edwards RR, Cahalan C, Mensing G, Smith M, Haythornthwaite JA. Pain, catastrophizing, and depression in the rheumatic diseases. Nat Rev Rheumatol. 2011;7(4):216–224. | ||
Sullivan MJ, D’Eon JL. Relation between catastrophizing and depression in chronic pain patients. J Abnorm Psychol. 1990;99(3):260–263. | ||
Sullivan MJL, Thorn B, Haythornthwaite JA, et al. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17(1):52–64. | ||
Sullivan MJ, Martel MO, Tripp DA, Savard A, Crombez G. Catastrophic thinking and heightened perception of pain in others. Pain. 2006;123(1–2):37–44. | ||
Keefe FJ, Brown GK, Wallston KA, Caldwell DS. Coping with rheumatoid arthritis pain: catastrophizing as a maladaptive strategy. Pain. 1989;37(1):51–56. | ||
Estlander AM, Knaster P, Karlsson H, Kaprio J, Kalso E. Pain intensity influences the relationship between anger management style and depression. Pain. 2008;140(2):387–392. | ||
Sullivan MD, Ballantyne JC. Must we reduce pain intensity to treat chronic pain? Pain. 2016;157(1):65–69. | ||
Han C, Pae CU. Pain and depression: a neurobiological perspective of their relationship. Psychiatry Investig. 2015;12(1):1–8. | ||
Khouzam HR. Psychopharmacology of chronic pain: a focus on antidepressants and atypical antipsychotics. J Postgrad Med. 2016;128(3):323–330. | ||
Aichhorn W, Whitworth AB, Weiss EM, Marksteiner J. Second-generation antipsychotics: is there evidence for sex differences in pharmacokinetic and adverse effect profiles? Drug Saf. 2006;29(7):587–598. | ||
Sramek JJ, Murphy MF, Cutler NR. Sex differences in the psychopharmacological treatment of depression. Dialogues Clin Neurosci. 2016;18(4):447–457. | ||
Usall J, Suarez D, Haro JM, Group SS. Gender differences in response to antipsychotic treatment in outpatients with schizophrenia. Psychiatry Res. 2007;153(3):225–231. | ||
Paller CJ, Campbell CM, Edwards RR, Dobs AS. Sex-based differences in pain perception and treatment. Pain Med. 2009;10(2):289–299. | ||
Mulsant BH, Houck PR, Gildengers AG, et al. What is the optimal duration of a short-term antidepressant trial when treating geriatric depression? J Clin Psychopharmacol. 2006;26(2):113–120. | ||
Park SJ, Lee R, Yoon DM, Yoon KB, Kim K, Kim SH. Factors associated with increased risk for pain catastrophizing in patients with chronic neck pain: a retrospective cross-sectional study. Medicine. 2016;95(37):e4698. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.