Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 13
Pharmacological Actions of Indoxyl Sulfate and AST-120 That Should Be Recognized for the Strategic Treatment of Patients with Chronic Kidney Disease
Authors Nagata D , Yoshizawa H
Received 16 October 2020
Accepted for publication 17 November 2020
Published 4 December 2020 Volume 2020:13 Pages 359—365
DOI https://doi.org/10.2147/IJNRD.S287237
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pravin Singhal
Daisuke Nagata, Hiromichi Yoshizawa
Division of Nephrology, Department of Internal Medicine, Jichi Medical University, Tochigi, Japan
Correspondence: Daisuke Nagata Tel +81 285 58 7346
Fax +81 285 44 4869
Email [email protected]
Abstract: Although there are many uremic substances in the body, the most studied and well-known molecule that predominantly binds to plasma proteins is indoxyl sulfate (IS). Many research groups have reported IS to have toxic effects on the kidney and cardiovascular system. It is difficult to remove IS with regular hemodialysis or hemodiafiltration. On the other hand, AST-120 has the capacity to bind to indole, which is a precursor of IS in the intestinal tract and excrete it in feces. IS production in the liver is efficiently suppressed by AST-120 administration. However, large-scale clinical studies have not shown that AST-120 suppresses hard endpoints such as doubling serum creatinine, end-stage renal disease, and death. In patients with accelerated chronic kidney disease (CKD) progression, AST-120 is expected to suppress those hard renal endpoints, but only when compliance to treatment is high. It is necessary to validate the renal protective effect of AST-120, as expected from the basic study on IS, including more patients with slowly progressive CKD in a large-scale clinical study in the future.
Keywords: chronic kidney disease, CKD, uremic toxin, indoxyl sulfate, IS, AST-120, hemodialysis, medication adherence
Introduction
Indoxyl sulfate (IS) has been studied as a uremic substance that accumulates in the plasma of chronic kidney disease (CKD) patients. Subsequent studies have shown that IS is primarily eliminated by the kidney. In addition, these studies have examined its role in kidney disease,1 subsequently assessing how much IS is involved in kidney disease pathogenesis. This review would like to reevaluate the toxicity of IS to the body and explain attempts to reduce the plasma concentration of IS and reduce the toxic effect on the human body. Moreover, in this review, we elucidate why AST-120, which is clinically applied to reduce toxic effects, is not as useful as expected from basic studies.
Characteristics of Indoxyl Sulfate
The chemical structure of IS is shown in Figure 1. IS is a metabolite of tryptophan, an essential amino acid. Tryptophan is metabolized to indole by the gut flora and is absorbed in the intestinal epithelium. Indole binds to sulfuric acid in the liver and becomes IS (see Figure 2). It has a low molecular weight of 213 daltons and is mostly bound to plasma proteins. The role of the gut flora in IS production has been shown to reduce urinary IS excretion with antibiotic administration.2 Sulfation of IS in the liver was revealed because indole injection into dogs with resected liver did not increase the plasma levels of IS.3
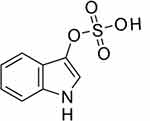 |
Figure 1 Structure of indoxyl sulfate: C8H8NO4S. |
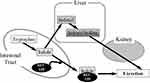 |
Figure 2 Metabolism of indoxyl sulfate and the beneficial effect of AST-120. |
Excretion of IS in the Kidney
Since IS is bound to plasma proteins, its removal is expected to be difficult by regular dialysis, as reported by several research groups.4–6 On the other hand, in the normal kidney, IS is secreted from the renal tubule into the ureteral cavity to provide maximum clearance.7 IS bound to the protein is in equilibrium with the unbound IS. When passing through the capillaries surrounding the proximal tubules, unbound IS is taken up into the tubule cells by organic anion transporters (OAT1 and OAT3) in the basolateral membrane,8,9 and is further secreted into the lumen by OAT4 on the luminal side.10 When unbound IS is secreted in this manner, the IS bound to the plasma protein is dissociated, and a new equilibrium state is maintained, so that IS is secreted one after another.
Research on the Removal and Suppression of IS Production
As mentioned above, IS is a protein-binding uremic toxin, and it is difficult to remove it by regular hemodialysis. Furthermore, IS has also been examined in hemodiafiltration (HDF) and it was reported that its removal is difficult even in HDF.11,12 It is easily expected that the diffusion should be increased to remove IS, and in principle, the methods such as lowering the IS concentration on the dialysate side, increasing the flow rate of the dialysate, and increasing the size of the dialyzer are used. However, although it has been demonstrated to be effective,13,14 it is not clear whether this will lead to improved clinical renal prognosis.
As mentioned above, IS is derived from tryptophan, so naturally, its production increases as food protein intake increases. Therefore, the easiest way to reduce IS production is to limit protein intake.15 A crossover study was conducted, using a low protein diet (LPD; 0.6 g/kg BW/day) and a very low protein diet (VLPD; 0.3 g/kg BW/day) supplemented with ketoanalogues in CKD patients every other week. They reported that serum IS levels decreased by 37% in VLPD.16 Several studies have reported that the administration of AST-120 to inhibit indole absorption in the intestinal tract lowers IS plasma levels in CKD patients.17,18 (Figure 2) As mentioned above, since it is considerably difficult to remove IS with regular HD or HDF because of its high protein binding activity, the method of binding indole to AST-120 and excreting it in feces is considered the most efficient method to remove IS.
Accumulation of Knowledge from Basic Research
The toxicity of IS to the kidney, heart, and vascular system is recognized from basic cell culture or animal experiments. Here, we concisely summarize the typical results of basic research (Table 1). IS accumulates in the plasma due to renal failure, which could cause renal injury. Some studies using 5/6-nephrectomized rats showed the direct effect of IS.19 However, it was shown that glomerular sclerosis and tubular damage might occur.20 Evidence that IS might be associated with inflammation and fibrosis was also revealed in studies using cultured tubular cells.21,22 Next, let us recapitulate on the reports that IS might cause angiopathy. As a result of the cell culture system, IS was shown to impair cultured human umbilical vein endothelial cell (HUVEC) proliferation and wound repair.23 Our research group has shown that IS might promote the exacerbation of atherosclerotic lesions and thrombosis by inducing the proliferation of vascular smooth muscle cells.24 A 5/6-nephrectomy model in ApoE KO mice resulted in atherosclerosis in the aorta, which was significantly suppressed by the administration of AST-120;25 it was suggested that IS may promote vascular endothelial damage even in vivo.
 |
Table 1 Summary of Results of Similar Studies Conducted to Investigate the Association Between Indoxyl Sulfate, CKD, and CVD |
Hereafter, we summarize some results of clinical studies. Increased plasma levels of IS are associated with the risk of hospitalization due to heart failure and cardiac death in patients with cardiomyopathy and CKD stages G 1–3.26 It has also been reported that there is a correlation between plasma IS concentration and the risk of vascular mortality in CKD patients.27 In hemodialysis patients, although the association between blood IS concentration and cardiovascular mortality is unclear,28,29 an increase in heart failure events has been reported.30
Results of Large-Scale Clinical Trials with AST-120
Clinically, there is insufficient evidence that IS accelerates renal disease progression, and IS is currently one of the surrogate markers for tubular injury severity. In such a situation, oral adsorbents that adsorb protein-binding uremic toxins have been clinically applied in several countries, including Japan. AST-120, a typical oral adsorbent charcoal, was developed in Japan. AST-120 was useful in delaying the initiation of dialysis in a Japanese Phase 3 study31 and was approved in 1991.
In addition, the CAP-KD trial32 was conducted in Japan after marketing of AST-120. The participants included 460 CKD patients with serum creatinine (sCr) <5.0 mg/dL and were already taking renin-angiotensin system inhibitors. It was shown that the administration of AST-120 can significantly suppress the decrease in eGFR for a short observation period of one year. However, it was impossible to suppress the composite endpoint consisting of doubling sCr, sCr ≥ 6.0 mg/dL, end-stage renal disease (ESRD), and death.
In 2015, the results of the EPPIC trial,33 which was conducted in North America, Latin America, EU, and Russia/Ukraine, were reported. This clinical trial consisted of EPPIC-1 and EPPIC-2 with approximately 1000 participants each, and the only difference between the two is that QOL assessment was included in EPPIC-2. A large number of participants (approximately 2000 in CKD stages 3–5) were randomized to the 9 g/day AST-120 group and placebo group. The observation period was approximately 3.5 years, including the enrollment period. However, AST-120 administration was unable to suppress the primary endpoint which is the initiation of dialysis, transplantation, and doubling of sCr levels. However, the secondary endpoint, the decline rate in the eGFR, was significantly suppressed.
In 2016, the K-STAR trial was conducted in South Korea,34 and in 2017, the results of its post-hoc analysis were published.35 The clinical trial included about 580 participants, and the observation period was about three years. The composite primary endpoints consisted of doubling sCr levels, decreasing eGFR by more than 50%, and introducing renal replacement therapy. This clinical trial also included measurement of plasma IS concentration. The primary endpoint was not suppressed in the AST-120 group. However, even in this trial, the decline in the eGFR tended to be suppressed.
In 2018, the results of the post hoc analysis of the EPPIC trial were published. In this analysis, risk factors were explored as the primary endpoint of the EPPIC trial and showed that urinary protein/creatinine ratio (UP/UCr)> 1.0 and hematuria are independent risk factors for the primary endpoint occurrence and decrease in eGFR.36 Therefore, narrowing down to the group of patients with elevated UP/UCr manifested hematuria at baseline, it suppressed the primary endpoint of the EPIPIC trial. This result is significant in that AST-120 may suppress the deterioration of renal function if the patients are appropriately selected. A sub-analysis of the EPPIC trial has also been published, focusing on the patient population collected in the United States.37 This analysis showed that the primary endpoint of the EPPIC study was significantly suppressed. The Kaplan-Meier curve, which plots the time to reach the placebo group’s primary endpoint in this study, was similar to what was expected before the commencement of the study, resulting in a somewhat accelerated CKD progression compared to other countries. Without the accelerated CKD progression, the clinical effect of AST-120 cannot be confirmed.
Considerations on Medication Adherence
It is known that AST-120 is relatively difficult to take orally, especially in elderly CKD patients. In clinical trials conducted in Japan and South Korea, the dose administered was 6 g/day, and in the EPPIC trial, the dose was 9 g/day. If the patients attempt to take oral adsorbent in capsules, they should take 30 capsules/day at 6 g/day and 45 capsules/day at 9 g/day. This challenges compliance and undoubtedly an obstacle. Recently, easy-to-take quick-disintegrating tablets have been launched in Japan, and the barriers to taking the drug have been considerably eased. For cases where adherence is low, the expected clinical effect cannot be obtained. Although we conducted a document search in Pubmed using “adherence or compliance” and “AST-120” as keywords, only the sub-analyses of CAP-KD, EPPIC, and K-STAR were found in the clinical studies that explored the relation between renal protection and AST-120 administration (on September 27, 2020). No studies have specifically examined adherence and the effect of AST-120 on improving renal prognosis. Previously, we examined the possibility that differences in the adherence of AST-120 could cause a difference in the rate of decrease in eGFR in patients visiting the CKD outpatient department. The number of participants was at most 100, and the follow-up was conducted after one year (UMIN-CTR ID: R000026467). Unfortunately, we were not able to detect significant differences in eGFR decline in this trial. This suggests that the participants’ number in this study might be overwhelmingly lacking in statistical power to prove the importance of adherence. Adherence in the EPPIC study was reported to be adequate at over 90%, and in the K-STAR study, the residual drug was rigorously checked at each visit (Table 2). High adherence might be a prerequisite for improving the renal prognosis of AST-120, but only in patients with rapid renal function decline as collected by EPPIC USA. Hard endpoints such as doubling sCr, ESRD, or death might not be identified easily. It is an important to note the rate of decrease in eGFR can be suppressed if adherence is high, at least in such populations, and it is necessary to improve the dosage form further to increase compliance in the future.
Conclusions
Various basic and clinical studies have revealed that IS has a detrimental effect in the living body. In particular, an increase in plasma IS concentration might promote renal dysfunction and cardiovascular disease in patients with renal failure. IS is difficult to remove with regular HD or HDF because of its high protein binding activity. Therefore, the method of binding indole, a precursor of IS, to AST-120 and excreting it in feces to remove IS is considered the most straightforward and most efficient method. In large-scale clinical trials using AST-120, the rate of decrease in eGFR was shown to be suppressed, but hard endpoints such as doubling sCr, ESRD, or death have yet to be proven. Moreover, the efficacy of AST-120 cannot be obtained if medication adherence is poor. Also, in slow-growing CKD patients, AST-120 seems to be less effective. In the future, if a prospective clinical trial is conducted using a new alternative hard endpoint as shown by Levey et al,38 the decrease in the eGFR is 30–40% from the baseline in 2–3 years, the effect of AST-120 might be clearly shown, but maximum ingenuity will be required to maintain high adherence.
Acknowledgments
We thank Ms. Keiko Fukuda for her technical support. This review was supported in part by the Japan Agency for Medical Research and Development (AMED) under Grant Number JP18ek0310010 (to DN) and JSPS KEKENHI Grant Number JP19K08685 (to DN). We would like to thank Editage for English language editing.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Pasternack A, Kuhlbaeck B, Tallgren LG. Serum indican in renal disease. Acta Med Scand. 1964;176:751–756. doi:10.1111/j.0954-6820.1964.tb00683.x
2. Brummer P, Kasanenk A. Serum indican and urinary indican excretion in achlorhydria. Acta Med Scand. 1955;152:123–128. doi:10.1111/j.0954-6820.1955.tb03469.x
3. Houssay BA. Phenolemia and indoxylemia: their origin, significance, and regulation. Am J Med Sci. 1936;192:615–626. doi:10.1097/00000441-193611000-00004
4. Vanholder RC, De Smet RV, Ringoir SM. Assessment of urea and other uremic markers for quantification of dialysis efficacy. Clin Chem. 1992;38:1429–1436. doi:10.1093/clinchem/38.8.1429
5. Lesaffer G, De Smet R, Lameire N, et al. Intradialytic removal of protein-bound uraemic toxins: role of solute characteristics and of dialyser membrane. Nephrol Dial Transplant. 2000;15:50–57. doi:10.1093/ndt/15.1.50
6. Niwa T. Removal of protein-bound uraemic toxins by haemodialysis. Blood Purif. 2013;35(Suppl 2):20–25. doi:10.1159/000350843
7. Sirich TL, Aronov PA, Plummer NS, et al. Numerous protein-bound solutes are cleared by the kidney with high efficiency. Kidney Int. 2013;84:585–590. doi:10.1038/ki.2013.154
8. Nigam SK, Wu W, Bush KT, et al. Handling of drugs, metabolites, and uremic toxins by kidney proximal tubule drug transporters. Clin J Am Soc Nephrol. 2015;10:2039–2049. doi:10.2215/CJN.02440314
9. Wu W, Bush KT, Nigam SK. Key role for the organic anion transporters, OAT1 and OAT3, in the in vivo handling of uremic toxins and solutes. Sci Rep. 2017;7:4939.
10. Mutsaers HA, van den Heuvel LP, Ringens LH, et al. Uremic toxins inhibit transport by breast cancer resistance protein and multidrug resistance protein 4 at clinically relevant concentrations. PLoS One. 2011;6:e18438. doi:10.1371/journal.pone.0018438
11. Krieter DH, Hackl A, Rodriguez A, et al. Protein-bound uraemic toxin removal in haemodialysis and post-dilution haemodiafiltration. Nephrol Dial Transplant. 2010;25:212–218. doi:10.1093/ndt/gfp437
12. Meert N, Waterloos M-A, Van Landschoot M, et al. Prospective evaluation of the change of predialysis protein-bound uremic solute concentration with postdilution online hemodiafiltration. Artif Organs. 2010;34:580–585. doi:10.1111/j.1525-1594.2010.01005.x
13. Meyer TW, Peattie JWT, Miller JD, et al. Increasing the clearance of protein-bound solutes by addition of a sorbent to the dialysate. J Am Soc Nephrol. 2007;18:868–874. doi:10.1681/ASN.2006080863
14. Camacho O, Rosales MC, Shafi T, et al. Effect of a sustained difference in hemodialytic clearance on the plasma levels of p-cresol sulfate and indoxyl sulfate. Nephrol Dial Transplant. 2016;31:1335–1341. doi:10.1093/ndt/gfw100
15. Poesen R, Mutsaers HAM, Windey K, et al. The influence of dietary protein intake on mammalian tryptophan and phenolic metabolites. PLoS One. 2015;10:e0140820. doi:10.1371/journal.pone.0140820
16. Marzocco S, Dal Piaz F, Di Micco L, et al. Very low protein diet reduces indoxyl sulfate levels in chronic kidney disease. Blood Purif. 2013;35:196–201. doi:10.1159/000346628
17. Niwa T, Emoto Y, Maeda K, et al. Oral sorbent suppresses accumulation of albumin-bound indoxyl sulphate in serum of haemodialysis patients. Nephrol Dial Transplant. 1991;6:105–109. doi:10.1093/ndt/6.2.105
18. Schulman G, Agarwal R, Acharya M, et al. A multicenter, randomized, double-blind, placebo-controlled, dose-ranging study of AST-120 (Kremezin) in patients with moderate to severe CKD. Am J Kidney Dis. 2006;47:565–577. doi:10.1053/j.ajkd.2005.12.036
19. Niwa T, Ise M, Miyazaki T. Progression of glomerular sclerosis in experimental uremic rats by administration of indole, a precursor of indoxyl sulfate. Am J Nephrol. 1994;14:207–212.
20. Enomoto A, Takeda M, Tojo A, et al. Role of organic anion transporters in the tubular transport of indoxyl sulfate and the induction of its nephrotoxicity. J Am Soc Nephrol. 2002;13:1711–1720. doi:10.1097/01.ASN.0000022017.96399.B2
21. Miyazaki T, Ise M, Seo H, et al. Indoxyl sulfate increases the gene expressions of TGF-beta 1, TIMP-1 and pro-alpha 1(I) collagen in uremic rat kidneys. Kidney Int Suppl. 1997;62:S15–22.
22. Bolati D, Shimizu H, Higashiyama Y, et al. Indoxyl sulfate induces epithelial-to-mesenchymal transition in rat kidneys and human proximal tubular cells. Am J Nephrol. 2011;34:318–323. doi:10.1159/000330852
23. Dou L, Bertrand E, Cerini C, et al. The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int. 2004;65:442–451.
24. Yamamoto H, Tsuruoka S, Ioka T, et al. Indoxyl sulfate stimulates proliferation of rat vascular smooth muscle cells. Kidney Int. 2006;69:1780–1785. doi:10.1038/sj.ki.5000340
25. Yamamoto S, Zuo Y, Ma J, et al. Oral activated charcoal adsorbent (AST-120) ameliorates extent and instability of atherosclerosis accelerated by kidney disease in apolipoprotein E-deficient mice. Nephrol Dial Transplant. 2011;26:2491–2497. doi:10.1093/ndt/gfq759
26. Shimazu S, Hirashiki A, Okumura T, et al. Association between indoxyl sulfate and cardiac dysfunction and prognosis in patients with dilated cardiomyopathy. Circ J. 2013;77:390–396. doi:10.1253/circj.CJ-12-0715
27. Barreto FC, Barreto DV, Liabeuf S, et al. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol. 2009;4:1551–1558. doi:10.2215/CJN.03980609
28. Lin CJ, Wu CJ, Pan CF, et al. Serum protein-bound uraemic toxins and clinical outcomes in haemodialysis patients. Nephrol Dial Transplant. 2010;25:3693–3700. doi:10.1093/ndt/gfq251
29. Shafi T, Meyer TW, Hostetter TH, et al. Free levels of selected organic solutes and cardiovascular morbidity and mortality in hemodialysis patients: results from the Retained Organic Solutes and Clinical Outcomes (ROSCO) investigators. PLoS One. 2015;10:e0126048. doi:10.1371/journal.pone.0126048
30. Cao X-S, Chen J, Zou J-Z, et al. Association of indoxyl sulfate with heart failure among patients on hemodialysis. Clin J Am Soc Nephrol. 2015;10:111–119. doi:10.2215/CJN.04730514
31. Koshikawa S, Koide K, Yamane Y, et al. The effect of AST-120 on delaying initiation of dialysis therapy in end-stage renal disease. Kidney Dial. 1992;32:783–794.
32. Akizawa T, Asano Y, Morita S, et al. Effect of a carbonaceous oral adsorbent on the progression of CKD: a multicenter, randomized, controlled trial. Am J Kid Dis. 2009;54:459–467. doi:10.1053/j.ajkd.2009.05.011
33. Schulman G, Berl T, Beck GJ, et al. Randomized placebo-controlled EPPIC trials of AST-120 in CKD. J Am Soc Nephrol. 2015;26:1732–1746. doi:10.1681/ASN.2014010042
34. Cha RH, Kang SW, Park CW, et al. A randomized, controlled trial of oral intestinal sorbent AST-120 on renal function deterioration in patients with advanced renal dysfunction. Clin J Am Soc Nephrol. 2016;11:559–567. doi:10.2215/CJN.12011214
35. Cha R-H, Kang SW, Park CW, et al. Sustained uremic toxin control improves renal and cardiovascular outcomes in patients with advanced renal dysfunction: post-hoc analysis of the Kremezin Study against renal disease progression in Korea. Kidney Res Clin Pract. 2017;36:68–78. doi:10.23876/j.krcp.2017.36.1.68
36. Schulman G, Berl T, Beck GJ, et al. Risk factors for progression of chronic kidney disease in the EPPIC trials and the effect of AST-120. Clin Exp Nephrol. 2018;22:299–308. doi:10.1007/s10157-017-1447-0
37. Schulman G, Berl T, Beck GJ, et al. The effects of AST-120 on chronic kidney disease progression in the United States of America: a post hoc subgroup analysis of randomized controlled trials. BMC Nephrol. 2016;17(1):141.
38. Levey AS, Inker LA, Matsushita K, et al. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the national kidney foundation and the US food and drug administration. Am J Kidney Dis. 2014;64:821–835. doi:10.1053/j.ajkd.2014.07.030
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

