Back to Journals » Drug Design, Development and Therapy » Volume 14
Pharmacological Activity and Mechanism of Tanshinone IIA in Related Diseases
Authors Guo R , Li L, Su J, Li S, Duncan SE, Liu Z, Fan G
Received 26 June 2020
Accepted for publication 24 September 2020
Published 5 November 2020 Volume 2020:14 Pages 4735—4748
DOI https://doi.org/10.2147/DDDT.S266911
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Rui Guo,1,2 Lan Li,1,2 Jing Su,1 Sheng Li,1 Sophia Esi Duncan,1 Zhihao Liu,1,2 Guanwei Fan1,2
1Tianjin Key Laboratory of Translational Research of TCM Prescription and Syndrome, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, People’s Republic of China; 2School of Integrative Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin, People’s Republic of China
Correspondence: Guanwei Fan
First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, No. 88, Changling Road, Liqizhuang Street, Xiqing District, Tianjin, People’s Republic of China
Tel +8613752503396
Email [email protected]
Abstract:
Salvia miltiorrhiza: (Danshen) is a significant (traditional Chinese medication) natural remedy, enhancing blood circulation and clear blood stasis. In this view, it is widely used against several heart diseases, eg, cardiomyopathy, arrhythmia, and congenital heart defects. Tanshinone IIA (tan-IIA) is the main fat-soluble component of Salvia miltiorrhiza. Modern pharmacological study shows that tan-IIA has anti-inflammatory and anti-oxidant activities. Tan-IIA induces remarkable cardioprotective effects via enhancing angiogenesis which may serve as an effective treatment against cardiovascular diseases (CVD). There is also evidence that tan-IIA has extensive immunomodulatory effects and plays a significant role in the development and function of immune cells. Tan-IIA reduces the production of inflammatory mediators and restores abnormal signaling pathways via regulating the function and activation of immune cells. It can also regulate signal transduction pathways, ie, TLR/NF-κB pathway and MAPKs/NF-κB pathway, thereby tan-IIA has an anti-inflammatory, anticoagulant, antithrombotic and neuroprotective role. It plays a protective role in the pathogenesis of cardiovascular disorders (ie, atherosclerosis, hypertension) and Alzheimer’s disease. It has also been revealed that tan-IIA has an anti-tumor role by killing various tumor cells, inducing differentiation and apoptosis, and has potential activity against carcinoma progression. In the review of this fact, the tan-IIA role in different diseases and its mechanism have been summarized while its clinical applications are also explored to provide a new perspective of Salvia miltiorrhiza. An extensive study on the mechanism of action of tan-IIA is of great significance for the effective use of Chinese herbal medicine and the promotion of its status and influence on the world.
Keywords: tanshinone-IIA, atherosclerosis, Alzheimer’ disease, cancer, anti-inflammation, anti-oxidative
Introduction
Salvia miltiorrhiza (named Danshen in Chinese) is a Chinese herbal remedy, also known as Chinese sage, composed of dried rhizomes and roots of Salvia miltiorrhiza Bge1 which was first published in Shennong Bencaojing. It has the effects of relieving pain, promoting blood circulation, and removing blood stasis.2 Modern pharmacological research has found that Salvia miltiorrhiza has dilated coronary artery,3 prevents myocardial ischemia,4 myocardial infarction5 improves microcirculation,6 and reducing myocardial oxygen consumption.7 In Asian countries, for hundreds of years, Salvia miltiorrhiza is widely used against various heart complications.8 A large number of experimental and clinical studies have reported that Salvia miltiorrhiza, whether it is the original drug or prepared (Danshen injection),9 Danshen dropping pills, Danhong injection and Danshen Gegen soup are beneficial to the heart during pathological processes, such as myocardial ischemia,10 myocardial infarction, reperfusion injury11 and so on.
Research on the chemical constituents of Salvia miltiorrhiza has been in place since the 1930s.12 Since then, many researchers have focused on separating and identifying components from this plant. At present, more than 200 compounds have been identified from Salvia miltiorrhiza Bge,13 according to the Chinese Academy of Sciences Chemical Database (www.organchem.csdb.cn) and the Chinese herbal medicine database.14 Tan-IIA is a representative of the fat-soluble15 component of Salvia miltiorrhiza (as shown in Figure 1), and other tanshinones and the hydrophilic component of Salvia miltiorrhiza (salvianolic acids) also play important roles in the pharmacological activities of danshen in treating various diseases. The terpenoids are easily reduced to diphenol derivatives,16 which are then oxidized17 and easily converted.18 Quinone compounds play a role in the transmission of electrons,19 as a product of metabolism of organisms,20,21 exhibits a variety of biological activities by promoting or interfering with various biochemical reactions of organisms,22 and promotes certain biochemical reactions as coenzymes23 for biological reactions.22 Or interfere with the action,24 thus showing a variety of pharmacological effects, such as anti-atherosclerosis,25 anti-myocardial ischemia,26 anti-arrhythmia,27 repair of vascular endothelial cells,28 improve coronary blood circulation,29 anti-cardiac hypertrophy, and anti-tumor (as shown in Figure 2).
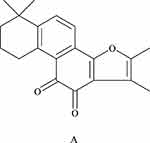 |
Figure 1 The chemical structures of the major lipophilic components (tanshinone-IIA) are shown in A. |
 |
Figure 2 Pharmacological activity And effects of tanshinone-IIA. |
Over the past few years, the compounds (particularly tan-IIA) isolated from the Salvia miltiorrhiza have been evaluated for their biological activities. Numerous proposed mechanisms have been reported on the role of tan-IIA in cardiovascular protection, including apoptosis and autophagy, anti-inflammatory,30 antioxidant, antithrombotic, anti-proliferation31 of vascular smooth muscle cells, inhibition of expression of vascular endothelium32 and leukocyte adhesion molecules,33 and improve acute myocardial ischemia.33 At the same time, studies have found that tan-IIA has a significant contribution to the activation, development, and proper functioning of immune cells. Tan-IIA is involved in both the innate and the acquired immune response which facilitates all stages of inflammatory pathways (from initiation to progression). Despite the in-depth study of the pharmacological effects of tan-IIA on cardiovascular system in-vitro, in-vivo, and clinical trials, its mechanistic target is remaining elusive. This article briefly reviews the research status of the pharmacological effects of tan-IIA in cardiovascular diseases and provides a reference for clinical rational drug use.
Tanshinone IIA Has a Significant Contribution to the Development, Activation, and Function of Immune Cells
Function and Activation of Immune Cells
Dendritic Cells
Dendritic cells (DC) are the largest antigen-presenting cells (APC) in the body. These cells induce the innate immune responses via presenting antigens to thymus-dependent lymphocytes (T cells).34 During the up-regulation of antigen-presenting molecules (ie, MHCI, MHC II, and CD1), CAMs (cellular adhesion molecules ie, CD54, CD58, CD11a/CD18, and CD50) and costimulatory molecules (ie, CD40, CD80, and CD86),35 due to endocytosis and antigen processing along with the weakening capacity, DC gradually matures, and then the ability of antigen presentation to T cells is significantly enhanced. Therefore, tan-IIA weakens the developmental process of immune system disorders by inhibiting the adaptive immunity, mediated by dendritic cells. A study confirms that tan-IIA has the potency to enhance the CD4(+) T cells polarization into Treg cells via DCs targeting to induce the upregulation of TGF-β1 (transforming growth factor β1) as well as via in-vitro naïve CD4(+) T cells direct targeting. Additionally, tan-IIA has a considerable role against neuroinflammation and may serve as a candidate therapeutic approach to cure the neuroinflammatory disorders eg, optic neuritis, transverse myelitis, and neuromyelitis optica.36 Li et al reported that tan-IIA attenuated CD86 expression on DC, reduced CD54 and MHC II expression level, recovered the capacity for endocytosis, and overcome the inflammatory cytokines (eg, IL-12 and IL-1) secretion as well in dose-dependent manner.37 Because of the underlined significant activities of tan-IIA, it may serve as an effective therapeutic approach against the progression of the atherosclerotic lesion.
T Lymphocytes or T Cells
T lymphocytes or T cells consider the principal lymphocytes components, associated with a variety of biological responses eg, inhibiting B cells from antibodies production, cytokines production, target cell killing, and specific response to mitogens and antigens.38 Cellular immunity is dependent on T cells immune response production. Cellular immunity is comprised of two types: specific binding and destructing the plasma membrane of target cells via forming a transmembrane pore, and direct killing of target cells.39 Most of the activated CD4(+) T cells can stimulate the aggregation of cytokines, and secrete the IL-12, IFN-γ (interferon-γ) and other pro-inflammatory cytokines that ultimately resulting in inflammation.40 According to the reported studies,41 tan-IIA elevates the level of anti-inflammatory cytokines, such as IL-10, while decreases the level of inflammatory cytokines, such as IL-2, IL-4. It has also been reported that tan-IIA elevates the level of T cell subsets such as CD3(+), CD4(+), and CD8(+). In view of this fact, tan-IIA may work as a lead compound for the development of effective medical treatment against liver laceration (liver injury). Yan et al studied42 that tan-IIA is associated with the downregulation of serum and brain IL-17 and IL-23 in the experimental autoimmune encephalomyelitis (EAE) rats. They also studied that this compound can lower the amount of CD4(+) T cells, CD8(+) T cells, and microglia and macrophages in the spinal cord. Their results confirm that tan-IIA relieves EAE and supports its use as a new treatment for multiple sclerosis.
Production of Cytokines
Cytokines (CK) are low-molecular-weight soluble proteins that are induced by immunogens, mitogens, or other stimulants to produce a variety of cell features.43 In the development of inflammation, cytokines show a very important role, mainly the relationship between pro-inflammatory and anti-inflammatory cytokines44 that promote each other and restrict each other. The dynamic changes of the two cytokines determine the development and outcomes of inflammation. A pleiotropic cytokine ie, IL-6 has dual effects of pro-inflammatory as well as anti-inflammatory effects.45 A reported study46 demonstrated that in the streptozotocin (STZ) rat model of DPNP, tan-IIA had efficient antihyperalgesic and antiallodynic abilities. This compound elevated the level of IL-10 (a cytokine with potent anti-inflammatory efficiency) and had an inhibitory potential against proinflammatory cytokines such as IL-1β, IL-6, and TNF-α. Based on these remarkable effects, tan-IIA might be used as a leading anti-inflammatory drug. IL-8 and monocyte chemoattractant protein 1 (MCP-1) are chemokines that induce monocyte adhesion to endothelial cells. IL-8 may attract T cells and cause smooth muscle cell (SMC) proliferation and migration. At the same time, MCP-1 can stimulate local infiltration, aggregation, and monocytes/macrophages proliferation.47
The data revealed that the tan-IIA analgesic effects in neuropathic pain are mostly activated by down-regulating SNL-induced astrocytic activation through blockage of JNK/MCP-1 cascade. MMP activation causes degradation of the extracellular matrix which results in the disruption of the fibrous cap and finally leads to the instability and rupturing of plaque. Among the subtypes of collagenases, type-IV collagenases, such as MMP-2 and MMP-948 have a role in local inflammatory cell infiltration in plaques. In plaques, the cell’s infiltration is the important element that causes injury to the walls of blood vessels and reduced the intima defense functions. Based on the results of Zhou et al, tan-IIA can decrease the expression level of MMP-9 and MMP-2 and inhibit the expression of p50 and p65. So, the migration and invasive ability of HNE-1NPC cells are blocked via tan-IIA by decreasing the expression level of matrix metalloproteinase.
Inflammation-Related Signal Transduction Pathway
Toll-Like Receptor/NF-κB Pathway
The Toll-like receptor (TLR) family is the main receptor for host cells to recognize various microbial pathogenic components. NF-κB is situated at the hub of the downstream signaling cascade of TLR. When biological stress activates cells, as a result, NF-κB is stimulated which translocates into the nucleus. In the nucleus, it regulates the expression level of inflammatory cytokines such as MCP, and cell adhesion molecules eg, ICAM-1and VCAM-1, and also initiate innate and acquired immune responses against pathogenic microbes47,49 (Figure 3). The downstream signaling pathways of TNF-α and TLR4/NF-κB are critical for the inflammatory signaling cascades.50 The TNF-α is an inflammatory cytokine and its expression level reflects the pathogenesis and severity of the inflammatory process. According to Meng et al,51 in-vitro study, in VSMCs, LPS‑induced inflammatory responses were found to be inhibited via tan-IIA by partially suppressed TLR4/TAK1/NF‑κB signaling cascade. The results showed that by using tan‑IIA, the neuronal TLR‑4 and NF‑κB expression levels were found to be reduced, while inflammatory cytokines production and neuronal oxidative stress levels were found to be elevated.52 Given these results, tan‑IIA is a significant approach for enhancing HIE through NF‑κB signaling mediated via TLR‑4. Du et al also revealed53 that this compound remarkably suppressed the enhanced transcriptional level of some matrix metalloproteinases and proinflammatory factors activated via TNF-α in RA-FLSs, which prevent the inflammatory reactivity, leading to prevent the knee joint disruption.
 |
Figure 3 NF-κB inflammation signaling pathway diagram. |
Mitogen-Activated Protein Kinase Pathway
Mitogen-activated protein kinases (MAPKs) are a class of Ser/Thr protein kinases in cells that contribute to various biological and physiological behavioral processes of cells, including gene transcription, cell differentiation and proliferation, cell cycle regulation, apoptosis, and inflammatory reactions.54 MAPKs comprise three well-characterized subfamilies ie, extracellular regulated protein kinase (ERK), c-Jun N-terminal protein kinase (JNK), and p38 MAPKs.55 The ERKs are one of the key signaling cassettes, have a significant contribution to cellular growth, development, and differentiation, while JNK and p38 both have a key role in cellular apoptosis. Li et al studied55 that tan-IIA has an inhibitory activity against glutamate-induced apoptosis via regulating the expression of proteins vital to cellular apoptosis and MAPK activation, including the overexpression of Bcl-2 protein, reduced level of Bax and cleaved caspase-3, and suppression of JNK and p38 MAPK activation. Fang et al results56 indicated that tan-IIA hinders the THP1 cells adhesion to activated-endothelial cells which are activated via TNF-α. Moreover, a mechanistic study illustrates the correlation of p38 MAPK/NF-κB cascade with tan-IIA mediated pharmacological effects.
Pharmacological Effects of Tanshinone-IIA in Diseases
Atherosclerosis
Worldwide, atherosclerosis and subsequent cardiovascular diseases cause millions of deaths each year. Atherosclerosis is a multi-arterial inflammatory disease57 characterized by oxidative stress, inflammatory responses, and immune disorders.58 This is a condition in which the arteries are narrowed and the elasticity of the arterial wall is lost due to excessive accumulation of viscous plaque in the intima of the arteries.59 The mechanism of atherosclerosis is associated with the destruction of lipoprotein metabolism and inflammation and has become the main focus of atherosclerosis research.60 Previous studies have found that in atherosclerosis, tan-IIA acts by inhibiting low density lipoprotein (LDL) oxidation,61 migration and development of smooth muscle cells, monocytes adhesion to the arterial endothelium, expression of pro-inflammatory cytokines, aggregation of platelets62 and cholesterol accumulation mediated by macrophages.63 However, current treatments for the inflammatory properties of atherosclerosis are still very limited. The tan-IIA cardioprotective effects are mainly associated with its anti-inflammatory and antioxidant activities. Tan-IIA has some potential to stabilize plaques in atherosclerosis. This section describes the protective effect of tan-IIA in atherosclerosis and its mechanism of action providing a new perspective for the clinical application of tan-IIA.
Anti-Inflammation
The inflammatory reaction has a key contribution to the occurrence as well as the development of diseases. Inflammation is a key factor in the development of atherosclerosis.63 Over time, many anti-inflammatory strategies have become potential treatments for atherosclerotic disease.64 Lipopolysaccharide (LPS) is the main constituent of the gram-negative bacterial cell wall and is responsible for the inflammatory response in animals as well as in humans. LPS transmits signals into cells through its receptor TLR4 and stimulates the nuclear factor NF-κB which can induce the expression of a series of inflammatory genes, leading to the release of a variety of inflammatory mediators and cytokines, which ultimately causes local or systemic inflammatory response syndrome. Therefore, by effectively blocking the abnormal activation of NF-κB may become an effective way of clinical anti-inflammatory target. Tan-IIA reduces atherosclerosis by inhibiting the inflammatory response.
Chang et al study showed that tan-IIA dose-dependently blocked the adhesion of human vascular endothelial cells by reducing IKK/NF‑κB signaling cascade activation and further attenuates the expression of VCAM-1, ICAM-1, and fractalkine.65 According to Wang et al, tan-IIA suppressed the development of atherosclerosis through inhibition of vascular inflammation, VSMCs apoptosis, and proliferation and migration of macrophages caused by ox-LDL.66 Xu et al provided evidence that tan-IIA reduces lesion size and atherosclerotic plaques stabilization in ApoE/mice by suppressing ROS production induced via oxidized LDL, the expression of TNF-α, IL-6 and MCP-1, and matrix metallo proteinase-9 (MMP-9) activity.67 Zhao et al revealed that tan-IIA may have a role in the vulnerable plaques stabilization, mainly due to its NF-κB suppression, anti-inflammatory effects, phosphorylation of adenosine monophosphate-activated protein kinase (AMPK) and the RAGE axis which tends to reduced matrix degradation (MMPs-induced) and expression of an inflammatory factor in ApoE −/− mice.68 Chen et al studies indicate that tan-IIA attenuate atherosclerosis by inhibiting miR-375 tend to activate KLF4, enhance autophagy and M2 polarization of macrophages.69 Li et al study suggested that tan-IIA exerts an effect on atherosclerotic lesions by inhibiting DC maturation and reduced the expression level of pro-inflammatory cytokines. While hindering their potential to activate cytokine secretion and T-cell proliferation.37
TLRs are well-characterized pattern recognition receptors that induce the innate immune response and also impact the adaptive immune responses at different levels.69 TLRs an important link between atherosclerosis and inflammation,70 making it an attractive target for the treatment of cardiovascular diseases. Zhao et al results suggested that tan-IIA could stabilize vulnerable atherosclerotic plaque in ApoE−/− mice by reducing inflammation and immune response in a dose-dependent manner, and this anti-inflammatory and immune-regulating effect may be obtained by TLR4/MyD88/NF-κB signaling cascade.71 These results also suggest that tan-IIA can induce an anti-inflammatory effect on RAW264.7 cells (LPS-induced) via reducing TLR4-MyD88-NF-κB signaling cascade, and regulating miRNA expression and a series of cytokine production.72 Inflammatory stimuli induce the expression of VCAM-1 on the endothelial cells surface. In the initial phases of AS, VCAM-1 enhance the adhesion and migration of macrophages and leukocytes, and induced macrophages to take up lipids which gave them a foamy appearance.73 VCAM-1 also promotes the adhesion of lymphocytes to endothelial cells.
In the later stages of AS, VCAM‐1 is a key mediator of leukocyte recruitment to sites of inflammation and stimulates macrophages aggregation which ultimately induces MMP secretion, thereby degrading the fibrous cap.74 Zhu et al found that STS reduced the production of malondialdehyde (MDA), elevated the activity of superoxide dismutase (SOD), reduced the level of TNF-α and IL-6, and downregulated the expression of intracellular chloride channel 1 (CLIC1), ICAM-1, and VCAM-1 through its inhibition of CLIC1 expression as well as membrane translocation in the atherosclerotic mice.75 The current study highlighted the clinical applications of tan-IIA on a pharmacological basis for the treatment of advanced atherosclerosis.
Regulate Endothelial Dysfunction (ED)
Tan-IIA and its derivatives remarkably inhibited oxidized LDL (oxLDL) uptake and the content of macrophage foam cell formation (induced by oxLDL). Tan-IIA also has an inhibitory effect against TSA of LOX-1 expression in macrophages, exploring the significant effect against atherosclerosis.76 ED is thought to predict the occurrence of atherosclerosis and is therefore considered an early marker of the disease77 and tan-IIA can control atherosclerosis by regulating endothelial dysfunction.
Studies have found that tan-IIA can improve endothelial function through the following mechanisms: Chen et al study78 suggested that this compound covers endothelial function by suppressing strain-induced endothelin-1 (ET-1) expression, increasing ETB receptors and the formation of nitric oxide (NO), lowering ETA receptors, and up-regulating endothelial nitric oxide synthase (eNOS) in Chronic intermittent hypoxia (CIH). According to Qian et al, this compound has a protective role in endothelial cells against oxidative stress factors such as H2O2 and methylglyoxal.79 Zhu et al results indicated that tan-IIA may exert neuroprotective effects via increased pErk/Erk ratio and the expression of receptors for activated C kinase-1 (RACK1), and reduce the increased level of Beclin1 and LC3-II/I tends to inhibition of autophagy in the hippocampus (in mouse models of atherosclerosis).80 Tang et al results suggested that the underlined compound suppresses the atherosclerotic lesions in ApoE−/− mice which may be due to decreased serum levels of oxLDL. The compound also decreases the mRNA expression of SR-A, CD36, and peroxisome proliferator-activated receptor-gamma (PPARγ) in aortas.81
Anti-Oxidants
Oxidative stress has a key role in atherogenesis which increases the demand for investigating significant antioxidants for the effective treatment of atherosclerosis. Recent studies have shown that tan-IIA reduce atherosclerosis by attenuating oxidative stress. Chen et al experiment suggested that tan-IIA prevents oxidative stress via lowering the production of oxLDL and elevates the activities of glutathione peroxidase (GPx) and SOD which might have a significant role in the atherosclerosis treatment. Tang et al results suggested that in rat model tan-IIA has the potency to attenuate atherosclerotic calcification (AC) which might be due to its inhibitory activity against oxLDL production (independent of the serum levels of lipids), calcium and 25-OH vitamin D (VD). Elevation in Cu/Zn SOD activity as well as mRNA and protein expression via tan-IIA might protect LDL against oxidation caused by superoxide anion in vessel.80 Li et al results found that tan-IIA protected cultured macrophages from H2O2-induced cell death, increased GPx-1 mRNA levels, also significantly increased glutathione peroxidase (GPx) activities and protection was mediated in large part by tan-IIA induction of GPx gene expression and activity in animal models of atherosclerosis.82
Diabetes Mellitus
Diabetes mellitus (DM) is a chronic metabolic disease characterized83 via hyperglycemia caused by impairment in insulin secretion or function, accompanied by abnormal metabolism of carbohydrates, proteins, and fats, which often leads to abnormal acid-base balance.84 DM is a heterogeneous and complex disease that can be classified into type 1 and type 2 DM.85 Although confounding factors such as dyslipidemia and hypertension are primarily associated with this chronic process. There is now sufficient evidence that diabetes alone can cause a large number of molecular changes in the heart.77 Based on several reported studies, tan-IIA has a key contribution to the pathogenesis of diabetes.
Anti-Inflammation
Tan-IIA has been used in TCM for the treatment of a verity of inflammatory and cardiovascular disorders. Based on the correlation between inflammation and Type 2 diabetes, tan-IIA can serve as a candidate target for the treatment of diabetes via regulating inflammatory processes. Yuan et al studies have shown that in experimental rats, the underlined compound prevents inflammatory processes and overcome symptoms of Type 2 diabetes through 5ʹ AMPK signaling cascade stimulated via NF-κB.86 Feng et al proved that the neuropathic pain in diabetic rats’ model has been lowered by tan-IIA through stimulating the Nrf2/ARE signaling cascade and attenuating the NF-kB signaling cascade.78 Sun et al study showed that tan-IIA has remarkable cardioprotective effects given the diabetic cardiomyopathy. Tan-IIA also enhanced Akt and glycogen synthase kinase-3β phosphorylation and block the phosphorylation of NF-κB to lower the level of TNF-α, MPO and IL-6 activities by kinin B2 receptor-Akt-GSK-3β dependent pathway.79 Li et al results revealed that STS can active the canonical Wnt pathway in partly, inhibit the expressed fractalkine induced by high glucose, and play a role of anti-inflammatory by regulating canonical Wnt pathway, thereby, it will provide an effective therapeutic target for DM and its complications.87 Zhu et al studies also proved that pretreatment of ghrelin combined with STS reduces the apoptosis rate of HUVECs induced by high glucose environment and inhibits the expression of fractalkine via β-catenin/Wnt signaling pathway for DM and its complication.88
Regulate Endothelial Dysfunction (ED)
It has been suggested that the dysfunction of endothelium-dependent vasorelaxation has a key role in the development of cardiovascular complications in diabetes.89 According to Li et al studies, Tan-IIA has significantly blocked the protein phosphatase 2A-A (PP2A) translocation from cytoplasm to the membrane and consequently decreased PP2A-A/eNOS interaction which leads to the prevention of eNOS dephosphorylation to cure diabetes via eNOS/NO cascade.90 Across the globe, diabetic retinopathy is considered to be the most predominant and severe form of DM. Based on the results of Fan et al, VEGF and ICAM‑1 expressions are remarkably downregulated via tan-IIA (in a dose‑dependent manner) in hyperglycemia which reveals that under HG conditions, tan-IIA has an inhibitory effect on the vascularization, proliferation, and migration of human retinal endothelial cells (HREC).91
Anti-Oxidants
Tan-IIA has a potential role against oxidative stress and inflammatory processes. Oxidative stress contributes to the initiation as well as the progression of diabetic kidney disease. According to Chen et al, tan-IIA reduced the level of MDA and elevated the level of SOD because of its significant antioxidant property.92 Chen et al experiment conformed that tan-IIA lowers the MDA content, 78-kDa glucose-regulated protein (Grp78), and expression of C/EBP-homologous protein (CHOP) via inducing SOD activity, decreased neuronal apoptosis, enhanced learning and memory through suppressing ER stress activation.93
Alzheimer’s Disease
Alzheimer’s disease (AD) is considered to be the worst form of dementia and the most prevalent among neurological disorders. AD is characterized by neuropeptides’ abnormal regulation and aggregation of beta-amyloid (Aβ) precursors in the brain. Nerve inflammation, oxidative stress, and blocked neurotransmission are alterations that occur in the initial phase of sporadic AD, which manifests as mild cognitive impairment.94,95 With the increase in aging, it has become a major problem in today’s society. But, there is a lack of key approaches for the cure of AD. The clinical symptoms of this neurological disorder are manifested by increased forgetfulness or cognitive dysfunction, loss of language, and behavior. The neuropathological features of this disease include Aβ plaques, polymeric hyperphosphorylation of neurofibrillary tangles, amyloid angiopathy, nerve loss, and synaptic dysfunction.96,97 In recent years, the famous TCM ie, Salvia miltiorrhiza which is commonly used in the cure of cardiovascular and cerebrovascular complications, exerts various neuroprotective effects and is increasing the attention for their use against AD.82
Anti-Inflammation
Recent studies indicated that inflammation is a part of a complex adaptive mechanism (“remodeling”) that persists throughout the life cycle, and has the function of preventing or mitigating the endogenous process of tissue destruction and degenerative changes.94 Lack of sufficient response against the inflammatory process can lead to severe inflammation and can spread locally (that is, from cell to cell) and systemic levels eg, via exosomes. AD is mainly linked with the neuroinflammatory process in the brain which leads to neuronal death. Nuclear factor of activated B-cells (NF-B) has a critical role in physiological inflammatory processes and can be a candidate target for AD therapy based on inflammation.98,99 Besides, several reported studies have shown that tan-IIA has a significant contribution to the regulation of neuroinflammatory diseases. Tan-IIA treatment protects neurons through its anti-inflammatory activities. Li et al study showed that in an AD model, tan-IIA administration can attenuate the development of astrocytes, lower the NF-κB level, and elevated the level of NeuN, Nissl body and IκB, as a result, tan-IIA exerts its neuroprotective and anti-inflammatory effects.76 Lu et al study proved tan-IIA may represent a potential treatment in neurodegenerative diseases, such as AD to support the survival of neurons by reducing expression levels of glial fibrillary acidic protein, C3d, CD11b, C3c, C1q, IL-6 and IL-1β in brain tissues.85 Zhang et al studies have shown that tan-IIA could lower the elevated expression of TNF-α, IL-1β and IL-6, attenuate the expression of monocyte chemoattractant protein 1(MCP-1) and COX-2, decreased the protein expression of iNOS, and caused protein expression of nNOS in the spastic cerebral palsy (SCP) rats, which results in the regulation of the NF-κB and p38MAPK signaling cascades.100 Jiang et al study revealed that the underlined compound significantly blocks the up-regulation of iNOS, matrix metalloproteinase‑2 (MMP‑2) as well as NF‑κB/p65 in the RNA and protein expression levels of AD rats through the NF-κB pathway to reduces AD risk.86 In summary, the underlined data indicate a more theoretical basis for the effective treatment of tan-IIA on behalf of neurodegenerative diseases and introduce a new perspective for the AD clinical treatment.
Anti-Oxidants
Oxidative stress-induced via amyloid β-peptide (Aβ) may have a key contribution to Alzheimer’s disease (AD) pathogenesis.101,102 It has been indicated that tan-IIA prevents oxidative stress and apoptosis. The basis of the mechanism is the ability to perform physiological adaptations by regulating various molecular and biochemical signal transductions that occur from the intracellular level of the entire brain to the network system level.103 Liu et al proved that tan-IIA has a role in the protection of cultured cortical neurons against Aβ25-35-induced neurotoxicity through its antioxidative effects via lowering the Aβ25-35-induced increase of caspase-3 activity and decreased cytochrome C translocation from mitochondria into the cytosol, also enhanced the Aβ25-35-induced Bcl-2/Bax ratio reduction in cortical neurons.104 Tan-IIA potentially decreased elevated level of acetylcholinesterase (AChE) activity and malondialdehyde (MDA) level caused via STZ, and considerably blocked STZ induced reduction in SOD and glutathione peroxidase (GSH-Px) activities in the parietal cortex and hippocampus to ameliorating neuronal damage, restoring cholinergic function, attenuating oxidative stress and blocking p38 MAPK signal pathway activation.105 These results strongly suggest that tan-IIA may be effective in treating AD associated with oxidative stress.
Induce Apoptosis of Tumor Cells
The aggregation of β-amyloid (Aβ) and neuronal death in the brain are pathological indications of AD.106 Cell death is an important process for neuronal death in AD. Lin et al study revealed that Tan-IIA considerably enhanced the spatial learning and balance memory deficits caused via Ab1-42 in rats by shielding neuronal loss and decreasing the enhanced phosphorylation of tau protein via inhibiting ERK and GSK-3β signaling cascades.104 Qian et al study proved that tan-IIA has a neuroprotective potential against the Aβ-induced cytotoxicity (through activation of Bcl-xL pNeuroprotection). The underlined compound also mediates neuropeptides that are correlated with the neuronal functions.107 Zhong et al found that tan-IIA treatment may inhibit apoptosis by down-regulation of p53 and pp53 in rats, and in turn to protect neurons.108
Cancer
Across the globe, cancer is the second most prominent cause of death.109 Many studies have confirmed the potential anticancer activities of tan-IIA. In the past decade, tan-IIA anticancer activity has aroused great interest. Tan-IIA is a natural anti-cancer agent extracted from Salvia miltiorrhiza and has anti-tumor activity. The anticancer effects and potential mechanisms of tan-IIA have been extensively studied in various cancer cell lines. Tan-IIA induces apoptosis through different molecular mechanisms and inhibits the spread of cancer.
Induce Apoptosis of Tumor Cells
Apoptosis is the death of a single cell or a small group of cells in the body. The cell plasma membrane will not be ruptured and will not cause the autolysis of dead cells. The normal human body eliminates damaged and mutated cells in the body through the mechanism of cell death, and cell apoptosis is usually inhibited during tumor development. When tumors occur, they can successfully induce apoptosis of tumor cells and will have a positive effect not only for the treatment of the primary tumor but also for the treatment of tumor recurrence and metastasis. Tan-IIA exerts its anti-tumor effects by promoting cell apoptosis. According to Chen et al,110 tan-IIA suppresses the cell growth and accelerates the cellular apoptosis via downregulating of survivin in keloid fibroblasts. Given this, it is revealed that tan-IIA can effectively participate in keloid treatment. These results indicated111 that in nude mice, the underlined compound considerably reduced HepG2 cell-based tumor growth (in a dose-dependent manner), and stimulate cell death rate through elevated up-regulation of CYP2J2 expression. Based on the reported study,111 tan-IIA may stimulate the apoptotic process of hepatocellular carcinoma via miR30b-p53-PTPN11/SHP2 cascade, effect apoptotic molecules ie, Bax/Bcl2, cleaved caspase 3 and the regulating factors of the cell cycle, such as p21, CDK6, cyclin D1. According to the reported study,112 tan-IIA enhances the apoptotic process through blockage of Wnt/β-catenin-dependent MGMT expression. These results provide extensive knowledge and understanding to explore the mechanistic pathway through which Salvia miltiorrhiza act against tumor progression. The underlined results113 revealed that TSA chemosensitizer colon cancer cells and enhance cellular apoptosis, attenuation of NF-κB activation with inhibitor ie, pyrrolidine dithiocarbamate, elevated the enhanced apoptosis via lowering NF-κB signaling cascade. Tan-IIA114 caused cell death via a mitochondria-dependent pathway in the bladder carcinoma cells. The combined therapy of tan-IIA with a low dose of cisplatin effectively caused the death of bladder carcinoma cells. The underlined results revealed that tan-IIA can act as a leading anti-cancer agent in bladder carcinoma.
Inhibition of Tumor Cell Migration and Proliferation
Based on the existing studies on tan-IIA, it can inhibit tumor cell migration and proliferation. The results113 revealed that TSN block SGC-7901 cell proliferation and migration via downregulating FOXM1 and has a significant effect against cancer progression. Moreover, high-dose of tan-IIA115 attenuate astrocytoma cell growth and development, and migration. Tan-IIA also enhances the apoptotic process via the Notch-1 pathway. Because of these results, tan-IIA may use as a leading compound for the development of effective drugs against astrocytoma.
Side Effects and Toxicity
Even though Salvia miltiorrhiza has been used for many decades, the toxic effects of the tan-IIA (derived from Salvia miltiorrhiza) are still understudied. Traditional Chinese medicine injection is a unique drug variety in China and is widely used in clinical practice. However, due to its complex composition, its adverse reaction events are more frequent, and the safety of traditional Chinese medicine injection has attracted a lot of attention. With the increasing clinical applications, the adverse reactions of Danshen injection include allergic reaction, bradycardia, tachycardia, drug-induced hepatitis, diarrhea, muscle tremor, and uremia.
Cao et al evaluate the hormesis effect, hemolytic effect, and anaphylaxis of sodium tan-IIA sulfonate injection in cavy and rabbit. According to Wang et al survey, chorionic and dechorionated zebrafish embryos were used for the evaluation of developmental and acute toxicity of tan-IIA. Then they observed the lethality as well as teratogenicity at various concentrations of tan-IIA.116 The underlined study may prevent the risk of its use in clinical practice. The aqueous extract of Danshen (via injections) is mostly used in China as a traditional Chinese remedy against cardiovascular complications. These results revealed that chronic or sub-chronic administration of Danshen injection was found to be lower or non-poisonous in both male and female rats, and the no-observed-adverse-effect level (NOAEL) for sub-chronic administration of Danshen injection dose was 5.76g/kg bw/day, however, Danshen injection were found to be associated with focal inflammation in a dose-dependent manner.117
Conclusions and Future Perspectives
As a traditional Chinese medication for enhancing blood circulation and clearing blood stasis, Salvia miltiorrhiza has been commonly used against clinical cardiovascular and cerebrovascular diseases, with remarkable curative effect and minute adverse reactions. In summary, tan-IIA not only has antioxidant effects, anti-atherosclerosis, and other cardiovascular pharmacological effects and antibacterial and anti-inflammatory effects, but more importantly, it has anti-tumor effects. Its anti-tumor mechanism may be involved in the enhancing of tumor cell differentiation and apoptosis. At present, tan-IIA has been widely used in the clinical non-tumor field. Tan-IIA is a promising new candidate target to be used as an anti-tumor drug in clinical practice and contribute to the treatment of malignant tumors. In-depth research on tan-IIA is being carried out worldwide, which may expand the effective clinical uses of the underlined compound and its different dosage forms. At the same time, new formulations and synthetic analogs with elevated bioavailability and reduced side effects are also under development. In-depth research on tan-IIA and Salvia miltiorrhiza is of great significance to the effective use of Chinese herbal medicine and to promote the status and influence of Chinese medicine on the international stage.
Funding
This work was supported by grants from the National Key Subject of Drug Innovation (2019ZX09201005-007), the National Natural Science Foundation of China (81774050), Tianjin Science Foundation for Distinguished Young Scholars (17JCJQJC46200).
Disclosure
All authors of this study have no conflict of interest with other people or organizations in promoting the clinical use of tanshinone IIA. The authors report no conflicts of interest for this work.
References
1. Li MH, Chen JM, Peng Y, Wu Q, Xiao PG. Investigation of Danshen and related medicinal plants in China. J Ethnopharmacol. 2008;120(3):419–426. doi:10.1016/j.jep.2008.09.013
2. Hao D-C, Ge G-B, Xiao P-G. Anticancer drug targets of salvia phytometabolites: chemistry, biology and omics. Curr Drug Targets. 2018;19:1. doi:10.2174/1389450117666161207141020
3. Uto T, Tung NH, Ohta T, et al. Antiproliferative activity and apoptosis induction by trijuganone C isolated from the root of Salvia miltiorrhiza Bunge (Danshen). Phytother Res. 2018;32(4):657–666. doi:10.1002/ptr.6013
4. Zhou R, He L-F, Li Y-J, Shen Y, Chao R-B, Du J-R. Cardioprotective effect of water and ethanol extract of Salvia miltiorrhiza in an experimental model of myocardial infarction. J Ethnopharmacol. 2012;139(2):440–446. doi:10.1016/j.jep.2011.11.030
5. Irmak F, Kurt Yazar S, Sirvan SS, Serin M. Beneficial effects of Salvia miltiorrhiza in the healing of burn wounds: an experimental study in rats. J Plast Surg Hand Surg. 2018;52:229–233. doi:10.1080/2000656X.2018.1461631
6. Fei Y-X, Wang S-Q, Yang L-J, et al. Salvia miltiorrhiza Bunge (Danshen) extract attenuates permanent cerebral ischemia through inhibiting platelet activation in rats. J Ethnopharmacol. 2017;207:57–66. doi:10.1016/j.jep.2017.06.023
7. Hung Y-C, Pan T-L, Hu W-L. Roles of reactive oxygen species in anticancer therapy with salvia miltiorrhiza Bunge. Oxid Med Cell Longev. 2016;2016:5293284. doi:10.1155/2016/5293284
8. Wang L, Ma R, Liu C, et al. Salvia miltiorrhiza: a potential red light to the development of Cardiovascular Diseases. Curr Pharm Des. 2017;23(7):1077–1097. doi:10.2174/1381612822666161010105242
9. Lin M, Qi X, Chen J, et al. The complete chloroplast genome sequence of Actinidia arguta using the PacBio RS II platform. PLoS One. 2018;13(5):e0197393–e0197393. doi:10.1371/journal.pone.0197393
10. Han B, Zhang X, Zhang Q, et al. Protective effects of salvianolate on microvascular flow in a porcine model of myocardial ischaemia and reperfusion. Arch Cardiovasc Dis. 2011;104(5):313–324. doi:10.1016/j.acvd.2011.02.004
11. Liu B, Du Y, Cong L, Jia X, Yang G. Danshen (Salvia miltiorrhiza) compounds improve the biochemical indices of the patients with coronary heart Disease. Evid Based Complement Alternat Med. 2016;2016:9781715. doi:10.1155/2016/9781715
12. Li YG, Song L, Liu M, Hu ZB, Wang ZT. Advancement in analysis of salviae miltiorrhizae radix et rhizoma (Danshen). J Chromatogr A. 2009;1216(11):1941–1953. doi:10.1016/j.chroma.2008.12.032
13. Wang L, Li Y, Guo Y, et al. Herba epimedii: an ancient chinese herbal medicine in the prevention and treatment of osteoporosis. Curr Pharm Des. 2016;22(3):328–349. doi:10.2174/1381612822666151112145907
14. Lukas B, Novak J. The complete chloroplast genome of Origanum vulgare L. (Lamiaceae). Gene. 2013;528(2):163–169. doi:10.1016/j.gene.2013.07.026
15. Wu WY, Wang YP. Pharmacological actions and therapeutic applications of Salvia miltiorrhiza depside salt and its active components. Acta Pharmacol Sin. 2012;33(9):1119–1130. doi:10.1038/aps.2012.126
16. Ling S, Luo R, Dai A, Guo Z, Guo R, Komesaroff PA. A pharmaceutical preparation of Salvia miltiorrhiza protects cardiac myocytes from tumor necrosis factor-induced apoptosis and reduces angiotensin II-stimulated collagen synthesis in fibroblasts. Phytomedicine. 2009;16(1):56–64. doi:10.1016/j.phymed.2008.09.008
17. Dow J, Painovich J, Hale SL, Tjen-A-Looi S, Longhurst JC, Kloner RA. Absence of actions of commonly used chinese herbal medicines and electroacupuncture on myocardial infarct size. J Cardiovasc Pharmacol Ther. 2012;17(4):403–411. doi:10.1177/1074248412443310
18. Hartuti ED, Inaoka DK, Komatsuya K, et al. Biochemical studies of membrane bound Plasmodium falciparum mitochondrial L-malate: quinone oxidoreductase, a potential drug target. Biochimica et Biophysica Acta. 2018;1859(3):191–200. doi:10.1016/j.bbabio.2017.12.004
19. Schulz CE, Dutta AK, Izsak R, Pantazis DA. Systematic high-accuracy prediction of electron affinities for biological quinones. J Comput Chem. 2018;39(29):2439–2451. doi:10.1002/jcc.25570
20. Schmidt S, Gonzalez D, Derendorf H. Significance of protein binding in pharmacokinetics and pharmacodynamics. J Pharm Sci. 2010;99(3):1107–1122. doi:10.1002/jps.21916
21. Leri M, Scuto M, Ontario ML, et al. Healthy effects of plant polyphenols: molecular mechanisms. Int J Mol Sci. 2020;21:4.
22. Yasar U. Two-sided action of Danshen on cytoprotective endogenous substances, epoxyeicosatrienoic acids. Chem Biol Interact. 2018;291:152. doi:10.1016/j.cbi.2018.06.023
23. Xu S, Liu P. Tanshinone II-A: new perspectives for old remedies. Expert Opin Ther Pat. 2013;23(2):149–153. doi:10.1517/13543776.2013.743995
24. Dong XL, Yu WX, Li CM, et al. Danshen (Salvia miltiorrhiza) protects ovariectomized rats fed with high-saturated fat-sucrose diet from bone loss. Osteoporos Int. 2017;29(1):223–235. doi:10.1007/s00198-017-4254-2
25. Kwok T, Leung PC, Lam C, et al. A randomized placebo controlled trial of an innovative herbal formula in the prevention of atherosclerosis in postmenopausal women with borderline hypercholesterolemia. Complement Ther Med. 2014;22(3):473–480. doi:10.1016/j.ctim.2014.03.010
26. Ren-an Q, Juan L, Chuyuan L, et al. Study of the protective mechanisms of compound danshen tablet (fufang danshen pian) against myocardial ischemia/reperfusion injury via the Akt-eNOS signaling pathway in rats. J Ethnopharmacol. 2014;156:190–198. doi:10.1016/j.jep.2014.08.023
27. Naveed M, Wenhua L, Gang W, et al. A novel ventricular restraint device (ASD) repetitively deliver Salvia miltiorrhiza to epicardium have good curative effects in heart failure management. Biomed Pharmacother. 2017;95:701–710. doi:10.1016/j.biopha.2017.07.126
28. Stumpf C, Fan Q, Hintermann C, et al. Anti-inflammatory effects ofdanshenon human vascular endothelial cells in culture. Am J Chin Med. 2013;41(05):1065–1077. doi:10.1142/S0192415X13500729
29. Chen Y-F, Lee N-H, Pai P-Y, et al. Tanshinone-induced ERs suppresses IGFII activation to alleviate Ang II-mediated cardiac hypertrophy. J Receptors Signal Transduction. 2017;37(5):493–499. doi:10.1080/10799893.2017.1360349
30. zhang X, Ma Z, Liang Q, et al. Tanshinone IIA exerts protective effects in a LCA-induced cholestatic liver model associated with participation of pregnane X receptor. J Ethnopharmacol. 2015;164:357–367. doi:10.1016/j.jep.2015.01.047
31. Luo J, Song W, Yang G, Xu H, Chen K. Compound Danshen (Salvia miltiorrhiza) dripping pill for coronary heart disease: an overview of systematic reviews. Am J Chin Med. 2015;43(1):25–43. doi:10.1142/S0192415X15500020
32. Wing-Shing Cheung D, Koon C-M, Ng C-F, et al. The roots of Salvia miltiorrhiza (Danshen) and Pueraria lobata (Gegen) inhibit atherogenic events: A study of the combination effects of the 2-herb formula. J Ethnopharmacol. 2012;143(3):859–866. doi:10.1016/j.jep.2012.08.011
33. Shang Q, Xu H, Huang L. Tanshinone IIA: A promising natural cardioprotective agent. Evid Based Complement Alternat Med. 2012;2012:716459. doi:10.1155/2012/716459
34. Domínguez-Andrés J, Joosten LAB, Netea MG. Induction of innate immune memory: the role of cellular metabolism. Curr Opin Immunol. 2019;56:10–16. doi:10.1016/j.coi.2018.09.001
35. Collin M, Bigley V. Human dendritic cell subsets: an update. Immunology. 2018;154(1):3–20.
36. Gong Y, Liu Y-C, Ding X-L, Fu Y, Cui L-J, Yan Y-P. Tanshinone IIA ameliorates CNS autoimmunity by promoting the differentiation of regulatory T cells. Neurotherapeutics. 2020;17(2):690–703. doi:10.1007/s13311-019-00789-2
37. Li HZ, Lu YH, Huang GS, Chen Q, Fu Q, Li ZL. Tanshinone II A inhibits dendritic cell-mediated adaptive immunity: potential role in anti-atherosclerotic activity. Chin J Integr Med. 2014;20(10):764–769. doi:10.1007/s11655-012-1213-9
38. Larson SR, Atif SM, Gibbings SL, et al. Ly6C(+) monocyte efferocytosis and cross-presentation of cell-associated antigens. Cell Death Differ. 2016;23(6):997–1003. doi:10.1038/cdd.2016.24
39. Montoro J, Pinana JL, Sanz J, Guerreiro M. T lymphocytes as therapeutic arsenal for patients with hematological malignancies. Curr Opin Oncol. 2018;30(6):425–434.
40. Doitsh G, Greene WC. Dissecting how CD4 T cells are lost during HIV infection. Cell Host Microbe. 2016;19(3):280–291. doi:10.1016/j.chom.2016.02.012
41. Qin XY, Li T, Yan L, Liu QS, Tian Y. Tanshinone IIA protects against immune-mediated liver injury through activation of T-cell subsets and regulation of cytokines. Immunopharmacol Immunotoxicol. 2010;32(1):51–55. doi:10.3109/08923970903120997
42. Yan J, Yang X, Han D, Feng J. Tanshinone IIA attenuates experimental autoimmune encephalomyelitis in rats. Mol Med Rep. 2016;14(2):1601–1609. doi:10.3892/mmr.2016.5431
43. Ouyang W, O’Garra A. IL-10 family cytokines IL-10 and IL-22: from basic science to clinical translation. Immunity. 2019;50(4):871–891. doi:10.1016/j.immuni.2019.03.020
44. Ge Y, Huang M, Yao YM. Autophagy and proinflammatory cytokines: interactions and clinical implications. Cytokine Growth Factor Rev. 2018;43:38–46. doi:10.1016/j.cytogfr.2018.07.001
45. Musolino C, Allegra A, Innao V, Allegra AG, Pioggia G, Gangemi S. Inflammatory and anti-inflammatory equilibrium, proliferative and antiproliferative balance: the role of cytokines in multiple myeloma. Mediators Inflamm. 2017;2017:1852517. doi:10.1155/2017/1852517
46. Zhang B, Yu Y, Aori G, et al. Tanshinone IIA attenuates diabetic peripheral neuropathic pain in experimental rats via inhibiting inflammation. Evid Based Complement Alternat Med. 2018;2018:2789847.
47. Yoshimura A, Ito M, Chikuma S, Akanuma T, Nakatsukasa H. Negative regulation of cytokine signaling in immunity. Cold Spring Harb Perspect Biol. 2018;10:7. doi:10.1101/cshperspect.a028571
48. Raja J, Denton CP. Cytokines in the immunopathology of systemic sclerosis. Semin Immunopathol. 2015;37(5):543–557. doi:10.1007/s00281-015-0511-7
49. Anthoney N, Foldi I, Hidalgo A. Toll and Toll-like receptor signalling in development. Development. 2018;145(9):dev156018. doi:10.1242/dev.156018
50. Zhao X, Huo R, Yan X, Xu T. IRF3 negatively regulates toll-like receptor-mediated NF-κB signaling by targeting TRIF for degradation in teleost fish. Front Immunol. 2018;9:867. doi:10.3389/fimmu.2018.00867
51. Meng Z, Si CY, Teng S, Yu XH, Li HY. Tanshinone IIA inhibits lipopolysaccharide‑induced inflammatory responses through the TLR4/TAK1/NF‑κB signaling pathway in vascular smooth muscle cells. Int J Mol Med. 2019. doi:10.3892/ijmm.2019.4100
52. Fang C, Xie L, Liu C, et al. Tanshinone IIA improves hypoxic ischemic encephalopathy through TLR4mediated NFkappaB signal pathway. Mol Med Rep. 2018;18(2):1899–1908.
53. Du H, Wang Y, Zeng Y, et al. Tanshinone IIA suppresses proliferation and inflammatory cytokine production of synovial fibroblasts from rheumatoid arthritis patients induced by TNF-alpha and attenuates the inflammatory response in AIA mice. Front Pharmacol. 2020;11:568. doi:10.3389/fphar.2020.00568
54. Achkar IW, Abdulrahman N, Al-Sulaiti H, Joseph JM, Uddin S, Mraiche F. Cisplatin based therapy: the role of the mitogen activated protein kinase signaling pathway. J Transl Med. 2018;16(1):96. doi:10.1186/s12967-018-1471-1
55. Li H, Han W, Wang H, et al. Tanshinone IIA inhibits glutamate-induced oxidative toxicity through prevention of mitochondrial dysfunction and suppression of MAPK activation in SH-SY5Y human neuroblastoma cells. Oxid Med Cell Longev. 2017;2017:4517486. doi:10.1155/2017/4517486
56. Fang J, Chen Q, He B, et al. Tanshinone IIA attenuates TNF-α induced PTX3 expression and monocyte adhesion to endothelial cells through the p38/NF-κB pathway. Food Chem Toxicol. 2018;121:622–630. doi:10.1016/j.fct.2018.09.063
57. Aluganti Narasimhulu C, Fernandez-Ruiz I, Selvarajan K, et al. Atherosclerosis–do we know enough already to prevent it? Curr Opin Pharmacol. 2016;27:92–102. doi:10.1016/j.coph.2016.02.006
58. Gistera A, Hansson GK. The immunology of atherosclerosis. Nat Rev Nephrol. 2017;13(6):368–380. doi:10.1038/nrneph.2017.51
59. Libby P, Hansson GK. Inflammation and immunity in diseases of the arterial tree: players and layers. Circ Res. 2015;116(2):307–311. doi:10.1161/CIRCRESAHA.116.301313
60. Dron JS, Lazarte J, Hegele RA. Recent Highlights ofATVB. Arterioscler Thromb Vasc Biol. 2018;38:11. doi:10.1161/ATVBAHA.118.311581
61. Hartley A, Haskard D, Khamis R. Oxidized LDL and anti-oxidized LDL antibodies in atherosclerosis – novel insights and future directions in diagnosis and therapy. Trends Cardiovasc Med. 2019;29(1):22–26. doi:10.1016/j.tcm.2018.05.010
62. Ahmadsei M, Lievens D, Weber C, von Hundelshausen P, Gerdes N. Immune-mediated and lipid-mediated platelet function in atherosclerosis. Curr Opin Lipidol. 2015;26(5):438–448. doi:10.1097/MOL.0000000000000212
63. Tabas I, Bornfeldt KE. Macrophage phenotype and function in different stages of atherosclerosis. Circ Res. 2016;118(4):653–667.
64. Fava C, Montagnana M. Atherosclerosis is an inflammatory disease which lacks a common anti-inflammatory therapy: how human genetics can help to this issue. A narrative review. Front Pharmacol. 2018;9:55. doi:10.3389/fphar.2018.00055
65. Chang -C-C, Chu C-F, Wang C-N, et al. The anti-atherosclerotic effect of tanshinone IIA is associated with the inhibition of TNF-α-induced VCAM-1, ICAM-1 and CX3CL1 expression. Phytomedicine. 2014;21(3):207–216. doi:10.1016/j.phymed.2013.09.012
66. Wang B, Ge Z, Cheng Z, Zhao Z. Tanshinone IIA suppresses the progression of atherosclerosis by inhibiting the apoptosis of vascular smooth muscle cells and the proliferation and migration of macrophages induced by ox-LDL. Biol Open. 2017;6(4):489–495. doi:10.1242/bio.024133
67. Xu S, Little PJ, Lan T, et al. Tanshinone II-A attenuates and stabilizes atherosclerotic plaques in apolipoprotein-E knockout mice fed a high cholesterol diet. Arch Biochem Biophys. 2011;515(12):72–79. doi:10.1016/j.abb.2011.08.006
68. Zhao D, Tong L, Zhang L, Li H, Wan Y, Zhang T. Tanshinone II A stabilizes vulnerable plaques by suppressing RAGE signaling and NF-kappaB activation in apolipoprotein-E-deficient mice. Mol Med Rep. 2016;14:4983–4990. doi:10.3892/mmr.2016.5916
69. Chen W, Li X, Guo S, et al. Tanshinone IIA harmonizes the crosstalk of autophagy and polarization in macrophages via miR-375/KLF4 pathway to attenuate atherosclerosis. Int Immunopharmacol. 2019;70:486–497.
70. Cole JE, Kassiteridi C, Monaco C. Toll-like receptors in atherosclerosis: a ‘Pandora’s box’ of advances and controversies. Trends Pharmacol Sci. 2013;34(11):629–636. doi:10.1016/j.tips.2013.09.008
71. Chen Z, Gao X, Jiao Y, et al. Tanshinone iia exerts anti-inflammatory and immune-regulating effects on vulnerable atherosclerotic plaque partially via the TLR4/MyD88/NF-κB signal pathway. Front Pharmacol. 2019;10:850. doi:10.3389/fphar.2019.00850
72. Fan G, Jiang X, Wu X, et al. Anti-inflammatory activity of tanshinone IIA in LPS-stimulated RAW264.7 macrophages via miRNAs and TLR4–NF-κB pathway. Inflammation. 2015;39(1):375–384. doi:10.1007/s10753-015-0259-1
73. Kong D-H, Kim YK, Kim MR, Jang JH, Lee S. Emerging roles of vascular cell adhesion molecule-1 (VCAM-1) in Immunological disorders and cancer. Int J Mol Sci. 2018;19(4):1057.
74. Foster CA. VCAM-1/alpha 4-integrin adhesion pathway: therapeutic target for allergic inflammatory disorders. J Allergy Clin Immunol. 1996;98(6 Pt 2):S270277. doi:10.1016/S0091-6749(96)70075-1
75. Zhu J, Xu Y, Ren G, et al. Tanshinone IIA Sodium sulfonate regulates antioxidant system, inflammation, and endothelial dysfunction in atherosclerosis by downregulation of CLIC1. Eur J Pharmacol. 2017;815:427–436. doi:10.1016/j.ejphar.2017.09.047
76. Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res. 2018;122(4):624–638. doi:10.1161/CIRCRESAHA.117.311586
77. Mudau M, Genis A, Lochner A, Strijdom H. Endothelial dysfunction: the early predictor of atherosclerosis. Cardiovasc J Afr. 2012;23(4):222–231. doi:10.5830/CVJA-2011-068
78. Chen L, Guo Q-H, Chang Y, Zhao Y-S, Li A-Y, Ji E-S. Tanshinone IIA ameliorated endothelial dysfunction in rats with chronic intermittent hypoxia. Cardiovasc Pathol. 2017;31:47–53. doi:10.1016/j.carpath.2017.06.008
79. Qian S, Qian Y, Huo D, Wang S, Qian Q. Tanshinone IIa protects retinal endothelial cells against mitochondrial fission induced by methylglyoxal through glyoxalase 1. Eur J Pharmacol. 2019;857:172419. doi:10.1016/j.ejphar.2019.172419
80. Zhu J, Liao S, Zhou L, Wan L. Tanshinone IIA attenuates Aβ25-35-induced spatial memory impairment via upregulating receptors for activated C kinase1 and inhibiting autophagy in hippocampus. J Pharm Pharmacol. 2016;69(2):191–201. doi:10.1111/jphp.12650
81. Tang F, Wu X, Wang T, et al. Tanshinone II A attenuates atherosclerotic calcification in rat model by inhibition of oxidative stress. Vascul Pharmacol. 2007;46(6):427–438. doi:10.1016/j.vph.2007.01.001
82. Li Y-I, Elmer G, Leboeuf RC. Tanshinone IIA reduces macrophage death induced by hydrogen peroxide by upregulating glutathione peroxidase. Life Sci. 2008;83(1516):557–562. doi:10.1016/j.lfs.2008.08.003
83. Pirillo A, Norata GD, Catapano AL. LOX-1, OxLDL, and atherosclerosis. Mediators Inflamm. 2013;2013:152786. doi:10.1155/2013/152786
84. Cheng X, Zhang D-L, Li X-B, et al. Syntheses of diacyltanshinol derivatives and their suppressive effects on macrophage foam cell formation by reducing oxidized LDL uptake. Bioorg Chem. 2014;52:24–30. doi:10.1016/j.bioorg.2013.11.001
85. Tao Z, Shi A, Zhao J. Epidemiological Perspectives of Diabetes. Cell Biochem Biophys. 2015;73(1):181–185. doi:10.1007/s12013-015-0598-4
86. Yuan FY, Zhang M, Xu P, et al. Tanshinone IIA improves diabetes mellitus via the NF-kappaB-induced AMPK signal pathway. Exp Ther Med. 2018;16(5):4225–4231.
87. Li FQ, Zeng DK, Jia CL, et al. The effects of sodium tanshinone IIa sulfonate pretreatment on high glucose-induced expression of fractalkine and apoptosis in human umbilical vein endothelial cells. Int J Clin Exp Med. 2015;8(4):5279–5286.
88. Zhu Q, Zeng D, Li F. Ghrelin combined with sodium tanshinone IIA sulfonate pretreatment reduces apoptosis and fractalkine expression induced by high-dose glucose in human umbilical vein endothelial cells. Minerva Endocrinol. 2019.
89. Pan C, Lou L, Huo Y, et al. Salvianolic acid B and Tanshinone IIA attenuate myocardial ischemia injury in mice by NO production through multiple pathways. Ther Adv Cardiovasc Dis. 2011;5(2):99–111. doi:10.1177/1753944710396538
90. Li Y-H, Xu Q, Xu W-H, Guo X-H, Zhang S, Chen Y-D. Mechanisms of protection against diabetes-induced impairment of endothelium-dependent vasorelaxation by Tanshinone IIA. Biochimica et Biophysica Acta. 2015;1850(4):813–823. doi:10.1016/j.bbagen.2015.01.007
91. Fan K, Li S, Liu G, Yuan H, Ma L, Lu P. Tanshinone IIA inhibits high glucose-induced proliferation, migration and vascularization of human retinal endothelial cells. Mol Med Rep. 2017;16(6):9023–9028. doi:10.3892/mmr.2017.7743
92. Chen X, Wu R, Kong Y, et al. Tanshinone IIA attenuates renal damage in STZ-induced diabetic rats via inhibiting oxidative stress and inflammation. Oncotarget. 2017;8(19):31915–31922. doi:10.18632/oncotarget.16651
93. Chen J, Bi Y, Chen L, Zhang Q, Xu L. Tanshinone IIA exerts neuroprotective effects on hippocampus-dependent cognitive impairments in diabetic rats by attenuating ER stress-induced apoptosis. Biomed Pharmacother. 2018;104:530–536. doi:10.1016/j.biopha.2018.05.040
94. Pilipenko V, Narbute K, Amara I, et al. GABA‐containing compound gammapyrone protects against brain impairments in Alzheimer’s disease model male rats and prevents mitochondrial dysfunction in cell culture. J Neurosci Res. 2019;97(6):708–726. doi:10.1002/jnr.24396
95. Di Rosa G, Brunetti G, Scuto M, et al. Healthspan enhancement by olive polyphenols in c. elegans wild type and parkinson’s models. Int J Mol Sci. 2020;21:11. doi:10.3390/ijms21113893
96. Wu TY, Chen CP, Jinn TR. Traditional Chinese medicines and Alzheimer’s disease. Taiwan J Obstet Gynecol. 2011;50(2):131–135. doi:10.1016/j.tjog.2011.04.004
97. Calabrese V, Cornelius C, Dinkova-Kostova AT, Calabrese EJ, Mattson MP. Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid Redox Signal. 2010;13(11):1763–1811. doi:10.1089/ars.2009.3074
98. Knapp M, Tu X, Wu R. Vascular endothelial dysfunction, a major mediator in diabetic cardiomyopathy. Acta Pharmacol Sin. 2019;40(1):1–8. doi:10.1038/s41401-018-0042-6
99. Calabrese V, Santoro A, Monti D, et al. Aging and Parkinson’s Disease: inflammaging, neuroinflammation and biological remodeling as key factors in pathogenesis. Free Radic Biol Med. 2018;115:80–91. doi:10.1016/j.freeradbiomed.2017.10.379
100. Mizamtsidi M, Paschou SA, Grapsa J, Vryonidou A. Diabetic cardiomyopathy: a clinical entity or a cluster of molecular heart changes? Eur J Clin Invest. 2016;46(11):947–953.
101. Liu T, Jin H, Sun Q-R, Xu J-H, Hu H-T. The neuroprotective effects of tanshinone IIA on β-amyloid-induced toxicity in rat cortical neurons. Neuropharmacology. 2010;59(78):595–604. doi:10.1016/j.neuropharm.2010.08.013
102. Di Giacomo V, Chiavaroli A, Recinella L, et al. Antioxidant and neuroprotective effects induced by cannabidiol and cannabigerol in rat CTX-TNA2 astrocytes and isolated cortexes. Int J Mol Sci. 2020;21:10. doi:10.3390/ijms21103575
103. Calabrese V, Santoro A, Trovato Salinaro A, et al. Hormetic approaches to the treatment of Parkinson’s disease: perspectives and possibilities. J Neurosci Res. 2018;96(10):1641–1662. doi:10.1002/jnr.24244
104. Lin L, Jadoon SS, Liu S-Z, et al. Tanshinone IIA ameliorates spatial learning and memory deficits by inhibiting the activity of ERK and GSK-3β. J Geriatr Psychiatry Neurol. 2019;32(3):152–163. doi:10.1177/0891988719837373
105. Liu C, Wu Y, Zha S, et al. Treatment effects of tanshinone IIA against intracerebroventricular streptozotocin induced memory deficits in mice. Brain Res. 2016;1631:137–146. doi:10.1016/j.brainres.2015.11.040
106. Li K, Wei Q, Liu -F-F, et al. Synaptic dysfunction in alzheimer’s disease: aβ, tau, and epigenetic alterations. Mol Neurobiol. 2017;55(4):3021–3032. doi:10.1007/s12035-017-0533-3
107. Qian YH, Xiao Q, Xu J. The protective effects of tanshinone IIA on beta-amyloid protein (142)-induced cytotoxicity via activation of the Bcl-xL pathway in neuron. Brain Res Bull. 2012;88(4):354–358. doi:10.1016/j.brainresbull.2012.03.007
108. Li J, Wang F, Zhou J, Li W. [Effects of tanshinone IIA on the expressions of p53, pp53 and apoptosis in the rats with Alzheimer’s disease]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2015;40(11):1210–1216. Chinese.
109. Calabrese EJ. A FAILED CANCER PARADIGM: implications for cancer risk assessment and patients. J Cell Commun Signal. 2019;13(3):271–272. doi:10.1007/s12079-019-00526-6
110. Chen G, Liang Y, Liang X, Li Q, Liu D. Tanshinone IIA inhibits proliferation and induces apoptosis through the downregulation of survivin in keloid fibroblasts. Ann Plast Surg. 2016;76(2):180–186. doi:10.1097/SAP.0000000000000544
111. Bai Y, Zhang L, Fang X, Yang Y. Tanshinone IIA enhances chemosensitivity of colon cancer cells by suppressing nuclear factor-kappaB. Exp Ther Med. 2016;11(3):1085–1089. doi:10.3892/etm.2016.2984
112. Chiu S-C, Huang S-Y, Chang S-F, et al. Potential therapeutic roles of tanshinone IIA in human bladder cancer cells. Int J Mol Sci. 2014;15(9):15622–15637.
113. Yu J, Wang X, Li Y, Tang B. Tanshinone IIA suppresses gastric cancer cell proliferation and migration by downregulation of FOXM1. Oncol Rep. 2017;37(3):1394–1400. doi:10.3892/or.2017.5408
114. Chiu SC, Huang SY, Chang SF, et al. Potential therapeutic roles of tanshinone IIA in human bladder cancer cells. Int J Mol Sci. 2014;15:15622–15637. doi:10.3390/ijms150915622
115. White WL. Erratum to: why I hate the index finger. Hand. 2011;6(2):233. doi:10.1007/s11552-011-9321-0
116. Wang T, Wang C, Wu Q, et al. Evaluation of tanshinone IIA developmental toxicity in zebrafish embryos. Molecules. 2017;22(4):660. doi:10.3390/molecules22040660
117. Wang M, Liu J, Zhou B, et al. Acute and sub-chronic toxicity studies of danshen injection in sprague-dawley rats. J Ethnopharmacol. 2012;141(1):96–103. doi:10.1016/j.jep.2012.02.005
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
