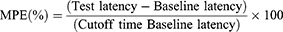Back to Journals » Journal of Experimental Pharmacology » Volume 15
Pharmacological Evaluation of the Anesthetic and Analgesic Potential of Injection Harsha 22: A Novel Polyherbal Local Anesthetic Formulation Intended for Parenteral Administration in Wistar Albino Rats
Authors Sasidharan S , Kaveri AN, Sithara MS, Nair J H
Received 21 December 2022
Accepted for publication 7 March 2023
Published 27 March 2023 Volume 2023:15 Pages 149—161
DOI https://doi.org/10.2147/JEP.S402277
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Shan Sasidharan,1,2 Asha Nair Kaveri,3 M S Sithara,2 Hareendran Nair J4
1Department of R&D, Pankajakasthuri Herbal Research Foundation, Thiruvananthapuram, Kerala, India; 2Small Animal Research Centre, Department of Toxicology and Pharmacology, CARe KERALA, Thrissur, Kerala, India; 3Department of Shalyatantra, Pankajakasthuri Ayurveda Medical College & P.G. Centre, Thiruvananthapuram, Kerala, India; 4Pankajakasthuri Herbal India Pvt. Ltd, Thiruvananthapuram, Kerala, India
Correspondence: Shan Sasidharan, Pankajakasthuri Herbal Research Foundation, Thiruvananthapuram, Kerala, India, Email [email protected]
Background: Local anaesthetics are medications that cause numbness that can be reversed by applying them topically. Local anaesthetics are clinically used to control pain during minor surgeries or to treat other acute and chronic pain. The present investigation intended to investigate the anesthetic as well as analgesic potential of Injection Harsha 22, a novel polyherbal formulation in Wistar albino rats.
Methods: The anesthetic potential of Injection Harsha 22 was evaluated by a heat tail-flick latency (TFL) test, whereas the analgesic effect was elevated by electrical stimulation testing. Here, lignocaine (2%) was used as the standard anesthetic drug.
Results: In TFL, Injection Harsha 22 showed anesthetic effects up to 90 minutes after application. Also, the duration of anesthesia in rats that were administered subcutaneously with Injection Harsha 22 was comparable to that of the rats treated with commercial lignocaine (2%). In an electrical stimulation test, single administration of Injection Harsha 22 to rats significantly prolonged analgesia compared with the normal control group. The median duration of analgesia in rats administered subcutaneously with Injection Harsha 22 and lignocaine solution was 40 minutes and 35 minutes, respectively. Furthermore, Injection Harsha 22 does not interfere with the hematopoietic system of the experiment animals.
Conclusion: Thus, the present investigation established the in vivo anesthetic and analgesic potential of Injection Harsha 22 in experimental animals. Hence, it can be concluded that Injection Harsha 22 can become a prominent substitute for lignocaine as a local anaesthetic agent after establishing its efficacy through stringent clinical trials in humans.
Keywords: anesthetic, analgesic, local anesthetic, polyherbal anesthetic formulation, Wistar rats
Introduction
One of the most dominant and challenging aspects of performing the various surgical procedures is pain control. Surgeons used anaesthetics to control pain to reduce the likelihood of experiencing discomfort or severe pain during restorative and surgical procedures.1 Hence, anesthetic drugs play a pivotal role in surgical procedures. In 2020, the Central Council of Indian Medicine issued a notification mandating that postgraduate students of two Ayurvedic programs (Shalya Tantra and Shalakya Tantra) be educated in multiple categories of modern surgeries as part of their curricula. This triggered a country-wide argument. Immediately, the modern doctors questioned how Ayurvedic surgeons would ensure anaesthesia for their patients and also handle complications during surgeries. The job of medicines is to help surgeries in three ways: anaesthesia, antibiotics and analgesics. Modern medicine performed admirably in this role, but not without disturbing and detrimental side effects associated with the medicines. Unfortunately, ayurvedic practitioners currently lack effective anaesthetic agents to carry out the surgical procedures safely and effectively. In the current scenario, ayurvedic professionals mandate a perfect, safe anaesthetic agent, as well as antimicrobial and wound healing drugs, in order to carry out surgeries smoothly. Regretfully, Ayurveda does not offer an ideal anaesthetic agent or antibiotics for performing the procedure effectively.2 Besides that, when performing surgeries, ayurvedic professionals mostly rely on modern medicine.
To overcome the aforementioned problem, the Pankajakasthuri Herbal Research Foundation launched a project to develop a novel herbal-based anaesthetic agent (injection and gel) as a supporting aid for Ayurvedic professionals to conduct surgical procedures. Through an intense research activity, Pankajakasthuri Herbal Research Foundation developed Injection Harsha 22, a polyherbal anesthetic formulation. The safety profile of the Injection Harsha 22 was established through the animal study.3
The Injection Harsha 22 was formulated using the hydroalcoholic extracts of herbs like Syzygium aromaticum, Myristica fragrans, Aconitum heterophyllum, Aconitum chasmanthum and Nardostachys jatamansi in injectable form. The essential oil from Syzygium aromaticum, one of the major ingredients in the anaesthetic formulation, is an effective, local and natural anaesthetic. In research studies, S. aromaticum oil was used to immobilize fish while managing, sorting, tagging, performing artificial reproduction procedures and surgery as well as to overwhelm sensory systems during various invasive procedures.4 M. fragrans essential oil is extremely effective in pain relief. Further, it is also reported to show anti-inflammatory and analgesic properties.5 In addition to this, it can be suggested as a relevant, economically advantageous and easily accessible anesthetic agent for fish.6 Furthermore, these two plants were rich with eugenol, which is well reported for its local anesthetic and analgesic activity. In addition to this, most of the ingredients used for the preparation of herbal anaesthetic are reported to have analgesic, antimicrobial and wound healing properties.
Aconitine is the primary bioactive and secondary metabolite alkaloid of Aconitum species, including A. heterophyllum and A. chasmanthum, and accounts for more than 60% of the total diester-diterpenoid alkaloids. Aconitine was tested in various pain models, and the results confirmed that it had significant analgesic effects in mouse pain models.7 In addition to this, intravenously administered Aconitum alkaloid samples to mice recorded significant anesthetic activity.8 N. jatamansi, a traditional medicinal plant frequently used to attenuate pain in Asia. (-)-Naringenin 4’,7-dimethyl ether, a bioactive compound isolated from Nardostachys jatamansi, was also reported to have significant analgesic activity in an animal experimental model.9
Thus, it is clear that the present formulation might have significant anesthetic as well as analgesic activity due to the rich blend of extracts from the above-mentioned herbs and can act as an ideal local anesthetic agent that will aid in performing mild surgeries and procedures. Furthermore, before proceeding with clinical studies, it is absolutely necessary to establish the preclinical information and potential of the formulation. Furthermore, in vivo efficacy data is absolutely essential for fulfilling all domestic and international regulatory guidelines regarding the product, which was intended for use in humans. As a result, the current study aimed to evaluate the anaesthetic and analgesic efficacy of Injection Harsha 22 in Wistar albino rats.
Materials and Methods
Materials
Lignocaine hydrochloride, the standard drug used in the study, was purchased from Ozone international, Mumbai. All the other chemicals and reagents used in this investigation were of analytical grade.
Collection and Authentication of Herbal Materials
The herbal materials selected for the present investigation, viz., S. aromaticum, M. fragrans, A. heterophyllum, A. chasmanthum, and N. jatamansi were collected from authorized dealers and identified and authenticated by Dr. R. Vijaya, Professor and Head, Department of Medicinal Plants, Pankajakasthuri Ayurveda Medical College and PG Centre, Killy, Kattakada, Trivandrum, Kerala, India.
Extraction of Herbal Materials
The air-dried, coarsely powdered S. aromaticum, M. fragrans, A. heterophyllum, A. chasmanthum, and N. jatamansi were separately subjected to maceration by placing in a stoppered macerating vessel with hydroalcoholic [water: ethanol (50:50)] as the extracting solvent. The extracts were separately weighed to obtain the extractive yield and stored in airtight bottles at 4°C for final formulation of test drug.
Extraction of Essential Oils
Essential oil from S. aromaticum was obtained using the steam distillation technique.
Preparation of Aseptic Area
Before the preparation of Injection Harsha 22, the walls and floor of the aseptic room were thoroughly washed with water, then disinfected by mopping with a 5% phenol solution. The bench was cleaned with 70% v/v isopropyl alcohol as well as sprayed in the atmosphere. The UV lights were switched on for 30 min prior to formulation and filling to the vial.
Formulation of Injection Harsha 22
The final formulation was strictly performed under an aseptic condition in a sterile cleanroom to avoid microbial contamination. First, each extract was carefully weighed and dissolved in sterile water for injection. This was subjected to homogenization in a high-speed homogenizer at 5000 rpm for 15 min, followed by 3000 rpm for 15 min in order to dissolve the extracts completely in the water. Next, it was subjected to ultrasonication for 75 minutes. The solution thus obtained was subjected to membrane filtration, first through a 0.45 μm membrane filter and then through a 0.22 μm membrane filter to obtain the “water phase”. Finally, oil phase (S. aromaticum oil) was added to the water phase and subjected to emulsification using a high-speed homogenizer. During the emulsification process, the first water phase was added to the homogenizer and subjected to thorough mixing at 700 rpm. Subsequently, the oil phase was added and allowed to mix completely with the water phase. Then, the mixing speed was increased to 1500 rpm to obtain creamy brown free-flowing liquid.
Aseptic Filtration
After formulation, Injection Harsha 22 was sterilized by filtration through 0.22 µm disposable membrane filter. The membrane filtration assembly fitted with the membrane filter was sterilized previously in the autoclave (Labline Pvt. Ltd., Delhi, India) at 121°C and 15 Lbs pressure for 15 minutes.
Aseptic Filling and Packing
After filtration, the preparations were filled with 2 mL volume in vials and packed by sterilised air tight rubber closure and sealed with sterile aluminium caps. The final packed vials were terminally sterilized by autoclaving at 121°C and 15 Lbs pressure for 15 minutes.
Sterility Testing
Sterility is the important parameter for parenteral drug; hence, we subjected the sterility test of Injection Harsha 22. Sterility testing must be performed for aerobic and anaerobic bacteria and fungi by using fluid thioglycolate medium and soya bean-casein digest media under aseptic conditions. The 1 mL sterile formulation was taken and diluted with 100 mL sterile water for injection. This was followed by the addition of 5 mL of solution to each medium and incubation for at least 14 days at 20–25°C in the fluid thioglycolate medium and at 20–25°C in the soybean casein digest medium to detect the growth of any microorganisms in the formulation.
Care, Maintenance and Ethical Approval
Wistar albino rats of both sexes, aged 8 to 12 weeks and weighing 200–250 g, were obtained from the in-house breeding centre of the small animal house of CARe KERALAM Ltd., Koratty, Thrissur, Kerala, India. The rats were acclimatized for 2 weeks under natural light–dark cycles. The rats were then housed in standard laboratory conditions, including an air-conditioned environment with adequate fresh air supply via the IVC system (air changes 15 per hour), a room temperature of 22 to 24°C, a relative humidity of 65–70%, and a 12-hour light/dark cycle. The temperature and relative humidity were documented daily. The experimental rats were fed ad libitum throughout the acclimatization and study periods. Pelletized laboratory rodent feed (produced by the Feed Plant of the School of Animal Nutrition and Feed Technology, Kerala Veterinary & Animal Sciences University, Kerala, India) was provided throughout the study period. The study proposal was endorsed by the Institutional Animal Ethics Committee (IAEC) of CARe KERALAM Ltd. [CKL/TOX/IAEC/2021-2/158].
Evaluation of the in vivo Anesthetic Effect Through Radiant Heat Tail-Flick Latency (TFL) Test
The in vivo anaesthetic effect of Injection Harsha 22 was assessed using the radiant heat tail-flick latency (TFL) test.10 Wistar Albino rats were placed in transparent polypropylene chambers without any pre-medication, and then the middle regions of their tails were exposed to a thermal source. TFL is the time lapse between the onset of stimulus application and the animals moving their tails away from the thermal source.11,12 TFL represents the rat’s sensitivity towards the temperature. In order to measure the local anesthetic effects on normal skin, treatments were administered subcutaneously to the rat tail by application of drug or vehicle solutions in the distal portion of the tail (3 cm).
Animals were treated in the following manner (4 animals in each group): Group 1 – Control, dose is 0.9% normal saline, 0.05 mL subcutaneous route; Group 2 – Test, dose is injection Harsha 22, 0.05 mL subcutaneous route; Group 3 – Standard, dose is standard lignocaine 2% w/v, 0.05 mL subcutaneous route.
Tail flick latencies were measured immediately following application and at 10-minute intervals using a Tail flick analgesiometer (Dolphin Pharmacy Instruments (p) Ltd.). Each rat’s baseline TFL was determined one day before the experiment. Animals with a basic value of 5 seconds were not included. Based on the red-hot appearance of the metal wire connecting the two electric terminals, the current intensity was set at 75. Injection Harsha 22 (Test), Lignocaine 2% (Standard), and 0.9% normal saline (Control) were applied in turn to the rats’ tails, which were placed in a perspex case, with the ventral surface of the distal 5–6 cm of the tail placed over a 0.5 cm hole, beneath which a heated wire was placed (thermal stimulus).
The maximum possible effects (MPEs) were calculated using the following formula:
The mean of three different measurements taken at 10-minute intervals was used to calculate the baseline latency.
Induction of Local Anaesthesia by Injection Harsha 22
Injection Harsha 22 was administered into the tail base, linea alba, left paralumbar and right paralumbar regions of the rats (Figure 1). To verify the local anaesthetic potential, each Injection Harsha 22 infiltrated site was pricked three times ten minutes after administration for pain sensation scoring using a numerical rank score.13
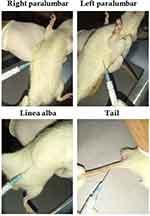 |
Figure 1 The four anatomical sites selected for administering the Injection Harsha 22. |
Analgesic Activity
The electrical stimulation testing was used to find out the analgesia in animals and humans during an in vivo analgesia duration test.14 Analgesia was tested using a current generator and a vocal response to electrical stimulation (starting at 1 mA and increasing to a maximum of 8 mA) at the skin area directly overlying the abdomen at the injection site. The experimental rats’ abdominal hair was shaved, and they were thoroughly screened preceding to injection of test drug to establish their vocalization threshold, or the current required to produce a vocalization response.
Animals were treated in the following manner: (6 animals in each group): Group 1 – Control, dose is 0.9% normal saline, 0.05 mL subcutaneous route; Group 2 – Test, dose is injection Harsha 22, 0.05 mL subcutaneous route; Group 3 – Standard, dose is local anesthetic lignocaine 2% injection, 0.05 mL subcutaneous route.
Injection Harsha 22, lignocaine (2%), and normal saline (0.9%) were injected subcutaneously on the abdomens of the experimental rats, and analgesia was determined at fixed time intervals (5, 10, 20, 30, 40, 50, 60, and 70 min). If all of the rats in one group did not vocalize in response to electrical stimulation at 2 mA above threshold, it indicates 100% analgesia; if half of the rats in one group vocalized in response to electrical stimulation, it indicates 50% analgesia; and if all of the rats in one group vocalized in response to electrical stimulation, it indicates 0% analgesia (no analgesic effect).
Hematological Index
For the haematological analyses, blood samples were collected from the retro-orbital sinus of the rats into clean test tubes containing EDTA 24 h after the completion of the study. The haematological index of rats was measured at the end of the drug administration period. Standard haematological and biochemistry tests were used to identify haematological parameters, enzymes, substrates, and metabolic products. Red blood cell count, white blood cell count, lymphocyte count, granulocyte count, haemoglobin, hematocrit, and platelet count were the indicators used to find out the hematological index of rats.
Serum Biochemical Index
Blood samples were collected from the retro-orbital sinus of the rats 24 h after the completion of the study, centrifuged for 10 minutes at 4000 rpm at 4°C, and stored at 20°C. The blood biochemical index of rats in each group was measured at the end of the drug administration period. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total protein, serum albumin, total bilirubin, urea, creatinine, uric acid, total protein, total cholesterol, triglyceride, HDL, VLDL, and LDL were the main biochemical indicators.
Statistical Analysis
The significance of the results was determined using one-way ANOVA with Dunnets post-test. The statistical significance level was set at p<0.05. GraphPad Prism, version 5.0, was used for all statistical analysis. The areas under the number of flinches against time curves (AUC) were calculated by the trapezoidal rule.
Results
Sterility Testing
The Injection Harsha 22 was evaluated for its sterility test by using fluid thioglycolate and soya bean casein digest medium to check the growth of any microorganism in the respective mediums after the incubation period. From the results, it was found that Injection Harsha 22 recorded no growth of any bacteria or fungi at the end of incubation period. Hence, all the formulations are sterile and found to be free from microorganisms and the results are shown in Table 1.
 |
Table 1 Sterility Test Result of Injection Harsha 22 |
Tail Flick Latency Test Demonstrated Excellent Anesthetic Effect of Injection Harsha 22
In vivo anesthetic effect of Injection Harsha 22 was studied in Wistar albino rats by Tail flick latency test. Tail flick latency obtained from control and treatment groups is shown in Figure 2. The highest reaction time for the Injection Harsha 22 and test group was 10.0 sec at 10 min, while it was 2.8 sec for control group. At all-time points, the tail-flick latency time of Harsha 22 and test group recorded almost similar reaction time. Rats treated with normal saline (negative control) did not show any significant difference in the reaction time on tail-flick throughout the 60 min observation. Also, the duration of anesthesia in rats that were administrated subcutaneously with Injection Harsha 22 was comparable to that of the rats treated with commercial 2% lignocaine (Figure 2).
 |
Figure 2 Tail flick latencies of rats after treatment with Injection Harsha 22 at different time intervals. Here Control: Normal saline, Test- Injection Harsha 22 and Standard- Lignocaine. |
Figure 3A depicts the %MPE and total anesthesia, given by the area under the curve for each group (Figure 3B). The findings indicated that subcutaneous administration of Injection Harsha 22 on rat tail led to significant increase in TFL (p<0.001) when compared to the rats in the control group. Injection Harsha 22 had an anaesthetic effect up to 90 minutes after application. The % MPE in the test group increased rapidly and then progressively recovered to its baseline level, as in the control group. In contrast, the % MPE in the control group remained nearly stable. In both the %MPE and area under the curve parameters, the Injection Harsha 22 displayed an anesthetic effect comparable to that of standard 2% lignocaine. The AUC for all treatment groups was statistically higher (p<0.001) than for the normal control group. Up (Figure 3B). No statistically significant difference was recorded in the AUC between Injection Harsha 22 and standard lignocaine used in the present investigation.
Local Anesthetic
Table 2 shows the pain sensation scoring used to assess the local anaesthetic potential of Injection Harsha 22. Loss of pain sensation that lasted for 1 h was significantly higher (p < 0.05) at all four anatomical sites: the tail, linea alba, left paralumbar and right paralumbar regions, in comparison with the pre-treatment sensation test.
Analgesic Activity
The outcome of in vivo analgesia duration using electrical stimulation test in rats is depicted in Figure 4. The results clearly indicated that a single administration of Injection Harsha 22 to rats provided significantly prolonged analgesia compared with the normal control group. The analgesic effect of Injection Harsha 22 was evident within 5 min following subcutaneous administration. In addition to this, the effect reached maximum at 5 min (100%) and remain constant up to 30 min. The lignocaine also showed similar analgesic effect, beginning at 5. For Injection Harsha 22, the analgesic effect gradually decreased towards 40 min (50%). With reference to MPA value, the Injection Harsha 22 demonstrated stronger analgesic activity than the standard lignocaine.
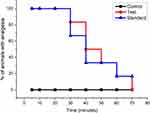 |
Figure 4 Percentage (%) of animals with analgesia after treatment. Here Control: Normal saline, Test- Injection Harsha 22 and Standard- Lignocaine. |
The median duration of analgesia in rats administered with subcutaneous injections of Injection Harsha 2 and lignocaine 2% solution was 40 minutes and 35 minutes, respectively. The percentage of animals with analgesia was evaluated by measuring vocal response to the electrical stimulation and it showed a significant difference (p<0.05) among the Injection Harsha 2 (test) and lignocaine (standard) group rats when compared with the rats in the normal control group. However, there was no significant difference in vocalization response among the test group animals when compared with standard drug treated animals.
Hematological and Serum Biochemistry Parameters
There were no significant differences in haematological and serum biochemical parameters between Injection Harsha 22 treated and control rats (Tables 3–5). All the values in the treatment group as well as the control group were within the normal range.
 |
Table 3 Effect of Injection Harsha 22 on the Hematological Parameters in Experimental Groups |
 |
Table 4 Effect of Injection Harsha 22 on the Serum Lipid Profile |
 |
Table 5 Effect of Injection Harsha 22 on the Serological Parameters in Experimental Groups |
Discussion
Local anesthetics are used by many healthcare professionals, including anesthesiologists, primary care providers, emergency department physicians, surgeons to treat painful conditions, avoid pain during a surgical procedure or operation, or relieve pain after surgery. Mepivacaine, lidocaine, etidocaine, bupivacaine, and other local anaesthetics are commonly used in clinical practice by modern medicine. Unfortunately, ayurvedic professionals lack effective anesthetic and analgesic agents to carry out mild surgical procedures. As a result, our R&D team initiated a project to develop a polyherbal anaesthetic agent that would make it easier for Ayurvedic professionals to perform surgical procedures.
The present polyherbal anesthetic agent, Injection Harsha 22 was formulated using S. aromaticum, M. fragrans, A. heterophyllum, A. chasmanthum and N. jatamansi. From the literature review, it was clear that most of the herbs selected for the formulation had been reported for anesthetic and analgesic activity earlier. The major ingredient used for the formulation was S. aromaticum which had been reported earlier for anesthetic and analgesic activity.15,16 Eugenol, the major compound present in S. aromaticum, is also reported to have anesthetic and analgesic activity.17–19 Another major ingredient in the formulation was M. fragrans which was also reported to have anesthetic properties.6 Eugenol is also a major phytochemical present in M. fragrans. Aconitine, major compound present in both A. heterophyllum and A. chasmanthum, was also reported to have anesthetic and analgesic activities.7
Local anesthetics derived from plant alkaloid have been successfully used in a wide range of situations to relieve and control pain. The most widely recognized mode of action for local anesthetics is the interaction with voltage-gated Na+ channels to inhibit sensory and motor functions.20 Anesthetic molecules penetrate through the lipid barriers of nerve sheaths and diffuse across the lipid bilayers of cell membranes so that they access the intracellular or cell-interior binding sites on Na+ channels embedded in membranes. Local anesthetics also diffuse into lipid bilayers and act on membrane-constituting lipids, modifying the physicochemical properties of neuronal and cardiomyocyte membranes.21 Unlike conventional local anesthetics to act on Na+, K+ and Ca2+ channels, neurotoxins specifically block voltage-gated Na+ channels,22 so they should be an ideal local anesthetic. Natural neurotoxins, many of which belong to plant alkaloids, potentially produce long-duration local anesthesia. Since plant the medicinal plants selected for the formulation of Injection Harsha rich with terpenoids, alkaloids and flavonoids including eugenol, aconitine have amphiphilic structures as well as local anaesthetics, these phytochemicals are expected to affect the activity of voltage gated Na+ channels through the common mechanisms.
Following the formulation of any herbal anesthetics, they should be tested in vivo to determine their efficacy in experimental animals. Numerous procedures are employed to study the outcome of local anesthetic formulations, which diverge in their clinical efficacy and durations.23 Evaluating the efficacy of local anaesthetics requires the ability to precisely detect the quantum of pain relief attained after in vivo electrical stimulation, a method widely used to assess the pain after applying any kind of anesthetic agent. Pain perception is made up of two major elements: sensory and reactive. The sensory component is proportional to the nociceptive stimulus intensity. The affective, emotional responses to painful sensations are referred to as the reactive component. Infringement stimulation uses a variety of methods, including pinprick and forceps pinch, but the intensity of these methodologies is not always consistent.24 Furthermore, they may cause skin damage in the animal, intern leads difficulty in evaluating the anesthetic potential of the test drugs. In pain research, somatomotor signs in animals such as tail flick and autonomic disturbances elicited by a nociceptive stimulus are thought to be indicators of “pain”.25 The TFL is the most common perspective technique for assessing the severity of acute or chronic pain.26 It employs radiant heat to assess the pain threshold and the effect of anaesthesia as well as to quantify the latency of the response to thermally noxious stimuli. Radiant heat is a type of constant infringement stimulation that does not harm the skin when a proper cut-off time is used.27,28
The present study demonstrated that subcutaneous application of Injection Harsha 22 on rat tails led to a noteworthy upsurge in tail flick latency when compared to the rats in the control group and anesthetic effect persisted up to 90 minutes after application. The % MPE in the test group increased speedily and then progressively returned to the baseline level. Also, the treatment with the Injection Harsha 22 had a statistically higher AUC than the normal control group. There was no statistically significant difference in the anesthetic effect between Injection Harsha 22 and standard lignocaine used.
Despite their ease of observation, most rodent somatomotor reflexes are poor predictors of the emotional component of pain perception. The nociceptive stimulus-induced vocalization response is an effective measure of the affective component of pain perception in animals.26–29 Vocalization is a distinct behavior that can be induced by a wide range of suprathreshold stimuli. Previous investigations mainly aimed at assessing the pharmacological parameters of pains especially in rodents used the vocalization response as a parameter to improve the sensitivity of pain measurement tests.26,30 The vocal communication response in mice has been employed for the pharmacological assessment of the analgesic effects of systemically administered drugs.26 In 1952, the method of applying electrical stimuli to mice’s tails and the use of “squeaking” as a nociceptive marker were described to evaluate the analgesic effect of any test materials.26 Grant et al demonstrated that the rodent vocalization response and subcutaneous injection of test drugs on the abdomen can be used to assess the pharmacological properties of local anesthetic agents.14 Furthermore, this methodology is a simple, dependable, and cost-effective method for evaluating local anaesthetics in vivo, and it has the potential to be an important tool in the development of newer, safer, and more effective local anaesthetic formulations.
The outcomes from the current investigation recorded a noteworthy increase in the analgesia duration among the test and standard treatment groups when related to the control (no treatment) group. There were no significant changes in analgesia duration between the Injection Harsha 22 and lignocaine group.
The hematopoietic system is an important target for evaluating plant extract toxicity in animal models.30 Toxins associated with the test drug can harm this system through indirect pathways, such as oxidative hemolysis in the circulation and immunotoxic reactions with blood components.31 In our study, the values obtained from the hematological and serum biochemical parameters of Injection Harsha 22 and control rat were similar. This clearly indicated that Injection Harsha 22 did not interfere with the hematopoietic system. In addition to this, anemia, infection, and/or immune system dysfunction are examples of conditions that could affect the normal values of hematopoietic system.32 Because none of these conditions were recorded in the study, the Injection Harsha 22 can be concluded to be safe for haematological parameters when used within this time frame.
Conclusion
The results of the radiated heat tail-flick latency (TFL) test and the in vivo analgesia duration test with electrical stimulation indicated that the test material, Injection Harsha 22, had comparable local anaesthetic activity to that of a commercially available lignocaine 2% formulation. Hence, it can be concluded that Injection Harsha 22 will become a prominent substitute for lignocaine after establishing its efficacy through stringent clinical trials in humans. Since this formulation is purely of herbal origin, it will be a great asset for the Ayurvedic professional in carrying out the surgical procedures recommended by the Central Council of Indian Medicine.
Data Sharing Statement
All data pertaining to this study are within the paper.
Ethical Approval
The Institutional Animal Ethics Committee (IAEC) of CARe KERALAM Ltd. examined and approved the application with the approval number [CKL/TOX/IAEC/2021-2/158]. The experimental rats were cared for and used in accordance with OECD guidelines for laboratory animal care and use.
Acknowledgment
The authors express their gratitude to “Aadhyathma Chinthalayesan” of Chinthalaya Ashram, Pothencode, Trivandrum, Kerala, India, for his compassion and blessings. We are grateful to Pankajakasthuri Herbal Research Foundation, Kattakada, Thiruvananthapuram, Kerala, India, and Pankajakasthuri Herbals India Pvt. Ltd Poovachal, Kattakada, Thiruvananthapuram, Kerala, India, for helping us with the research. We would also like to thank all of the Directors and Employees of Pankajakasthuri Herbal Research Foundation and Pankajakasthuri Herbals India Pvt. Ltd for their assistance in the completion of this project. The authors would like to thank CARe KERALAM Ltd. in Koratty, Thrissur, Kerala, India, for providing the necessary infrastructure for the animal experiments.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Disclosure
The authors disclosed no conflicts of interest in this work.
References
1. Abebe MM, Arefayne NR, Temesgen MM, Admass BA. Evidence-based perioperative pain management protocol for day case surgery in a resource limited setting: systematic review. Ann Med Surg. 2022;80:104322. doi:10.1016/j.amsu.2022.104322
2. Yadav S, Jain S, Chaudhary J, Bansal R, Sharma M. The role of Ayurveda management in preventing surgical site infections instead of surgical antibiotic prophylaxis. J Ayurveda Integr Med. 2017;8(4):263–265. doi:10.1016/j.jaim.2017.03.003
3. Sasidharan S, Kaveri Nair A, Sithara MS, Hareendran Nair J. A study on the acute dermal irritation of a novel polyherbal anesthetic formulation for parenteral administration in Wistar albino rats. Pharm Pharmacol Int J. 2022;10(5):
4. Javahery S, Nekoubin H, Moradlu AH. Effect of anaesthesia with clove oil in fish (review). Fish Physiol Biochem. 2012;38(6):1545–1552. doi:10.1007/s10695-012-9682-5
5. Zhang WK, Tao SS, Li TT, et al. Nutmeg oil alleviates chronic inflammatory pain through inhibition of COX-2 expression and substance P release in vivo. Food Nutr Res. 2016;60:30849. doi:10.3402/fnr.v60.30849
6. Al-niaeem KS, Mohammed FA, Al-Hamadany QH. The anaesthetic effect of nutmeg powder, Myrisitca fragrans on young common carp, Cyprinus carpio. Biol Appl Environ Res. 2017;2:279–286.
7. Deng J, Han J, Chen J, et al. Comparison of analgesic activities of aconitine in different mice pain models. PLoS One. 2021;16(4):e0249276. doi:10.1371/journal.pone.0249276
8. Gu RR, Meng XH, Zhang Y, et al. (-)-naringenin 4’,7-dimethyl ether isolated from Nardostachys jatamansi relieves pain through inhibition of multiple channels. Molecules. 2022;27(5):1735. doi:10.3390/molecules27051735
9. Kekuda PTR, Sudharshan SJ, Chinmaya A, Valleesha NC, Murthuza S, Rajeshwara Achur N. Central nervous system (CNS) depressant and Analgesic activity of methanolic extracts of Nardostachys jatamansi DC and Coscinium fenestratum Colebr. in experimental animal model. J Pharm Res. 2009;2(11):1716.
10. You P, Yuan R, Chen C. Design and evaluation of lidocaine- and prilocaine-coloaded nanoparticulate drug delivery systems for topical anesthetic analgesic therapy: a comparison between solid lipid nanoparticles and nanostructured lipid carriers. Drug Des Devel Ther. 2017;11:2743–2752. doi:10.2147/DDDT.S141031
11. Li Z, Chai Y, Gong C, Du G, Liu J, Yang J. Basic evaluation of the antinociceptive effects of lidocaine and bupivacaine on the tail nerves of healthy rats. Clin Pharmacol Toxicol. 2013;113:31–36. doi:10.1111/bcpt.12061
12. Li A, Yang F, Xin J, Bai X. An efficient and long-acting local anesthetic: ropivacaine-loaded lipid-polymer hybrid nanoparticles for the control of pain. Inter J Nanomed. 2019;14:913–920. doi:10.2147/IJN.S190164
13. Flecknel PA. The relief of pain in laboratory animals. Lab Anim. 1984;18(2):147–160. doi:10.1258/002367784780891226
14. Grant GJ, Piskoun B, Lin A, Bansinath M. An in vivo method for the quantitative evaluation of local anesthetics. J Pharmacol Toxicol Methods. 2000;43(1):69–72. doi:10.1016/S1056-8719(00)00079-4
15. Batiha GE, Alkazmi LM, Wasef LG, Beshbishy AM, Nadwa EH, Rashwan EK. Syzygium aromaticum L. (Myrtaceae): traditional uses, bioactive chemical constituents, pharmacological and toxicological activities. Biomolecules. 2020;10(2):202. doi:10.3390/biom10020202
16. Cortés-Rojas DF, de Souza CR, Oliveira WP. Clove (Syzygium aromaticum): a precious spice. Asian Pac J Trop Biomed. 2014;4(2):90–96. doi:10.1016/S2221-1691(14)60215-X
17. Saydmohammed M, Pal AK. Anesthetic effect of eugenol and menthol on handling stress in Macrobrachium rosenbergii. Aquaculture. 2009;298:162–167. doi:10.1016/j.aquaculture.2009.10.020
18. Daniel AN, Sartoretto SM, Schmidt G, et al. Anti-inflammatory and antinociceptive activities of eugenol essential oil in experimental animal models. Rev Bras Farmacogn. 2009;19:212–217. doi:10.1590/S0102-695X2009000200006
19. Guenette SA, Beaudry F, Marier JF, Vachon P. Pharmacokinetics and anesthetic activity of eugenol in male Sprague-Dawley rats. J Vet Pharmacol Ther. 2006;29:265–270. doi:10.1111/j.1365-2885.2006.00740.x
20. Tsuchiya H. Anesthetic agents of plant origin: a review of phytochemicals with anesthetic activity. Molecules. 2017;22:1369. doi:10.3390/molecules22081369
21. Tsuchiya H, Mizogami M. Interaction of local anesthetics with biomembranes consisting of phospholipids and cholesterol: mechanistic and clinical implications for anesthetic and cardiotoxic effects. Anesthesiol Res Pract. 2013;2013:297141. doi:10.1155/2013/297141
22. Vadhanan CP, Narendren G. Future local anesthetics—neurotoxins? Int J Anesthesiol Res. 2014;2:11–15. doi:10.14205/2310-9394.2014.02.01.3
23. Becker DE, Reed KL. Local anesthetics: review of pharmacological considerations. Anesth Prog. 2012;59(2):90–103. doi:10.2344/0003-3006-59.2.90
24. Wang CF, Gerner P, Schmidt B, et al. Use of bulleyaconitine A as an adjuvant for prolonged cutaneous analgesia in the rat. Anesth Analg. 2008;107:1397–1405. doi:10.1213/ane.0b013e318182401b
25. Ramabadran K, Bansinath M. Evaluation of analgesic actions of morphine in various pain models in experimental animals. In: Kapoor LD, editor. Opium Poppy: Botany, Chemistry and Pharmacology. New York: The Haworth Press; 1995:255–298.
26. Ouchi K, Sekine J, Koga Y, Nakao S, Sugiyama K. Establishment of an animal model of sedation using epidural anesthesia that uses the tail-flick test for evaluating local anesthetic effects in rats. Exp Anim. 2013;62(2):137–144. doi:10.1538/expanim.62.137
27. Yang Y, Qiu D, Liu Y, Chao L. Topical anesthetic analgesic therapy using the combination of ropivacaine and dexmedetomidine: hyaluronic acid modified long-acting nanostructured lipid carriers containing a skin penetration enhancer. Drug Des Devel Ther. 2019;13:3307–3319. doi:10.2147/DDDT.S211443
28. Jackson KJ, Carroll FI, Negus SS, Damaj MI. Effect of the selective kappa-opioid receptor antagonist JDTic on nicotine antinociception, reward, and withdrawal in the mouse. Psychopharmacol. 2010;210(2):285–294. doi:10.1007/s00213-010-1803-1
29. Gregory NS, Harris AL, Robinson CR, et al. An overview of animal models of pain: disease models and outcome measures. J Pain. 2013;14(11):1255–1269. doi:10.1016/j.jpain.2013.06.008
30. Jourdan D, Ardid D, Chapuy E, et al. Audible and ultrasonic vocalization elicited by a nociceptive stimulus in rat: relationship with respiration. J Pharmacol Toxicol Method. 1997;38(2):109–116. doi:10.1016/S1056-8719(97)00067-1
31. Ashafa AO, Yakubu MT, Grierson DS, Afolayan AJ. Toxicological evaluation of the aqueous extract of Felicia muricata Thunb. leaves in Wistar rats. Afri J Biotechnol. 2009;8:42–48.
32. Glatman Zaretsky A, Engiles JB, Hunter CA. Infection-induced changes in hematopoiesis. J Immunol. 2014;192(1):27–33. doi:10.4049/jimmunol.1302061
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.