Back to Journals » Cancer Management and Research » Volume 7
Pharmacokinetics, safety, and efficacy of APF530 (extended-release granisetron) in patients receiving moderately or highly emetogenic chemotherapy: results of two Phase II trials
Authors Gabrail N, Smith C, Yanagihara R, Spaczyński M, Cooper W, O’Boyle E, Boccia R
Received 13 August 2014
Accepted for publication 5 October 2014
Published 17 March 2015 Volume 2015:7 Pages 83—92
DOI https://doi.org/10.2147/CMAR.S72626
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Kenan Onel
Nashat Gabrail,1 Ronald Yanagihara,2 Marek Spaczyński,3 William Cooper,4 Erin O'Boyle,5 Carrie Smith,1 Ralph Boccia6
1Gabrail Cancer Center, Canton, OH, USA; 2St Louise Regional Hospital, Gilroy, CA, USA; 3Department of Gynecology, Obstetrics and Gynecologic Oncology, University of Medical Sciences, Poznan, Poland; 4TFS International, Flemington, NJ, USA; 5FibroGen, Inc., San Francisco, CA, USA; 6Center for Cancer and Blood Disorders, Bethesda, MD, USA
Background: Despite advances with new therapies, a significant proportion of patients (>30%) suffer delayed-onset chemotherapy-induced nausea and vomiting (CINV) despite use of antiemetics. APF530 is a sustained-release subcutaneous (SC) formulation of granisetron for preventing CINV. APF530 pharmacokinetics, safety, and efficacy were studied in two open-label, single-dose Phase II trials (C2005-01 and C2007-01, respectively) in patients receiving moderately emetogenic chemotherapy or highly emetogenic chemotherapy.
Methods: In C2005-01, 45 patients received APF530 250, 500, or 750 mg SC (granisetron 5, 10, or 15 mg, respectively). In C2007-01, 35 patients were randomized to APF530 250 or 500 mg SC. Injections were given 30 to 60 minutes before single-day moderately emetogenic chemotherapy or highly emetogenic chemotherapy. Plasma granisetron was measured from predose to 168 hours after study drug administration. Safety and efficacy were also evaluated.
Results: APF530 pharmacokinetics were dose proportional, with slow absorption and elimination of granisetron after a single SC dose. Median time to maximum plasma concentration and half-life were similar for APF530 250 and 500 mg in both trials, with no differences between the groups receiving moderately and highly emetogenic chemotherapy. Exposure to granisetron was maintained at a therapeutic level over the delayed-onset phase, at least 168 hours. Adverse events in both trials were as expected for granisetron; injection site reactions (eg, erythema and induration) were predominantly mild and seen in ≤20% of patients. Complete responses (no emesis, with no rescue medication) were obtained in the acute, delayed, and overall phases in ≥80% and ≥75% of patients in both trials with the 250 and 500 mg doses, respectively.
Conclusion: After a single injection of APF530, there were dose-proportional pharmacokinetics and sustained concentrations of granisetron over 168 hours. The 250 and 500 mg doses were well tolerated and maintained therapeutic granisetron levels for ≥5 days.
Keywords: cancer, chemotherapy-induced nausea and vomiting, subcutaneous
Background
Control of cancer chemotherapy-induced nausea and vomiting (CINV) is paramount in maintaining patients’ quality of life and their compliance with later chemotherapy.1 Regimens may be classified as moderately emetogenic chemotherapy (MEC) or highly emetogenic chemotherapy (HEC), on the basis of the emetogenic potential of the individual agents.2 Prevention of CINV is the goal; available antiemetics include serotonin (5-HT3) receptor antagonists, neurokinin 1 (NK-1) receptor antagonists, and dexamethasone. Guidelines on the use of these agents in CINV management, based on the emetogenic potential of the chemotherapy regimen, have been published.1,3,4 Delayed-onset CINV (occurring more than 24 hours after chemotherapy) is typically more difficult to control than acute-onset CINV (occurring 0–24 hours after chemotherapy),1,3,4 and despite available antiemetics, CINV is undertreated, with more than 30% of patients continuing to experience CINV with current antiemetic treatments.4,5
APF530 is a subcutaneous (SC), sustained-release formulation of 2% granisetron in a polymer vehicle designed to provide a therapeutic level of the drug for ≥120 hours.6 A double-blind, placebo-controlled, intrapatient dose-escalation Phase I study in healthy male subjects determined that single SC doses of APF530 are safe and well tolerated at 125, 250, 500, and 1,000 mg, with mild injection site reactions, no serious adverse events (AEs), and no clinically significant laboratory abnormalities or electrocardiographic (ECG) changes.7,8
The two previously unpublished Phase II studies presented here assessed the pharmacokinetic properties, safety, and efficacy of three doses of APF530, in patients receiving MEC or HEC.
Methods
Pharmacokinetics, safety, and efficacy of single SC injections of APF530 were assessed in two open-label multicenter Phase II trials in patients receiving MEC or HEC regimens. The first trial (C2005-01) was a sequential ascending dose study. The second study (C2007-01) was a randomized study with two doses of APF530.
Patients
Inclusion criteria were similar for the two Phase II studies. Eligible patients were at least 18 years old, males or nonpregnant females with cytologically or histologically confirmed cancer, Eastern Cooperative Oncology Group (ECOG) performance status ≤2, and scheduled to receive a single-day MEC or HEC regimen. C2005-01 was initially designed to enroll only patients with MEC; however, during the conduct of the trial, the decision was made to enroll patients receiving both MEC and HEC. Emetogenicity was defined according to Hesketh criteria.2 Prior chemotherapy was allowed. In C2005-01, corticosteroids were permitted, although not required, in compliance with the current standard of care and were used in 84% of patients; in C2007-01, patients were required to be able to receive standard doses of dexamethasone as specified in the protocol.
Patients were not eligible if they received radiation therapy within 7 days prior to receiving APF530 or had scheduled radiation therapy or chemotherapy during the 14 (C2005-01) or 7 (C2007-01) days after receiving APF530. Use of CYP3A4 inhibitors was not permitted. In C2005-01, nausea of greater than mild severity or any vomiting within 24 hours prior to receiving APF530 was exclusionary. Patients with head and neck cancer or upper gastrointestinal cancer were not eligible.
In C2005-01, patients were not eligible if they received antiemetics or other prohibited medications within 10 days prior to receiving APF530. In C2007-01, patients were not eligible if they received granisetron, systemic corticosteroids, or other prohibited medications within 7 days prior to receiving APF530. Patients with a heart rate-corrected QT interval (QTc) interval >500 ms or a cardiac abnormality predisposing them to arrhythmia were also excluded.
The C2005-01 protocol was reviewed and approved by a central institutional review board, the Western Institutional Review Board. The C2007-01 protocol was reviewed and approved by an independent ethics committee for each investigational site. Both studies were conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent. C2007-01 was registered with European Union Drug Regulating Authorities Clinical Trials (EudraCT), as EudraCT number 2008-000469-53. C2005-01 was completed before requirements for trial registration.
Study design
C2005-01 patients were assigned to one of three ascending APF530 dose groups: 250 mg (containing granisetron 5 mg), 500 mg (containing granisetron 10 mg), and 750 mg (containing granisetron 15 mg). The decision to enroll in the next dose-escalation group was based on AEs and laboratory results with the lower dose. Doses were given by SC injection in the abdominal area 30 minutes before the start of chemotherapy. A local anesthetic, usually a lidocaine preparation or ethyl chloride spray, was applied to the area of the injection site prior to study drug administration. The study duration was 14 days. After a 7-day treatment and sample collection period, patients returned for a final follow-up visit 14±3 days after study drug administration. Patients were prescribed rescue medication (excluding granisetron) at the discretion of the investigator, to be used as needed in the event of vomiting.
In C2007-01, patients were randomly assigned to receive APF530 250 or 500 mg by SC injection 30 to 60 minutes before the scheduled chemotherapy. Dexamethasone 8 mg intravenously (IV) was given on day 0, in patients with MEC. Dexamethasone 20 mg IV was given on day 0, and 8 mg twice a day on days 1 to 3, in patients with HEC. The study duration was 14±3 days. Patients were prescribed rescue medication as in the 2005-01 study.
Study objectives
The primary objective of both trials was to define the pharmacokinetic properties of granisetron after a single SC dose of APF530 in cancer patients receiving chemotherapy. Secondary objectives were to assess the safety of single SC injections of APF530 in patients receiving single-day MEC or HEC. Evaluation of efficacy in preventing acute-onset (0–24 hours) or delayed-onset (24–168 hours) CINV was an exploratory objective.
Assessments
Plasma granisetron concentrations were measured predose, prior to chemotherapy infusion (C2005-01), and at 2, 6, 24, 48, 72, 96, 120, 144, and 168 hours after APF530 administration. In C2007-01, additional time points were added at 4, 12, and 18 hours after APF530 administration. Measured pharmacokinetic parameters included area under the concentration–time curve (AUC) at 0 to 24 hours, AUC at 0 to 168 hours, maximum plasma concentration (Cmax), time to Cmax (Tmax), the elimination rate constant, and half-life (t1/2) in both trials; and additionally, apparent total clearance, apparent volume of distribution, and mean residence time, in C2007-01.
Safety was assessed throughout the studies, based on vital signs, physical examination, clinical laboratory tests (only at screening in C2007-01), and 12-lead ECGs at screening (predose) and 168 hours (also at 48 hours in C2005-01), and included AEs, injection site reactions, and concomitant medications.
Efficacy was assessed by the number of emetic episodes, number of retching/dry heave episodes, nausea ratings, and rescue medication use during 7 days following the single dose of APF530, which were recorded in patient diaries and collected at each study visit (C2005-01). Alternatively, patients were interviewed at each visit, regarding the occurrence of nausea and emesis and use of rescue medication, with responses recorded (C2007-01).
Efficacy parameters included complete response (CR) (no emetic episodes and no use of rescue medication), complete control (CR with no more than mild nausea), and total response (CR with no nausea), and were summarized for the acute and delayed phases, for MEC and HEC.
Statistics
Noncompartmental methods and descriptive statistics were used to derive and analyze the pharmacokinetic parameters. Descriptive summaries were used for safety measurements by treatment group and preferred term. Efficacy was summarized by time interval and treatment group. Sample sizes were consistent with a previous pharmacokinetic study of palonosetron in patients with cancer receiving chemotherapy.9
Results
Patients
C2005-01 enrolled 45 male and female patients, and C2007-01 enrolled 35 female patients. Most patients had received prior chemotherapy (Table 1). Ovarian, breast, and lung cancers were most common (Table 2). Ovarian cancer affected 83% of women in C2007-01. More than half the patients received carboplatin-based chemotherapy, and 65% received an HEC regimen (Table 3).
  | Table 1 Patient demographics and baseline characteristics in both APF530 Phase II studies |
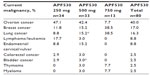  | Table 2 Current malignancies in patients in both APF530 Phase II studies |
  | Table 3 Current chemotherapy for patients in both APF530 Phase II studies |
Patient dispositions and protocol deviations
C2005-01 patients receiving MEC (22 [49%]) or HEC (23 [51%]) were enrolled at six clinical sites in the United States; 17 received APF530 250 mg, 15 received APF530 500 mg, and 13 received APF530 750 mg. Three of 15 patients in the 500 mg group did not complete the study: two had unrelated serious AEs (one dysphagia; one dyspnea, malaise, and diaphoresis) and one withdrew consent (Table 4). After completing the study, one patient experienced fever and septic shock within 4 weeks after receiving APF530 and later died of underlying non-small-cell lung cancer (NSCLC).
  | Table 4 Patient dispositions in both APF530 Phase II studies |
Six patients scheduled to receive chemotherapy on days 1 and 8, or on days 1, 8, and 15 were allowed to enter the study by the medical monitors, and one also had head and neck cancer. All patients who received study drug were included in the safety analysis. Four patients who did not receive the full dose of study drug were excluded from the pharmacokinetic and efficacy analyses (N=41); five patients with missing samples were also excluded from the pharmacokinetic analysis (N=36).
C2007-01 patients were randomized at three sites in Poland; 17 received APF530 250 mg, and 18 received APF530 500 mg (Table 4). Data from six patients with minor deviations from the protocol were allowed by the medical monitor: five were >65 years of age, and one had enrolled in another trial within 30 days. Twelve patients missing up to three blood samples were included in the pharmacokinetic analysis. Two patients received restricted medications, one did not receive the full oral dose of dexamethasone on two occasions, and two did not have final ECGs available for analysis. These patients were included in the final analysis, and their inclusion did not confound the study results.
Pharmacokinetics
Both studies met their primary objective in defining the pharmacokinetic properties of granisetron after a single SC dose of APF530 (Tables 5 and 6). The rate (Tmax) and extent of absorption (Cmax) of granisetron from APF530 were generally dose proportional and consistent between the two studies. Granisetron was absorbed slowly – Cmax occurred at approximately 24 hours (range, 19–32 hours), and the t1/2 was 26 to 34 hours. No dose-related differences in the rate of absorption of granisetron from the APF530 polymer were apparent. Importantly, exposure to granisetron was maintained over the delayed-onset phase, for at least 168 hours (Figures 1 and 2). Mean plasma concentrations, in C2005-01, 168 hours after dosing were 0.579, 1.946, and 2.386 ng/mL for the 250, 500, and 750 mg dose groups, respectively.
In C2005-01, the comparison of t1/2, Tmax, and the elimination rate constant among the dose groups revealed no significant differences (P ≥0.309, Kruskal–Wallis test), and regression analysis indicated dose proportionality for AUC and Cmax. Large variability was seen within each dose group in both trials; the use of separate patient groups for each dose was likely to have contributed.
In C2005-01, various factors were assessed for their effect on the plasma profile of granisetron after APF530 administration. Minor differences were seen in the pharmacokinetic profile related to chemotherapy history (naïve vs previously exposed), emetogenic classification, alcohol or tobacco use, and anthracycline as a component of the chemotherapy. The greatest difference in mean AUC values was between smokers and nonsmokers, but with the large interindividual variability within each group, no meaningful differences in the plasma profiles could be attributed to any of these variables.
Safety
Treatment-emergent AEs (TEAEs) in 82.2% of patients in C2005-01 and 51.4% of patients in C2007-01 did not generally appear to be dose related (Table 7). TEAEs were mostly mild to moderate and unrelated to the study drug.
  | Table 7 Treatment-emergent adverse events in both APF530 Phase II studies |
In C2005-01, no clinically significant laboratory abnormalities were reported following APF530 administration. In C2007-01, clinically significant low red blood cell counts and low hemoglobin concentrations were reported in four patients. No clinically meaningful changes in vital signs, physical examinations, or ECGs were reported in either study.
AEs related to APF530 occurred in 28.9% of patients in C2005-01. In addition to injection site reactions, events related to APF530, and occurring in at least two patients, in C2005-01 were mild to moderate constipation (three patients) and mild to moderate headache (two patients). In C2007-01, the only related AEs, other than injection site reactions, were mild or moderate constipation in four patients (11.6%).
Injection site reactions in studies C2005-01 and C2007-01, respectively, included erythema (8.9% and 5.7%), induration (6.7% and 8.9%), bruising (4.4% and 5.7%), and tenderness or pain (2.2% and 2.9%). There were 19 reactions among 80 patients, 17 mild and two of moderate intensity.
Serious AEs in C2005-01 occurred in four patients: one patient died (related to the underlying disease); and among the remaining patients, one had dyspnea, malaise, and hyperhidrosis, one had intractable diarrhea, and one had dysphagia. All patients recovered. None of the events was related to the study drug. In C2007-01, serious AEs occurred in three patients: one had thrombocytopenia and anemia, one had anemia, and one had thrombocytopenia and abdominal pain. None of the serious AEs was considered related to the study drug, and there were no deaths during the study.
Efficacy
Both acute-onset and delayed-onset CINV were controlled at all doses in both the US and European trials (Table 8). Among those treated with APF530 250 or 500 mg, CR was obtained in ≥83% of patients, in both the acute-onset and delayed-onset phases, and complete control was obtained in ≥76%, indicating that nausea was controlled almost as effectively as emesis; the nausea that did occur was mostly mild.
Discussion
The most important finding of these similarly designed Phase II trials was that granisetron exposure was maintained for 7 days with a single APF530 SC dose. In both studies, granisetron pharmacokinetics were similar and dose proportional with regard to the Cmax achieved and drug exposure over the acute-onset (24 hours) and delayed-onset (168 hours) phases.
In C2005-01, six of the 45 enrolled patients violated protocol in that they received additional chemotherapy within 14 days after receiving APF530. In the judgment of the medical monitor, chemotherapy received after day 7 would not affect assessment of the primary (pharmacokinetics) objective or efficacy. However, chemotherapy administered from days 7 to 14 could have affected the assessment of AEs in the second week of the study. In C2007-01, six of 35 enrolled patients did not meet the study entry criteria because of their age or participation in another trial within 30 days; these were considered minor deviations unlikely to affect the study outcome.
The granisetron transdermal patch (Sancuso®) is another extended-release form of granisetron but is unlike APF530. It is designed to provide extended release of granisetron for up to 7 days. Unlike APF530, the patch is intended for use with multiday chemotherapy regimens and must be applied 24 to 48 hours before the start of chemotherapy because of the time (48 hours) required to reach the granisetron Cmax. The Cmax achieved with the patch is about half that achieved with APF530 250 mg, and the total exposure over 7 days (AUC at 0 to 168 hours) is comparable with that of APF530 250 mg.10,11 Overall, the CR rate was 60% with the patch compared with 65% for oral granisetron.11 Detachment of the patch reduces the amount of granisetron delivered and may be the cause of at least some of the broad variability noted in the pharmacokinetic measurements.10
Interpreting the findings with APF530 SC in the context of granisetron IV is not straightforward because a minimal effective concentration of granisetron in prevention of CINV has not been defined. The recommended dose of granisetron for prevention of CINV is 10 μg/kg,12 which achieves a Cmax of 4.9 ng/mL.13 In an APF530 Phase I safety and pharmacokinetics study in normal volunteers in which granisetron IV (50 μg/kg) was used as a control, granisetron concentrations at 24 and 48 hours were 3.67 and 0.890 ng/mL, respectively. Assuming that an effective granisetron concentration is maintained for at least 48 hours, the minimal effective concentration is <1.0 ng/mL.13 With APF530 500 mg in C2005-01, the granisetron concentration at 168 hours was 1.96 ng/mL; it appears that an effective concentration of granisetron was maintained over at least 7 days with APF530 500 mg.
The dose of granisetron in APF530 SC raised no safety issues. Regulatory concerns regarding potential prolongation of the QTc by 5-HT3 inhibitors resulted in labeling changes regarding potential cardiac safety for granisetron.14 The labeling change was based on individual incidents of QT prolongation.12,15 However, no effect on QTc intervals had been seen in several earlier trials with IV and oral granisetron,16–20 and a recent study with transdermal granisetron also reported no significant effects on QTc or other ECG variables.13 The effect of high-dose APF530 on the QTc interval (QTc) was assessed in a blinded, placebo-controlled study in normal volunteers with APF530 SC 1,000 mg. No clinically significant QTc prolongation was seen with APF530 SC or granisetron IV.8
Based on the findings in these Phase II trials, a Phase III trial was conducted to assess the efficacy of APF530 SC 250 mg and APF530 SC 500 mg in comparison with the second-generation 5-HT3 inhibitor palonosetron. For the 500 mg dose of APF530, the CR rate was noninferior to that of palonosetron in the control of acute emesis following administration of MEC or HEC, and in control of delayed emesis following administration of MEC. In control of delayed emesis following HEC, CR rates with APF530 SC 500 mg were numerically superior to those of palonosetron, although superiority to palonosetron in this setting was not demonstrated.6,21
Conclusion
The pharmacokinetic properties of APF530 have been defined in two Phase II trials in cancer patients receiving a MEC or a HEC regimen. An effective plasma concentration of granisetron was maintained for 7 days with a single dose of APF530. APF530 was well tolerated, exhibiting AEs expected with granisetron. Injection site reactions occurred in fewer than 10% of patients and were mild in most patients. Preliminary efficacy data suggest that APF530 is an expanded option for prevention of acute and delayed CINV. APF530 is a novel delivery system that could particularly benefit chemotherapy patients in the outpatient setting, where convenience and patient compliance are important concerns. On the basis of the findings in this study, APF530 SC 250 mg and APF530 SC 500 mg were carried forward in a Phase III trial.
Author contributions
NG, RY, MS, and RB were the study site investigators, EO was the clinical representative, CS was the clinical study nurse at the Gabrail Cancer Center, and WC performed the statistical analysis. All authors were involved in study conception and design, data acquisition, and the drafting and critical revision of the manuscript’s intellectual content. All authors have read and approved the final manuscript, and agree to be accountable for all aspects of the work’s accuracy and integrity.
Acknowledgments
This study was sponsored by Heron Therapeutics, Inc. (formerly AP Pharma, Inc.). Medical writing support was provided by Richard McCabe, PhD, of SciStrategy Communications, supported by Heron Therapeutics, Inc. The authors would like to thank the many investigators and their clinical staff, who made this study possible.
Disclosure
Dr Nashat Gabrail has received research funding from Heron Therapeutics, Inc. (formerly AP Pharma, Inc.). for the conduct of the clinical trial. Dr Ronald Yanagihara has received research funding from AP Pharma, Inc. for the conduct of the clinical trial. Dr Marek Spaczyński has received funding and honoraria from AP Pharma, Inc. for the conduct of this clinical trial. William Cooper is a consultant for TFS International, the clinical research organization contracted to provide work for AP Pharma, Inc. Erin O’Boyle was previously an employee of, held stock options in, and is currently a consultant for AP Pharma, Inc. Carrie Smith has received funding from AP Pharma, Inc. as a consultant. Dr Ralph Boccia has received clinic funding for the trial only. The authors declare that they have full control of the primary data and agree to allow the journal to review these data. The authors report no other conflicts of interest in this work.
References
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Antiemesis – v2.2014. Fort Washington, PA: National Comprehensive Cancer Network; 2014. Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#supportive. Accessed November 19, 2014. | |
Hesketh PJ, Kris MG, Grunberg SM, et al. Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol. 1997;15(1):103–109. | |
Basch E, Prestrud AA, Hesketh PJ, et al; American Society of Clinical Oncology. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2011;29(31):4189–4198. | |
Roila F, Herrstedt J, Aapro M, et al; ESMO/MASCC Guidelines Working Group. Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol. 2010;21 Suppl 5:v232–v243. | |
Herrstedt J. Antiemetics: an update and the MASCC guidelines applied in clinical practice. Nat Clin Pract Oncol. 2008;5(1):32–43. | |
Raftopoulos H, Cooper W, O’Boyle E, Gabrail N, Boccia R, Gralla RJ. Comparison of an extended-release formulation of granisetron (APF530) versus palonosetron for the prevention of chemotherapy-induced nausea and vomiting associated with moderately or highly emetogenic chemotherapy: results of a prospective, randomized, double-blind, noninferiority phase 3 trial. Support Care Cancer. Epub September 2, 2014. | |
Barr J, O’Boyle E, Johnson M, et al. Phase I, double-blind, placebo-controlled, ascending single subcutaneous dose, safety, tolerability, and pharmacokinetic study of sustained release granisetron (APF530) [abstract e20587]. J Clin Oncol. 2013;31(Suppl):e20587. | |
Mason J, Boon TE, O’Boyle E, Dietz AJ. Comparison of the effects of subcutaneous APF530, intravenous granisetron, moxifloxacin, and placebo on the QT interval in humans [abstract e20539]. J Clin Oncol. 2013;31(Suppl). | |
Eisenberg P, MacKintosh FR, Ritch P, Cornett PA, Macciocchi A. Efficacy, safety and pharmacokinetics of palonosetron in patients receiving highly emetogenic cisplatin-based chemotherapy: a dose-ranging clinical study. Ann Oncol. 2004;15:330–337. | |
Sancuso® (Granisetron Transdermal System) [prescribing information]. Bridgewater, NJ: ProStrakan Inc; 2014. Available from: http://www.sancuso.com/forms/SANCUSO-Full_PI.pdf. Accessed November 19, 2014. | |
Boccia RV, Gordan LN, Clark G, Howell JD, Grunberg SM; Sancuso Study Group. Efficacy and tolerability of transdermal granisetron for the control of chemotherapy-induced nausea and vomiting associated with moderately and highly emetogenic multi-day chemotherapy: a randomized, double-blind, phase III study. Support Care Cancer. 2011;19(10):1609–1617. | |
Kytril® (granisetron hydrochloride) injection for intravenous use [prescribing information]. South San Francisco, CA: Genentech, Inc.; 2011. | |
Mason JW, Selness DS, Moon TE, O’Mahony B, Donachie P, Howell J. Pharmacokinetics and repolarization effects of intravenous and transdermal granisetron. Clin Cancer Res. 2012;18(10):2913–2921. | |
fda.gov [homepage on the Internet]. Safety: Kytril (granisetron hydrochloride) injection, tablets and oral solution [FDA drug safety communication]. US Food and Drug Administration; 2009 [updated October 21, 2009]. Available from: http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm187526.htm. Accessed October 7, 2014. | |
Kytril® (granisetron hydrochloride) tablets oral solution [prescribing information]. South San Francisco, CA: Genentech, Inc; 2011. Available from: http://www.gene.com/download/pdf/kytril_injection_prescribing.pdf. Accessed November 19, 2014. | |
Upward JW, Arnold BD, Link C, Pierce DM, Allen A, Tasker TC. The clinical pharmacology of granisetron (BRL 43694), a novel specific 5-HT3 antagonist. Eur J Cancer. 1990;26 Suppl 1:S12–S15. | |
Carmichael J, Philip PA, Forfar C, Harris AL. An open study to assess the safety, tolerance and pharmacokinetics of an intravenous infusion of granisetron given at 3 mg over 30 s in patients receiving chemotherapy for malignant disease. Cancer Chemother Pharmacol. 1995;37(1–2):134–138. | |
Jantunen IT, Kataja VV, Muhonen TT, Parviainen T. Effects of granisetron with doxorubicin or epirubicin on ECG intervals. Cancer Chemother Pharmacol. 1996;37(5):502–504. | |
Aapro M, Bourke JP. Rapid intravenous administration of granisetron prior to chemotherapy is not arythmogenic: results of a pilot study. Eur J Cancer. 2003;39(7):927–931. | |
Carmichael J, Harris AL. The cardiovascular safety of high-dose intravenous granisetron in cancer patients receiving highly emetogenic chemotherapy. Cancer Chemother Pharmacol. 2004;53(2):123–128. | |
Boccia RV, Cooper W, O’Boyle E. Sustainability of antiemetic responses with APF530 (sustained-release granisetron) during multiple cycles of moderately (MEC) and highly (HEC) emetogenic chemotherapy regimens: Results of a randomized Phase III trial [abstract 9626]. J Clin Oncol. 2013;31(Suppl):9626. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





