Back to Journals » Drug Design, Development and Therapy » Volume 17
Pharmacological Mechanism of Sancao Yuyang Decoction in the Treatment of Oral Mucositis Based on Network Pharmacology and Experimental Validation
Authors Liu Y, Ye Y , Xie G , Xu Y, Cheng M , Li C , Qu M , Zhu F
Received 19 October 2022
Accepted for publication 27 December 2022
Published 13 January 2023 Volume 2023:17 Pages 55—74
DOI https://doi.org/10.2147/DDDT.S391978
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Yunxia Liu,1,* Yun Ye,2,* Guanqun Xie,2 Yefeng Xu,1 Miao Cheng,1 Chunling Li,2 Mengqi Qu,2 Feiye Zhu3
1Oncology Department, Hangzhou Third People’s Hospital, Hangzhou, People’s Republic of China; 2College of Basic Medical Science, Zhejiang Chinese Medical University, Hangzhou, People’s Republic of China; 3Academy of Chinese Medical Sciences, Zhejiang Chinese Medical University, Hangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yunxia Liu, Oncology Department, Hangzhou Third People’s Hospital, Hangzhou, People’s Republic of China, Email [email protected] Feiye Zhu, Academy of Chinese Medical Sciences, Zhejiang Chinese Medical University, Hangzhou, People’s Republic of China, Email [email protected]
Purpose: The network pharmacology analysis, molecular docking and experimental verification were performed to explore the pharmacological mechanisms of Sancao Yuyang Decoction (SCYYD) in the treatment of oral mucositis (OM).
Methods: Active ingredients in SCYYD and their potential targets, as well as OM-related targets were screened from public databases. The core targets and signaling pathways of SCYYD against OM were determined by protein–protein interaction (PPI) network, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis. The ingredient-target-disease network and target-pathway network were constructed. Subsequently, molecular docking was carried out to predict the binding activity between active ingredients and key targets. Moreover, in vivo experiment was conducted to further verify the core targets predicted by network pharmacology analysis.
Results: A total of 119 bioactive ingredients were screened from the corresponding databases. One hundred and eighty-six putative targets were retrieved and bioinformatics analysis was performed to reveal the top 5 potential candidate agents and 10 core targets. GO and KEGG enrichment analysis showed that SCYYD exerted excellent therapeutic effects on OM through several pathways, such as HIF-1 and Ras signaling pathway. Subsequently, molecular docking showed that main ingredients in SCYYD had optimal binding activities to the key protein targets. Moreover, the result of in vivo experiment indicated that SCYYD not only inhibited inflammation response and promoted wound healing of oral mucosa in OM rats, but also reversed high expressions of SRC, HSP90AA1, STAT3, HIF1α, mTOR, TLR4, MMP9, and low expression of ESR1.
Conclusion: This study preliminarily uncovered the multiple compounds and multiple targets of SCYYD against OM using network pharmacology, molecular docking and in vivo verification, which provided a new insight of the pharmacological mechanisms of SCYYD in treatment of OM.
Keywords: oral mucositis, Sancao Yuyang Decoction, pharmacological mechanisms, network pharmacology, experimental verification
Introduction
Oral mucositis (OM) refers to the inflammatory and/or ulcerative lesions of oral mucosa which caused by multiple factors such as infectious disease, immune dysfunction and some medications, etc.1 It is also one of the common complications of cancer patients receiving radiotherapy and chemotherapy with prevalence of 40% to 70%.2,3 According to ESMO mucositis guidelines, the term “mucositis” is described as inflammation of mucosa resulting from ionising radiation or chemotherapeutic agents such as methotrexate, cyclophosphamide, 5-fluorouracil (5-FU), etc.1 It is characterized by erythema, erosion and development of ulcerative lesions in the oral mucosa with severe pain which affect eating, speaking and sleep.4 The eating patterns affected by severe pain of OM in cancer patients may reduce nutritional intake, and subsequently result in poor general condition and quality of life.5 Currently, there are many common clinical management strategies of OM such as standardized oral care, antibiotic, anti-inflammatory, growth factors, analgesics and some mouthwashes.6 Although drugs such as dexamethasone, cydiodine, and doxepin are usually applied for OM to alleviate inflammation and painful, the long-term application may lead to unexpected side effects such as susceptible to fungal infection, rash, more drowsiness, stinging or burning sensation and fatigue.1,7 Therefore, it is urgent to search for the effective therapeutic strategies for OM with few side effects.
Traditional Chinese medicine (TCM) is a complementary and alternative medicine which has been used for more than 2000 years to prevent or treat diseases in China. In recent years, increasing patients with OM choose TCM due to its remarkable efficacy and low toxicity.8,9 Studies have shown that many herbal medicines with anti-inflammatory, antioxidation and analgesic properties have good therapeutic effects on OM.10,11 Sancao Yuyang Decoction (SCYYD) is a Chinese medicine prescription that has been patented (Patent No. ZL 201810411853.2) for treating OM. It is composed of four Chinese medicines, namely Lithospermum Erythrorhizon (Zi cao), Prunellae Spica(Xia ku cao), Menthae Herba(Bo he) and licorice (Gan cao). Our previous clinical studies have shown that SCYYD can reduce local inflammation and improve symptoms such as erythema, erosion and ulcer of oral mucosa in OM patients.12 However, the mechanism of SCYYD on OM has not been fully elucidated and suitable approaches are needed for the further research.
TCM contains a large number of ingredients which acts synergistically by multiple mechanisms. Due to the complicated mechanisms of TCM, we should search appropriate approaches to analyze each component and its corresponding targets. Network pharmacology is a comprehensive research method integrating chemical informatics, bioinformatics, network biology and traditional pharmacology.13 It can reveal the relationship between multiple components and multiple targets from a systematic and holistic perspective.14 Based on network pharmacology and in vivo validation, this study predicted and validated the ingredients present in SCYYD, their protein targets, as well as the multiple mechanisms involved in the action of these ingredients on OM. The detail of workflow of this study is shown in Figure 1.
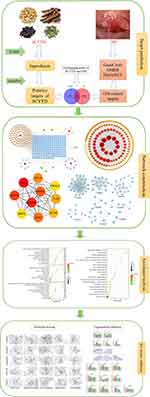 |
Figure 1 Detailed workflow of present study. |
Materials and Methods
Collection of Active Ingredients in SCYYD and Identification of Their Targets
All ingredients of the SCYYD were acquired from Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP, http://tcmspw.com/tcmsp.php). Oral bioavailability (OB) and druglikeness (DL) are two important properties of absorption, distribution, metabolism and excretion (ADME). OB ≥30% and BL ≥0.18 were applied as screening criteria in this study. The putative molecular targets of the active ingredients in SCYYD were retrieved from the SuperPred database (https://prediction.charite.de/).
Collection of OM-Associated Molecular Targets
The OM-associated molecular targets were screened from three sources, namely, GeneCards (https://www.genecards.org/), Online Mendelian Inheritance in Man (OMIM, https://omim.org/) and DisGeNET (https://disgenet.org/). “Oral mucositis” was applied as the keyword and duplicate genes were removed.
Construction of Ingredient-Target-Disease Network
The OM-associated targets and the drug-related targets were intersected, and a Venn diagram of the gene symbols was drawn to obtain the potential targets of SCYYD against OM. Then, Cytoscape 3.8.2 software was used to construct the ingredient-target-disease network.
Protein–Protein Interaction (PPI) Network Construction
The overlapping target genes of SCYYD-related targets and OM associated targets were imported into String database (http://string-db.org) to obtain the PPI network. The parameter organism was defined as Homo sapiens, and the minimum required interaction score was greater than 0.7. Cytoscape 3.8.2 software was applied to establish the PPI relationship network and Cytohubba and MCODE software were employed to identify the core proteins and clusters. Then, biological process (BP) of each cluster was analyzed using DAVID Bioinformatics Resources 6.7 (http://david.abcc.ncifcrf.gov/).
Enrichment of Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathways
The target genes were uploaded to the functional annotation tool of DAVID Bioinformatics Resources 6.7 (http://david.abcc.ncifcrf.gov/) to conduct the GO and KEGG pathway enrichment analyses. GO enrichment analysis included BP, cellular component (CC) and molecular function (MF) analysis. The parameters of P<0.05 and FDR <0.05 were recognized as significant of GO and KEGG enrichment. The “target-pathway” network was visualized using Cytoscape 3.8.2.
Computational Validation of Active Ingredients and Targets Interactions by Molecular Docking
The binding ability between active ingredients selected from ingredient-target-disease network and the key targets was analyzed using molecular docking. The SDF format files of active ingredients were downloaded from PubChem database (http://pubchem.ncbi.nlm.nih.gov) and converted to mol2 format files after energy minimization calculations by Chem3D software. PDF format files for the key target proteins were obtained from RCSB PDB database (https://www.rcsb.org/). Molecular docking of the main active ingredients and core target proteins was performed using CB-Dock2 online molecular docking (https://cadd.labshare.cn/cb-dock2/). The lower vina score is considered as more stable binding capacity between small molecular compounds and proteins.
Experimental Validation
Preparation of SCYYD Aqueous Extract
Four raw herbs of SCYYD were obtained from the pharmacy department of the Hangzhou Third People’s Hospital (Hangzhou, China). Lithospermum Erythrorhizon, Prunellae Spica, Menthae Herba and Licorice were mixed as a proportion of 2.5:5:1:5(w/w). The mixed herbs were soaked in 5 volumes of distilled water for 1h, and then decocted for 2 times and 0.5h each time. The decoction was concentrated to 1.825 g/mL and stored at −20°C before further use.
Animals
A total of forty male Sprague-Dawley rats weighing 180~200g were purchased from Shanghai Slac Laboratory Animal Co. Ltd (Shanghai, China). All of these rats were bred at Laboratory Animal Research Center of Zhejiang Chinese Medical University (Hangzhou, China) with a specific pathogen-free animal care facility. They were housed in a standard laboratory conditions (room temperature 23 ± 2°C; humidity 50±10%) with a 12h/12h light/dark cycle and free access to water and food. The animal experimental protocol was approved by Laboratory animal management and ethics committee of Zhejiang Chinese Medical University, Hangzhou, China (Approval No. IACUC-20210503-08). All experimental procedures were performed following the Regulations for the Administration of Affairs Concerning Experimental Animals approved by the State Council of the People’s Republic of China.
Establishment of OM Animal Model and Drug Administration
All the rats were randomly and equally divided into 5 groups (n = 8 in each group): normal control group (NC), OM model group (OM) and group of OM rats treated with SCYYD at low, medium and high dosage (OM+L, OM+M, OM+H). Except the NC rats, others were anesthetized by intraperitoneal injection of sodium pentobarbital (50 mg/kg). A 9mm2 piece of filter paper was soaked in saturated sodium hydroxide solution and placed in the labial fornix region of the inferior incisors for 15s, which created a uniform ulcer, and 25 mg/kg methotrexate was injected intraperitoneally.
After that, rats in low, medium and high dosage of SCYYD groups were administered intragastrically with SCYYD at the dosage of 7.3 g/kg, 14.6g/kg and 29.2 g/kg, respectively, once a day for 8 consecutive days. Rats in the other 2 groups were given the same dosage of 0.9% saline. The body weight was measured during the experiment. At the end of experiment, tissues of oral mucosa were collected for further analysis.
Evaluation of the Oral Mucosal Healing
To detect the mucosal healing, oral mucositis scoring system was used for scoring the healing of oral mucosa. The scoring system is shown in Table 1.
 |
Table 1 The Scoring System of Mucosal Healing |
Histopathology Evaluation
To evaluate the histopathological changes in the oral mucosa, hematoxylin and eosin (H&E) staining was performed. Tissue samples of oral mucosa from labial fornix region of the inferior incisors were fixed with 10% formalin and dehydrated with ethanol and xylene. Dehydrated samples were embedded in paraffin blocks. Each paraffin-embedded specimen was cut into sections at a thickness of 5 μm. Each section was stained with H&E and observed with NanoZoomer S60 Digital Slice Scanner (Hamamatsu, Japan).
RNA Extraction and qPCR
Total RNA was extracted from oral mucosa tissues by Total RNA Rapid Extraction Kit (Beijing Biotek Biotechnology Co., Ltd, Beijing, China) according to the operating manual. A Nanodrop one (Thermo Scientific, USA) was used to detected the RNA purity. Obtained RNA templates were reverse-transcribed into cDNA using HiFiScript Quick gDNA Removal cDNA kit (CoWin Biosciences, Jiangsu, China). qPCR was performed following SYBR green PCR Master Mix instructions (CoWin Biosciences, Jiangsu, China) with Roche 480 real-time system (Roche, USA). Primers used for qPCR were synthesized by Sangon Biotech (Shanghai) Co., Ltd (Shanghai, China) and the sequences are shown in Table 2. β-Actin was used as an endogenous control gene. The reaction profile was: one cycle at 95°C for 10 min; 45 cycles: 95°C 10 min; 95°C 10s; 60°C 30s, 72°C 32s; melting curve analysis was at 65°C~97°C. Relative mRNA expression was calculated and analyzed by the 2−ΔΔCt method.
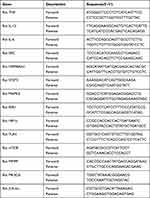 |
Table 2 Primer Sequences for qPCR |
Statistical Analysis
All data were presented as mean±SEM. SPSS16.0 software and GraphPad Prism 5 software were applied for statistical analysis. The results were analyzed using Student’s t-test and One-way ANOVA. A level of P<0.05 was deemed statistically significant.
Results
Identification of Active Ingredients in SCYYD
A total of 555 ingredients in SCYYD were acquired from TCMSP database, including 51 from Lithospermum Erythrorhizon, 60 from Prunellae Spica, 164 from Menthae Herba and 280 from Licorice. Twelve (23.5%) components among the 51 ingredients in Lithospermum Erythrorhizon met the criteria of OB ≥30 and DL ≥0.18, 11 (18.3%) components among 60 ingredients in Prunellae Spica measured up to the requirements of OB ≥30 and DL ≥0.18, 10 (6.1%) ingredients in Menthae Herba and 92 (32.9%) ingredients in Licorice researched the standard of OB ≥30 and DL ≥0.18 (Table 3). After removing the overlaps, 119 ingredients were selected as the putative active ingredients for further analyses (Supplementary Table S1).
 |
Table 3 Number of Ingredients in SCYYD with OB ≥30 and DL ≥0.18 |
Acquisition of Targets of Ingredients in SCYYD Against OM
The chemical structure and SMILES of ingredients in SCYYD were obtained from PubChem (https://pubchem.ncbi.nlm.nih.gov/). We retrieved the putative targets of ingredients in SCYYD from SuperPred database. After eliminating the overlaps, a total of 443 targets were obtained for further analyses. Two thousand one hundred and fifty-two OM-associated targets were collected from the GeneCards database, OMIM database and DisGeNET database. After looking for the intersection of the drug-related targets and OM-associated targets, 186 overlapping genes were found and the detailed information is shown in Figure 2 and Supplementary Table S2.
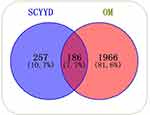 |
Figure 2 Venn diagram for the intersection of potential targets of SCYYD and OM-related genes. |
Ingredient-Target-Disease Network and Analysis
To reveal the relationship between four herbs in SCYYD, their active ingredients and potential targets of OM, the ingredient-target-disease network was constructed using Cytoscape 3.8.2 software. The network consisted of 299 nodes and 5656 edges (Figure 3 and Supplementary Table S3), suggesting that SCYYD exerts effects for OM through multiple ingredients and targets. According to the number of degrees, the top 10 key active ingredients of SCYYD in the treatment of OM were sitosterol, naringenin, kaempferol, quercetin, luteolin, Glabridin, Spinasterol, Mairin, Phaseolinisoflavan and Stigmasterol (Table 4). Among them, the top five belonged to more than two herbs respectively, namely, sitosterol was found in Lithospermum Erythrorhizon, Menthae Herba and Licorice, naringenin belonged to Menthae Herba and Licorice, kaempferol and quercetin were the compounds of Prunellae Spica and Licorice, luteolin belonged to Prunellae Spica and Menthae Herba.
 |
Table 4 The Top 10 Key Active Ingredients of SCYYD in the Treatment of OM |
PPI Network Construction and Core Targets Screening
To identify the information on the predicted interactions, a PPI network with 183 nodes and 1892 edges was constructed using String database and visualized by Cytoscape 3.8.2 (Figure 4A). Then, the top 10 genes, ranked by degree value, were calculated by Cytohubba and collected to be the core targets, namely, SRC (degree=86), HSP90AA1 (degree=82), STAT3 (degree=86), MAPK3 (degree=70), ESR1 (degree=68), HIF1α (degree=67), TLR4 (degree=65), mTOR (degree=64), MMP9 (degree=63), PIK3CA (degree=63) (Figure 4B and C). As shown in Figure 5 and Supplementary Table S4, eight clusters of SCYYD against OM were collected by MCODE software. The highest score was cluster 1 which contained 31 nodes and 245 edges. There were 64 terms of BP in cluster 1, 32 terms of BP in cluster 2, 33 terms of BP in cluster 3, 2 terms of BP in cluster 5, and 3 terms of BP in cluster 6(P<0.05 and FDR <0.05) (Supplementary Tables S5-S9). The top 10 entries of BP in cluster 1, cluster 2 and cluster 3 ranked according to their q-values are shown in Figure 6. The top 5 enriched BP terms in cluster 1 were negative regulation of gene expression (GO:0010629), positive regulation of transcription from RNA polymerase II promoter (GO:0045944), inflammatory response (GO:0006954), positive regulation of angiogenesis (GO:0045766) and negative regulation of endothelial cell apoptotic process (GO:2000352). The main BP terms in cluster 2 were cellular response to reactive oxygen species (GO:0034614), apoptotic process (GO:0006915), cellular response to cadmium ion (GO:0071276), peptidyl-serine phosphorylation (GO:0018105), cellular response to UV (GO:0034644). The BP terms in cluster 3 were focused on peptidyl-tyrosine phosphorylation (GO:0018108), transmembrane receptor protein tyrosine kinase signaling pathway (GO:0007169), protein phosphorylation (GO:0006468), cell migration (GO:0016477), positive regulation of kinase activity (GO:0033674).
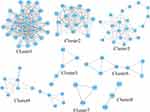 |
Figure 5 The gene clusters of SCYYD against OM. The cluster from 1 to 8 is based on their cluster score. |
 |
Figure 6 The BP for gene clusters of SCYYD against OM. (A) The top 10 BP terms of Cluster 1. (B) The top 10 BP terms of Cluster 2. (C) The top 10 BP terms of Cluster 3. |
GO and KEGG Pathway Enrichment Analysis and Target-Pathway Network Construction
GO enrichment analysis was performed to verify the biological functions of 186 putative targets of SCYYD against OM. A total of 404 terms were acquired (P<0.05 and FDR <0.05), including 283 for BP, 53 for CC, and 68 for MF (Supplementary Tables S10-S12) and the top 10 entries of BP, CC and MF terms ranked according to their q-values are shown in Figure 7. The top 5 enriched BP terms were cytokine-mediated signaling pathway (GO: 0019221), inflammatory response (GO: 0006954), protein phosphorylation (GO: 0006468), peptidyl-tyrosine phosphorylation (GO: 0018108) and platelet activation (GO: 0030168). The main enriched CC terms were response to plasma membrane (GO:0005886), receptor complex (GO:0043235), cell surface (GO:0009986), integral component of plasma membrane (GO:0005887) and membrane raft (GO:0045121). The MF analysis focused on protein serine/threonine/tyrosine kinase activity (GO:0004712), transmembrane receptor protein tyrosine kinase activity (GO:0004714), protein tyrosine kinase activity (GO:0004713), ATP binding (GO:0005524) and protein serine/threonine kinase activity (GO:0004674).
 |
Figure 7 The top 10 of GO enrichment analysis for target genes of SCYYD against OM. |
To further analyze the underlying mechanism on involved pathways of SCYYD against OM, KEGG pathway enrichment was carried out. A total of 160 signaling pathways were obtained (P<0.05 and FDR <0.05) (Supplementary Table S13) and the 20 most significant KEGG pathways closely associated with OM are depicted in Figure 8, including HIF-1 signaling pathway and Ras signaling pathway. A target-pathway network was constructed based on the top 20 significant KEGG pathways and their corresponding genes to identify the molecular mechanism of SCYYD on OM (Figure 9). The following pathways had the top highest number of genes: Pathways in cancer (53), Kaposi sarcoma-associated herpesvirus infection (27), Human cytomegalovirus infection (27), Hepatitis B (26), Chemical carcinogenesis-receptor activation (26), and Ras signaling pathway (26). Among these target genes from the top 20 pathways, PIK3CD, PIK3CB, PIK3CA, PIK3R1, MAPK1, MAPK3, MAP2K2, NFKB1, STAT3, IKBKB and mTOR were identified as significantly enriched genes. This result indicated that the effects of SCYYD on OM might be related with multiple pathways.
 |
Figure 8 The top 20 signaling pathways from KEGG enrichment analysis of target genes of SCYYD against OM. |
 |
Figure 9 The target-pathway network of SCYYD against OM. The blue nodes stand for pathways and the red nodes stand for target genes. The node size is proportional to the number of degree. |
Binding Ability Between the Active Ingredients and Key Targets by Molecular Docking
The binding activity between five top active ingredients selected from the ingredient-target-disease network (sitosterol, naringenin, kaempferol, quercetin, luteolin) and the core proteins (SRC, HSP90AA1, STAT3, ESR1, HIF1α) were carried out by CB-Dock2 online molecular docking. Higher absolute values of vina score indicate a stronger affinity and stability between the ingredients and targets. The absolute value greater than 4.25 indicates that the molecular and the target have a certain binding activity, the absolute value more than 5.0 indicates a good binding activity, and greater than 7.0 indicates that there is a strong binding activity between the ingredients and their corresponding targets.15 As shown in Table 5, except the absolute vina scores of each active ingredient with ESR1 were between 5.0 and 7.0, others were greater than 7.0, indicating that the five top active ingredients had a strong binding activity to SRC, HSP90AA1, STAT3 and HIF1α. The 3D map of each docking diagram is shown in Figure 10.
 |
Table 5 Binding Energy Between Key Ingredients and Target Proteins |
 |
Figure 10 The 3D map of each docking diagram. |
SCYYD Ameliorated the Symptoms of OM
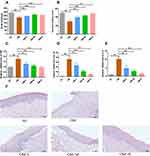
To verify the effect of SCYYD in the treatment of OM in the in vivo animal experiment, OM model rats were established and administered intragastrically with SCYYD. The body weight and mucosal healing score were measured, and then the histological changes of oral mucosa were examined by H&E staining. As shown in Figure 11A, the body weight of rats in OM group was significantly lower than those in the NC group (P<0.01). Compared with OM group, the body weight of rats in OM+M and OM+H groups was increased significantly (P<0.01). There was no significant difference in body weight between the OM+L group and the OM group (P>0.05).
The oral mucosa of rats were observed during the experiment. The oral mucosa of rats in OM group was presented as thick pseudomembrane on the surface of ulcer with obvious inflammatory edema around it. Treatment with different dosages of SCYYD accelerated the mucosa healing significantly (Supplementary Figure S1). As shown in Figure 11B, the mucosal healing score in OM group was decreased significantly compared to the NC group (P<0.01). After treatment with SCYYD at low, medium and high dosages, the mucosal healing score was significantly higher than those in OM group (P<0.01), indicating the SCYYD could heal the ulcer in oral mucosa effectively.
SCYYD Reduced the Inflammatory Factors of OM
To detect the anti-inflammation function of SCYYD, the inflammatory factors of oral mucosa were measured by qPCR. As shown in Figure 11C-E, the mRNA expressions of TNF, IL-1β and IL-6 in OM group were higher than that in the NC group (P<0.01). After treatment with different dosages of SCYYD, the expression of TNF was decreased in the OM+L, OM+M and OM+H group (P<0.01 or P<0.05), and the expressions of IL-1β and IL-6 was decreased in the OM+M and OM+H group (P<0.01).
SCYYD Ameliorated the Histopathology of OM
In histopathological findings, the NC group showed intact epithelium and absence of inflammatory cell infiltration. The oral mucosa of the OM group was severely ulcerated with disrupted epithelial layer and severe inflammatory cell infiltration. Although reepithelization was initiated in the OM+L group, recovery was incomplete and there were still some inflammatory cell infiltration. Reepithelization in the OM+M group was initiated and the healing process was much more accelerated with less inflammatory cell infiltration. The oral mucosa of OM+H group exhibited recovered epithelium with little inflammatory cell infiltration (Figure 11F).
Validation of the Main Key Targets of Treatment of OM by SCYYD
To further evaluate the key targets obtained from network pharmacologic analyses, we assessed the expressions of SRC, HSP90AA1, STAT3, MAPK3, ESR1, HIF1α, TLR4, mTOR, MMP9 and PIK3CA by qPCR. As shown in Figure 12, the mRNA expressions of SRC, HSP90AA1, STAT3, HIF1α, TLR4, mTOR and MMP9 in OM group were higher than that in the NC group (P<0.01 or P<0.05). After treatment with different dosages of SCYYD, the expressions of SRC, HSP90AA1, STAT3, HIF1α, TLR4 and mTOR were decreased in the OM+L, OM+M and OM+H group (P<0.01 or P<0.05), and the expression of MM9 was decreased in the OM+M and OM+H group (P<0.01 or P<0.05). Compared with the NC group, the expressions of ESR1 and PIK3CA were lower in the OM group (P<0.01), and expression of ESR1 was increased after treated by medium and high dosages of SCYYD (P<0.01 or P<0.05), but there was no significant difference of PIK3CA between OM group and groups of treatment with different dosages of SCYYD (P>0.05).
Discussion
OM is a recurrent disease with complicated etiology involving multiple signaling pathways. The most significant feature of OM is painful as well as inflamed lesions which may appear in any place of mouth. Therefore, many herbs with property of heat clearing and detoxifying are increasingly applied in the treatment of OM due to their anti-inflammatory and antioxidant effects.9,16 SCYYD possesses the effect of heat clearing and detoxifying and has been proven to be therapeutically effective in treating chemotherapy-induced OM by inhibiting inflammatory factors.12 In this study, we developed a rat model of OM with the use of production of mucosal injury (sodium hydroxide) and cancer chemotherapy (methotrexate) in combination which was easy to evaluate the therapeutic effect of drugs on OM due to it could provide a sufficient ulcer area that lasted for a relatively long period.17 After treating with different dosages of SCYYD, the symptoms of erythema, erosion and ulcerative lesions were improved and the inflammatory factors decreased. It indicated that SCYYD exerted the effects of anti-inflammatory and mucosal healing. However, the molecular mechanism of SCYYD on OM remained elusive due to its property of multi-component and multi-target. Network pharmacology, involving the systems biology and bioinformatics, provides a powerful and comprehensive research approach for studying the complicated mechanism of TCM. It has been widely used to reveal the multi-target and multi-pathway mechanisms of Chinese medicines and their active ingredients in the treatment of diseases in recent years.18–20 In the current study, we applied network pharmacology analysis and experimental validation to investigate the effects and underlying mechanisms of SCYYD against OM.
Appropriate pharmacokinetic properties can enable drugs to reach target organs and play a therapeutic role, which is important for drug screening and design.21 In this study, 119 bioactive ingredients were identified by the criteria of OB ≥30 and DL ≥0.18. Among them, we found the top key active compounds included sitosterol, naringenin, kaempferol, quercetin, and luteolin. Sitosterol is a phytosterol which can suppress the chronic inflammation by downregulating IL-6 and TNF-α.22 Naringenin, a citrus flavonoid, was reported to exert anti-inflammatory, antioxidant, anticancer and antiatherogenic effects.23 It was also reported that naringenin acted as an immunomodulator to provide modality for the development of novel anti-inflammatory agent.24 Kaempferol is a natural flavonoid which has various pharmacological activities such as anti-inflammatory, antioxidant, anticancer, antimicrobial, cardioprotective, neuroprotective, etc and acts as a therapeutic strategy in the treatment of diseases including arthritis, cardiovascular diseases, oxidative stress, allergies, diabetes, etc.25–27 The anti-inflammatory effect of kaempferol was associated with inhibiting activation of inflammatory cytokines through efficiently disturbing the transactivation of STAT3.28 Quercetin is a flavonoid which also has anti-inflammatory, antioxidant, and anticancer activities. Moreover, quercetin treatment was shown to alleviate the inflammation by inhibiting NF-κB pathway in 5-fluorouracil-induced or radiation-induced oral mucositis model.29,30 As a flavonoid, luteolin also possessed anti-inflammatory activity by regulating the transcription factors such as STAT3, NF-κB, and AP-1.31,32 All these literatures supported that multiple ingredients in SCYYD predicted by network pharmacology played considerable anti-inflammatory roles in the treatment of OM.
In the current study, a total of 186 putative genes were identified based on the network pharmacology analysis and the GO and KEGG enrichment was further enriched according to these genes. The BP enrichment results indicated that most of putative genes were related to inflammatory response, a key etiology factor of OM. Additionally, the KEGG results indicated that majority of pathways were associated with immune and inflammatory, tumor and virus infection. For example, HIF-1 signaling pathway can be activated by oxidative stress and then lead to the expression of various genes, including those for inflammatory, cytokine growth factors and chemokines.33 The biological function analysis of modules of target genes also showed that the mechanisms of SCYYD were related to inflammatory response and cellular response to reactive oxygen species. The results of GO and KEGG enrichment analysis indicated that the mechanism of SCYYD against OM may be associated with anti-inflammation and antioxidation.
To explore the basic mechanisms of SCYYD against OM, the core targets were identified by PPI network, namely, SRC, HSP90AA1, STAT3, MAPK3, ESR1, HIF1α, TLR4, mTOR, MMP9, PIK3CA. SRC, a cytoplasmic tyrosine kinase, is mainly expressed in human neutrophils to modulate multiple functions such as ROS production, NET formation, integrin activation and migration towards inflamed sites.34 HSP90AA1 encodes heat shock protein 90α(Hsp90α) which is a highly conserved chaperone protein in eukaryotes. Studies showed that HSP90AA1 might be a key gene in the development of inflammation and Hsp90 inhibitors were considered to be potent inhibitors of the inflammatory response.35,36 STAT3 is reported to exert an important role in homeostasis of the oral mucosa by regulating the production of inflammatory cytokines and its polymorphism may be a novel risk factor for the occurrence of OM.37 MAPK3 is reported to play a vital role in controlling the magnitude of an autoimmune.38 ESR1 encodes ERα protein which regulates cells and pathways in the innate and adaptive immune system and exerts the inhibitory effects on inflammatory response through limiting NF-κB pathway.39 HIF1α can be activated by some metallic ions, nitrogen monoxide, and cytokines such as IL-1βand TNF-a to exert its biological effect. It is reported that the expression of HIF1α substantially enhanced in oral mucosa of irradiated rat.40 TLR4, an important pattern recognition receptor, is activated by LPS or DAMPs, which activates both innate and adaptive immune cells and leads to production of pro-inflammatory cytokines.41 Radiotherapy- and/or chemotherapy-induced oral damage was also shaped via TLRs pathway.42 mTOR is an atypical serine/threonine protein kinase in the phosphatidylinositol kinase related kinase (PIKK) protein family. It is reported that downregulation of the binding of mTOR and NLRP3 could counteract the intestinal inflammation.43,44 MMPs are involved in the pathogenesis of OM, mostly due to the fact that they trigger injury within the tissue of the submucosa and disrupt the integrity between the epithelium and the basal membrane.45 PIK3CA, a gene coding for the p110α catalytic subunit of PI3K, significantly increased tumor susceptibility in an oral carcinogenesis mouse model.46
Consist with these studies, results of current animal experiment showed that the expressions of SRC, HSP90AA1, STAT3, HIF1α, TLR4, mTOR and MMP9 were decreased after treating with SCYYD, while expression of ESR1 was increased, indicating the SCYYD exert the effect through multiple targets. All of these findings have demonstrated the close relationship between the key genes and OM, which facilitated to further explore the therapeutic mechanisms of SCYYD against OM.
Then we applied molecular docking to verify the specific interaction between the main ingredients and the core targets. The results demonstrated that the main ingredients in SCYYD had optimal binding activities to the key protein targets, suggesting the active compounds in SCYYD may exert therapeutic action of SCYYD against OM via related target and corresponding signaling pathways, which can be further studied to investigate the natural medicine for OM.
However, there are still some limitations in the present study. Firstly, the public databases we used for screening the active ingredients of SCYYD and target genes related to OM were imperfect, leading to some other compounds and target genes may not be included in this study. Moreover, further studies needed to be completed to validate other targets and signaling pathways of SCYYD acting on OM. Despite the limitations, this study preliminary uncovered the underlying mechanisms of SCYYD for OM, which provided basis for the further investigation of new anti-inflammatory drugs for OM.
Conclusion
In summary, we applied the network pharmacology, molecular docking and in vivo experiment to explore the underlying mechanisms of SCYYD in the treatment of OM. The results suggested that the SCYYD acted on multiple targets and various signaling pathways to exert therapeutic effect. Although further researches are needed to reveal the exact mechanism, this study systematically and comprehensively clarified the possible targets and signal pathways of SCYYD against OM, providing an experimental basis for clinical treatment of OM.
Ethical Approval
This study is involving human data from public databases GeneCards, OMIM and DisGeNET. Due to GeneCards, OMIM and DisGeNET belong to public databases and users can download relevant data for free for research and publish relevant articles, the ethics committee of Hangzhou Third People’s Hospital confirms that this study would have had the need for ethics approval waived.
Acknowledgments
This study was supported by Hangzhou Medical and Health Science and Technology Key Project (No. 0020190586); Zhejiang Famous Traditional Chinese Medicine Expert Inheritance Studio Construction Project Liu Yunxia Famous Traditional Chinese Medicine Expert Inheritance Studio (No. GZS2020030).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Peterson DE, Boers-Doets CB, Bensadoun RJ., et al. Management of oral and gastrointestinal mucosal injury: ESMO Clinical Practice Guidelines for diagnosis, treatment, and follow-up. Ann Oncol. 2015;26(Suppl 5):v139–51. doi:10.1093/annonc/mdv202
2. Rubenstein EB, Peterson DE, Schubert M, et al. Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer. 2004;100(9 Suppl):2026–2046. doi:10.1002/cncr.20163
3. Pulito C, Cristaudo A, Porta C, et al. Oral mucositis: the hidden side of cancer therapy. J Exp Clin Cancer Res. 2020;39(1):210. doi:10.1186/s13046-020-01715-7
4. Jung YS, Park EY, Sohn HO. Oral health status and oral health-related quality of life according to presence or absence of mucositis in head and neck cancer patients. J Cancer Prev. 2019;24(1):43–47. doi:10.15430/JCP.2019.24.1.43
5. Park JW, Oh J, Ko SJ, et al. Effects of Onchung-eum, an herbal prescription, on 5-fluorouracil-induced oral mucositis. Integr Cancer Ther. 2018;17(4):1285–1296. doi:10.1177/1534735418805560
6. Wang G, Jia L. Herb medicine for relieving radiation induced oral mucositis: a systematic review and meta-analysis protocol. Medicine. 2019;98(50):e18337. doi:10.1097/MD.0000000000018337
7. Sio TT, Le-Rademacher JG, Leenstra JL, et al. Effect of doxepin mouthwash or diphenhydramine-lidocaine-antacid mouthwash vs placebo on radiotherapy-related oral mucositis pain: the alliance A221304 randomized clinical trial. JAMA. 2019;321(15):1481–1490. doi:10.1001/jama.2019.3504
8. Meyer-Hamme G, Beckmann K, Radtke J, et al. A survey of Chinese medicinal herbal treatment for chemotherapy-induced oral mucositis. Evid Based Complement Alternat Med. 2013;2013:284959. doi:10.1155/2013/284959
9. Lin Z, Chen J, Han S. The efficacy of heat-clearing (Qingre) and detoxifying (Jiedu) traditional Chinese medicine gargle for chemotherapy-induced oral mucositis: a systematic review and meta-analysis. Front Pharmacol. 2021;12:627628. doi:10.3389/fphar.2021.627628
10. Geng QS, Liu RJ, Shen ZB, et al. Transcriptome sequencing and metabolome analysis reveal the mechanism of Shuanghua Baihe Tablet in the treatment of oral mucositis. Chin J Nat Med. 2021;19(12):930–943. doi:10.1016/S1875-5364(22)60150-X
11. Sheng P, Xie J, Wu Y, et al. A network pharmacology approach for uncovering the mechanism of ‘Kouchuangling’ in radiation-induced oral mucositis treatment. Comb Chem High Throughput Screen. 2022;25. doi:10.2174/1386207325666220617151600
12. Yunxia L, Yefeng X, Shuai L, et al. Clinical study on prevention and treatment of Sancao Yuyang Decoction on oral mucositis of patients with osteosarcoma after high-dose Methotrexate chemotherapy. China J Traditional Chine Med Pharm. 2019;34(10):4942–4946.
13. Zhang R, Zhu X, Bai H, et al. Network pharmacology databases for traditional Chinese medicine: review and assessment. Front Pharmacol. 2019;10:123. doi:10.3389/fphar.2019.00123
14. Li S, Zhang B. Traditional Chinese medicine network pharmacology: theory, methodology and application. Chin J Nat Med. 2013;11(2):110–120. doi:10.1016/S1875-5364(13)60037-0
15. Hsin KY, Ghosh S, Kitano H. Combining machine learning systems and multiple docking simulation packages to improve docking prediction reliability for network pharmacology. PLoS One. 2013;8(12):e83922. doi:10.1371/journal.pone.0083922
16. Tanideh N, Namazi F, Andisheh Tadbir A, et al. Comparative assessment of the therapeutic effects of the topical and systemic forms of Hypericum perforatum extract on induced oral mucositis in golden hamsters. Int J Oral Maxillofac Surg. 2014;43(10):1286–1292. doi:10.1016/j.ijom.2014.05.013
17. Takeuchi I, Kawamata R, Makino K, Rat A. model of oral mucositis induced by cancer chemotherapy for quantitative experiments. Anticancer Res. 2020;40(5):2701–2706. doi:10.21873/anticanres.14241
18. He S, Wang T, Shi C, et al. Network pharmacology-based approach to understand the effect and mechanism of Danshen against anemia. J Ethnopharmacol. 2022;282:114615. doi:10.1016/j.jep.2021.114615
19. Dong Y, Zhao Q, Wang Y. Network pharmacology-based investigation of potential targets of astragalus membranaceous-angelica sinensis compound acting on diabetic nephropathy. Sci Rep. 2021;11(1):19496. doi:10.1038/s41598-021-98925-6
20. Zhou W, Zhang H, Wang X, et al. Network pharmacology to unveil the mechanism of Moluodan in the treatment of chronic atrophic gastritis. Phytomedicine. 2022;95:153837. doi:10.1016/j.phymed.2021.153837
21. Obach RS, Baxter JG, Liston TE, et al. The prediction of human pharmacokinetic parameters from preclinical and in vitro metabolism data. J Pharmacol Exp Ther. 1997;283(1):46–58.
22. Kurano M, Hasegawa K, Kunimi M, et al. Sitosterol prevents obesity-related chronic inflammation. Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863(2):191–198. doi:10.1016/j.bbalip.2017.12.004
23. Patel K, Singh GK, Patel DK. A Review on Pharmacological and Analytical Aspects of Naringenin. Chin J Integr Med. 2018;24(7):551–560. doi:10.1007/s11655-014-1960-x
24. Zeng W, Jin L, Zhang F, et al. Naringenin as a potential immunomodulator in therapeutics. Pharmacol Res. 2018;135:122–126. doi:10.1016/j.phrs.2018.08.002
25. Calderón-Montaño JM, Burgos-Morón E, Pérez-Guerrero C, et al. A review on the dietary flavonoid kaempferol. Mini Rev Med Chem. 2011;11(4):298–344. doi:10.2174/138955711795305335
26. Devi KP, Malar DS, Nabavi SF, et al. Kaempferol and inflammation: from chemistry to medicine. Pharmacol Res. 2015;99:1–10. doi:10.1016/j.phrs.2015.05.002
27. Imran M, Rauf A, Shah ZA, et al. Chemo-preventive and therapeutic effect of the dietary flavonoid kaempferol: a comprehensive review. Phytother Res. 2019;33(2):263–275. doi:10.1002/ptr.6227
28. Gong JH, Shin D, Han SY, et al. Blockade of Airway Inflammation by Kaempferol via Disturbing Tyk-STAT Signaling in Airway Epithelial Cells and in Asthmatic Mice. Evid Based Complement Alternat Med. 2013;2013:250725. doi:10.1155/2013/250725
29. Zhang J, Hong Y, Liuyang Z, et al. Quercetin prevents radiation-induced oral mucositis by upregulating BMI-1. Oxid Med Cell Longev. 2021;2021:2231680. doi:10.1155/2021/2231680
30. Lotfi M, Kazemi S, Ebrahimpour A, et al. Protective effect of quercetin nanoemulsion on 5-Fluorouracil-induced oral mucositis in mice. J Oncol. 2021;2021:5598230. doi:10.1155/2021/5598230
31. Aziz N, Kim MY, Cho JY. Anti-inflammatory effects of luteolin: a review of in vitro, in vivo, and in silico studies. J Ethnopharmacol. 2018;225:342–358. doi:10.1016/j.jep.2018.05.019
32. Wang S, Cao M, Xu S, et al. Luteolin alters macrophage polarization to inhibit inflammation. Inflammation. 2020;43(1):95–108. doi:10.1007/s10753-019-01099-7
33. Reuter S, Gupta SC, Chaturvedi MM, et al. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49(11):1603–1616. doi:10.1016/j.freeradbiomed.2010.09.006
34. Kao TI, Chen PJ, Wang YH, et al. Bletinib ameliorates neutrophilic inflammation and lung injury by inhibiting Src family kinase phosphorylation and activity. Br J Pharmacol. 2021;178(20):4069–4084. doi:10.1111/bph.15597
35. Zhang H, Huang J, Fan X, et al. HSP90AA1 promotes the inflammation in human gingival fibroblasts induced by Porphyromonas gingivalis lipopolysaccharide via regulating of autophagy. BMC Oral Health. 2022;22(1):366. doi:10.1186/s12903-022-02304-0
36. Li F, Song X, Su G, et al. AT-533, a Hsp90 inhibitor, attenuates HSV-1-induced inflammation. Biochem Pharmacol. 2019;166:82–92. doi:10.1016/j.bcp.2019.05.003
37. Watanabe A, Yamamoto K, Ioroi T, et al. Association of single nucleotide polymorphisms in STAT3, ABCB1, and ABCG2 with Stomatitis in patients with metastatic renal cell carcinoma treated with sunitinib: a retrospective analysis in Japanese patients. Biol Pharm Bull. 2017;40(4):458–464. doi:10.1248/bpb.b16-00875
38. Bendix I, Pfueller CF, Leuenberger T, et al. MAPK3 deficiency drives autoimmunity via DC arming. Eur J Immunol. 2010;40(5):1486–1495. doi:10.1002/eji.200939930
39. Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 2015;294(2):63–69. doi:10.1016/j.cellimm.2015.01.018
40. Feng CJ, Guo JB, Jiang HW, et al. Spatio-temporal localization of HIF-1alpha and COX-2 during irradiation-induced oral mucositis in a rat model system. Int J Radiat Biol. 2008;84(1):35–45. doi:10.1080/09553000701616080
41. Firmal P, Shah VK, Chattopadhyay S. Insight into TLR4-mediated immunomodulation in normal pregnancy and related disorders. Front Immunol. 2020;11:807. doi:10.3389/fimmu.2020.00807
42. Ji L, Hao S, Wang J, et al. Roles of Toll-like receptors in radiotherapy- and chemotherapy-induced oral mucositis: a concise review. Front Cell Infect Microbiol. 2022;12:831387. doi:10.3389/fcimb.2022.831387
43. Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168(6):960–976. doi:10.1016/j.cell.2017.02.004
44. Cosin-Roger J, Simmen S, Melhem H, et al. Hypoxia ameliorates intestinal inflammation through NLRP3/mTOR downregulation and autophagy activation. Nat Commun. 2017;8(1):98. doi:10.1038/s41467-017-00213-3
45. Charbaji N, Rosenthal P, Schäfer-Korting M, et al. Cytoprotective effects of opioids on irradiated oral epithelial cells. Wound Repair Regen. 2013;21(6):883–889. doi:10.1111/wrr.12115
46. Du L, Chen X, Cao Y, et al. Overexpression of PIK3CA in murine head and neck epithelium drives tumor invasion and metastasis through PDK1 and enhanced TGFβ signaling. Oncogene. 2016;35(35):4641–4652. doi:10.1038/onc.2016.1
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.