Back to Journals » Pharmacogenomics and Personalized Medicine » Volume 14
Pharmacokinetics of Tenofovir Alafenamide Fumarate and Tenofovir in the Chinese People: Effects of Non-Genetic Factors and Genetic Variations
Authors Li X, Tan XY, Cui XJ, Yang M, Chen C, Chen XY
Received 15 July 2021
Accepted for publication 28 September 2021
Published 14 October 2021 Volume 2021:14 Pages 1315—1329
DOI https://doi.org/10.2147/PGPM.S329690
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Xue Li,1,* Xin-Yi Tan,2,* Xue-Jun Cui,3 Ming Yang,1 Chao Chen,1 Xiao-Yun Chen2
1Phase I Clinical Research Laboratory of Shanghai LongHua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, People’s Republic of China; 2Department of Rheumatology of Shanghai LongHua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, People’s Republic of China; 3Institute of Spinal Disease, Shanghai University of Traditional Chinese Medicine, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiao-Yun Chen
Department of Rheumatology of Shanghai LongHua Hospital, Shanghai University of Traditional Chinese Medicine, No. 725, South Wanping Road, Shanghai, 200032, People’s Republic of China
Tel/Fax +86-21-64289981
Email [email protected]
Chao Chen
Phase I Clinical Research Laboratory of Shanghai LongHua Hospital, Shanghai University of Traditional Chinese Medicine, No. 725, South Wanping Road, Shanghai, 200032, People’s Republic of China
Tel +86-21-64385700-9707
Fax +86-21-64289981
Email [email protected]
Background: Tenofovir alafenamide fumarate (TAF) was approved for HBV treatment in China in 2018. Despite higher antiviral efficacy and less impact on renal function and bone mineral density, the pharmacokinetic profiles of TAF are highly variable. The objectives of this study were to investigate the pharmacokinetics of TAF in the Chinese population and explore the associations between TAF and genetic polymorphisms and non-genetic factors.
Patients and Methods: A total of 64 healthy Chinese subjects aged 18∼ 65 years old were planned to enroll. According to the dietary intake status, the subjects were divided into two groups (n = 32 per group). The concentrations of TAF and tenofovir were measured by HPLC-MS/MS, and the single-nucleotide polymorphisms were analyzed by MALDI-TOF MS.
Results: All the enrolled participants (18– 35 years) completed the clinical trial study. Similar to the results reported in other ethnic populations, the pharmacokinetic profiles of TAF and tenofovir were highly variable in the Chinese people, and the HFHC diet can significantly increase the systemic exposure of TAF. We determined both HFHC diet and rs7311358 (SLCO1B3) genotypes were independently associated with TAF AUC0-t, while HFHC diet, age and rs3740066 (ABCC2) variants were predictive of t1/2 of tenofovir (P < 0.05). The subjects with the AA genotype in rs7311358 had significantly higher TAF AUC0-t values (1.15 times) than those with a G allele, and the t1/2 of tenofovir in the rs3740066 TT genotype group was 1.23 times longer than that of CC genotype group. Furthermore, there was a trend of higher TAF AUC and shorter tenofovir t1/2 for the rs2032582 (ABCB1) T allele and rs3742106 (ABCC4) CC variant, respectively, although not statistically significant in the multiple linear regression analysis.
Conclusion: This study provided new evidence to suggest a critical link between both genetic and non-genetic factors and TAF pharmacokinetics in the Chinese people.
Keywords: tenofovir alafenamide fumarate, pharmacokinetics, SLCO1B3, ABCB1, ABCC2, ABCC4
Graphical Abstract:
Introduction
Hepatitis B is an infectious illness caused by hepatitis B virus (HBV) and affects the liver of Hominoidea, including humans. Worldwide estimates in 2015 from the data provided by World Health Organization (WHO) indicate that 257 million people were living with chronic hepatitis B virus (CHB) while 887,000 deaths were caused by hepatitis B associated with cirrhosis or hepatocellular carcinoma (HCC).1 HBV infection represents a major public health problem in China with more than 78 million HBV carriers, 28 million active hepatitis cases, and nearly 300,000 annual deaths resulting from HBV-related cirrhosis.2 To redress this problem, effective strategies involve timely vaccination to prevent infection or interventions with antiviral agents to treat hepatitis B disease. Regarding the latter, established HBV management guidelines recommend nucleos(t)ide analogues (NAs) such as entecavir or tenofovir disoproxil fumarate (TDF) as the first-line oral agents.1 However, side effects reported in some patients including nephrotoxicity and reduction in bone mineral density have limited their application.3–5
Tenofovir alafenamide fumarate (TAF), a novel oral tenofovir prodrug, is a nucleotide analogue developed to inhibit HIV-1 and HBV reverse transcription and was approved in 2016 by the FDA.6 Compared with TDF, which is quickly hydrolyzed to tenofovir in plasma, TAF exhibits higher stability in plasma and reaches higher intracellular levels in target cells. Notably, it was reported that TAF produced higher antiviral efficacy at doses ten times lower than TDF with less impact on renal function and bone mineral density.7,8 Chan et al reported that 4% of CHB patients receiving 25 mg TAF experienced serious adverse effects events (SAE), and 32% of patients exhibiting grade 3 or 4 laboratory abnormalities, the most common of which were elevations in ALT and AST.9 The laboratory abnormalities occurred early in treatment (within the first 1–3 months) and all resolved without sequelae. However, the pharmacokinetic profiles of TAF in different ethnic populations are highly variable.10,11 Therefore, for the rational application of TAF, it is important to identify factors that influence inter-individual variability.12,13
Drug-induced toxicity is closely related to the metabolism and achievable concentration of the drug involved. For the uptake and conversion of TAF to tenofovir, a variety of enzymes and drug transporters have been shown to be involved including carboxylesterase 1 (CES1), P-glycoprotein (P-gp), breast cancer resistance protein (BCRP) and the organic anion transporting polypeptides 1B1 and 1B3 (OATP1B1 and OATP1B3, respectively) (Figure 1) et al.14–17 Patient-to-patient variation in the activities of these enzymes and transporters all contribute to TAF pharmacokinetics, affecting both its efficacy and safety in individual patients. Importantly, previous studies have indicated that genetic differences in the enzymes and transporters involved in TAF metabolism may be associated with variations in treatment responses. Indeed, some evidence suggests race-associated differences in the pharmacokinetics and metabolism of NAs. For example, the AIDS Clinical Trials Group Study A5202 Team reporting on the patient disposition of tenofovir plasma concentrations found that race/ethnicity was associated with tenofovir oral clearance in treatment-naive adults living with HIV-1.18 This covariate relationship raises questions about the possibility of differences in the efficacy and risk of adverse events in different patient populations.
TAF was approved by China’s Drug Administration for the treatment of chronic HBV infection in 2018, and so far, relatively few pharmacokinetic studies have been done in China. Zhao et al performed a pharmacokinetic study in 8 healthy Chinese volunteers in 2019.7 However, considering the high variability of TAF, a larger scale clinical trial needs to be conducted. In addition, correlations between non-genetic and genetic factors in TAF metabolism have not been reported in Chinese subjects. Thus, the aim of this study was to investigate the pharmacokinetic characteristics of TAF in Chinese population, evaluating whether selected polymorphisms in candidate genes and non-genetic factors were associated with TAF metabolism.
Materials and Methods
Materials
TAF tablets (25 mg) were purchased from Gilead Sciences, Inc. (Foster City, CA, U.S.A). The reference standards of TAF, tenofovir and the respective stable isotope-labeled internal standards were supplied by Toronto Research Chemicals (Toronto, Canada). Whole Blood DNA Extraction Kits (QIAamp® DNA Blood Mini Kit) were purchased from Qiagen Inc (Valencia, CA, U.S.A). Complete Genotyping Reagent Kit for MassARRAY® Compact 384 was purchased from Sequenom Inc (CA, U.S.A). Formic acid, dimethyl sulfoxide, and ammonium hydroxide were purchased from Thermo Fisher Scientific (Framingham, MA, U.S.A), ammonium acetate from Sigma-Aldrich Fluka (St. Louis, MO), and high-performance liquid chromatography (HPLC) grade acetonitrile and methanol were from Merck KGaA (Darmstadt, Germany). Ultrapure water was prepared by the Milli-Q Reagent water system (Billerica, USA). All the other reagents and solvents were commercially available.
Study Design
This study was an open-label, single-center, single-dose study conducted at Longhua Hospital, Shanghai University of Traditional Chinese Medicine. The study protocol was approved by the Institutional Review Board of Longhua Hospital (Approved Number 2018LCSY005) and registered at Chinese Clinical Trials Registry (ChiCTR1900022594). We conducted the study in accordance with the Declaration of Helsinki and the Principle of Good Clinical Practice. All participants provided informed signed consent before joining the study. The planned enrollment was 64 healthy participants aged 18~65 years old, body mass index (BMI) 19~26 kg/m2. Exclusion criteria included a history of medical disease, any clinically significant abnormality detected by physical examination or routine laboratory analysis, HIV or hepatitis (B or C) infection, a history of tobacco use (three or more cigarettes per day) or consuming an average of fourteen alcohol units per week, pregnancy, or the use of drugs or herbal medications known to alter tenofovir metabolism. Additionally, all subjects were asked to refrain from consuming caffeinated products or grapefruit juice on the day of the study. All participants were hospitalized one day before the beginning of the study and randomly divided into two groups (fasted or fed, n = 32 evaluable participants per group). On the day of the experiment, the participants took 25 mg TAF P.O. on an empty stomach or after a high fat and high calorie Chinese diet (HFHC diet, 800–1000 calories, including fried rice with egg and steamed meatballs). Thereafter, blood samples (~4 mL) were collected from the upper limb vein into K2EDTA vacuum tubes at 0 (pre-dose) and 0.17, 0.33, 0.5, 0.75, 1, 1.5, 2, 3, 4, 5, 6, 8, 12, 24, 48, 72, 96 h post-dose intervals. Plasma was separated from whole blood by centrifugation at 3000 × g for 10 min at 4 °C, and cell pellets were collected at the same time for DNA extraction. All samples were preserved at −70 °C until analysis.
Determination of TAF and Tenofovir Plasma Concentrations
TAF and tenofovir plasma concentrations were determined using a liquid chromatography tandem mass spectrometry method (HPLC-MS/MS). Briefly, 800 μL acetonitrile and 50 μL internal standard working solution were added to each 200 μL plasma sample, and the mixture was vortexed for 1 min followed by centrifugation at 14,000 rpm for 10 min at 4 °C. The upper 900 μL supernatant was transferred to a glass tube and evaporated to dryness under a gentle stream of nitrogen. The residue was reconstituted with 100 μL of acetonitrile-water (20:80, v/v), which containing 0.1% v/v formic acid and 2 mM ammonium acetate, and a 20 μL aliquot was injected into HPLC-MS/MS system (LC-20AD-Triple Quad 6500, Shimadzu, Japan and Applied Biosystems/MDS Sciex, U.S.A). The mobile phase consisted of 0.1% v/v formic acid and 2 mM ammonium acetate in water (A) mixed with acetonitrile (B), and was operated with a gradient elution at 0.4 mL/min through an AQUASIL C18 column (5 μm, 2.1 × 100 mm; Thermo Scientific, U.S.A.). The column temperature was maintained at 40 °C. Ionization was conducted in positive ion mode and the selected transition ions were m/z 477.2→270.2 (TAF), m/z 482.1→270.1 (TAF-d5), m/z 288.1→176.1 (tenofovir) and m/z 294.1→182.1 (tenofovir-d6), respectively.
Calibration curves were generated using drug-free human plasma spiked with known concentrations of TAF (0.4~400 ng/mL) and tenofovir (0.2~60 ng/mL). Curves were linear (R2 > 0.99) over the assayed concentration range. The inter- and intra-day precision (determined as RSD) were ≤3.4% and 5.1%, and accuracy (determined as RE) was −1.3%~7.0% and −0.6%~1.3% for TAF and tenofovir, respectively. The extraction efficiencies for all the analytes as well as IS were consistent and reproducible, and no significant matrix effect was found. All samples were analyzed within established storage stability periods. Analyst 1.6.2 software (Applied Biosystems, U.S.A) was used for data acquisition and analysis.
Calculation of Pharmacokinetic Parameters
Noncompartmental analysis was performed using Phoenix WinNonlin software (version 8.2, Certara Corporation, St. Louis, MO) to determine the pharmacokinetic parameters of TAF and tenofovir. The maximum plasma concentration (Cmax) and the time to maximum concentration (Tmax) were obtained from direct observation of the plasma profiles. The area under the plasma concentration–time curve (AUC) from time zero to the last measurable concentration (Clast) was calculated using the Linear Trapezoidal method (AUC0-t). The terminal half-life (t1/2) was calculated as ln(2)/λz, where λz, the elimination rate constant, was determined from linear regression of time vs log concentration (the best fit method). Except for Tmax, which was expressed as median (range), other parameters are expressed as geometric mean (CV%) (range).
Selection of Single Nucleotide Polymorphisms (SNPs)
To evaluate the associations between TAF pharmacokinetic parameters and enzyme/transporter genotypes, genetic polymorphisms were chosen for analysis on the basis of prior evidence showing functional impact on the enzymes/transporters involved in TAF and tenofovir metabolism. Several public SNP databases were used in this study (NCBI dbSNP: https://www.ncbi.nlm.nih.gov/snp/ and Ensembl genome browser 102: https://asia.ensembl.org/index.html). After excluding synonymous coding SNPs, a total of 36 SNPs in 10 genes were selected as candidates (Supplemental Table 1): CES1 (CES1), SLCO1B1 (OATP1B1), SLCO1B3 (OATP1B3), ABCG2 (BCRP), ABCB1 (P-gp), ABCC2 (MRP2), ABCC4 (MRP4), ABCC10 (MRP7), SLC28A2 (CNT2), OCRL (OCRL-1).
SNP Genotyping Assays
Genomic DNA was extracted from the blood cell pellet using the QIAamp® DNA Blood Mini Kit (Qiagen Inc., Valencia, CA, U.S.A), and stored at −20 °C. Genotypes were determined by using matrix-assisted laser desorption/ionization-time of flight mass spectrometer (MALDI-TOF MS) coupled with the MassARRY Analyzer 4 (Sequenom Inc, CA, U.S.A). Agena SNP Assay Design Software 3.1 (Agena, San Diego, CA, U.S.A) was used to design primers. The PCR reactions (5 μL each) were carried out in 384-well plate according to the manufacturer’s instructions. A GeneAmp® 9700 384 Dual instrument was used to amplify PCR with the following program: 1) 2 min at 95 °C; 2) 45 cycles of 30 s at 95 °C, 30 s at 56 °C, and 60 s at 72 °C; 3) 5 min at 72 °C; 4) keep at 25 °C. SAP reactions were performed with the following program: 1) 40 min at 37 °C; 2) 5 min at 85 °C; 3) hold at 25 °C. The iPLEX Gold reaction was carried out in the following program: 1) 30 s at 94 °C; 2) 5 s at 94 °C and 5 cycles of 5 s at 52 °C and 5 s at 80 °C; 3) 40 extension cycles; 4) 3 min at 72 °C; 5) hold at 25 °C. The sample plate was transferred onto a SpectroCHIP array to perform nano dispensing. The assays and plates were defined in the MassARRY database. The spectra were required by using the MassARRY mass spectrometer and analyzed by using TyperAnalyzer Software 4.0 (Sequenom Inc, CA, U.S.A).
Statistical Analysis
Statistical analyses in this study were performed using Statistical Analysis System (SAS, version 9.4, SAS Institute Inc, Cary, NC, U.S.A). All data were presented as mean ± SD (continuous variables) or numbers (categorical variables). The allele frequencies of all genotypes were calculated, and the distribution of these genotypes according to Hardy–Weinberg equilibrium (HWE) was tested by the chi-square test (χ2-test). Only SNPs that produced a significance level of P ≥ 0.05 were further analyzed. Associations between demographic data and pharmacokinetic parameters were calculated with Spearman’s rank correlation (continuous variables) or Kruskal–Wallis H-test (categorical variables). Associations between different genotypes and pharmacokinetic parameters were tested by one-way ANOVA with post-hoc testing (when appropriate) by Bonferroni (variance homogeneity) or Games–Howell multiple comparisons procedure. Where the number of individuals with the homozygous variant genotype were small (<3 individuals), data were analyzed by combining the heterozygous and homozygous variant groups (variant carrier) to increase statistical power. Any independent variables with a P value of <0.10 (Spearman’s rank correlation and Kruskal–Wallis H-test) or <0.05 (ANOVA) in the univariate analysis entered into a model of multivariable regression analysis using the stepwise method. A P value of <0.05 was considered statistically significant for multivariable regression analysis.
Results
Demographic Data
Of the 255 subjects who signed informed consent, 64 subjects met the enrollment criteria and entered the clinical trial (Figure 2). All participants were ethnic Han. The primary reasons for exclusion included HIV or hepatitis (B or C) infection, liver or kidney function alterations, electrocardiographic abnormality or withdrawal of consent.
Comparisons of the participant demographic characteristics between the fasted (n = 32) and fed groups (n = 32) showed a similar distribution of age, weight, sex, and body mass index (BMI) (Table 1). The mean age was 27 years old and most subjects were male (81%). The body mass index ranged from 19.2~25.7 kg/m2 and 19.9~26.0 kg/m2, respectively, in the fasted and fed groups. No serious adverse events related to the drug were observed and all participants completed the study.
 |
Table 1 Demographic Characteristic of 64 Subjects |
Pharmacokinetics of TAF and Tenofovir
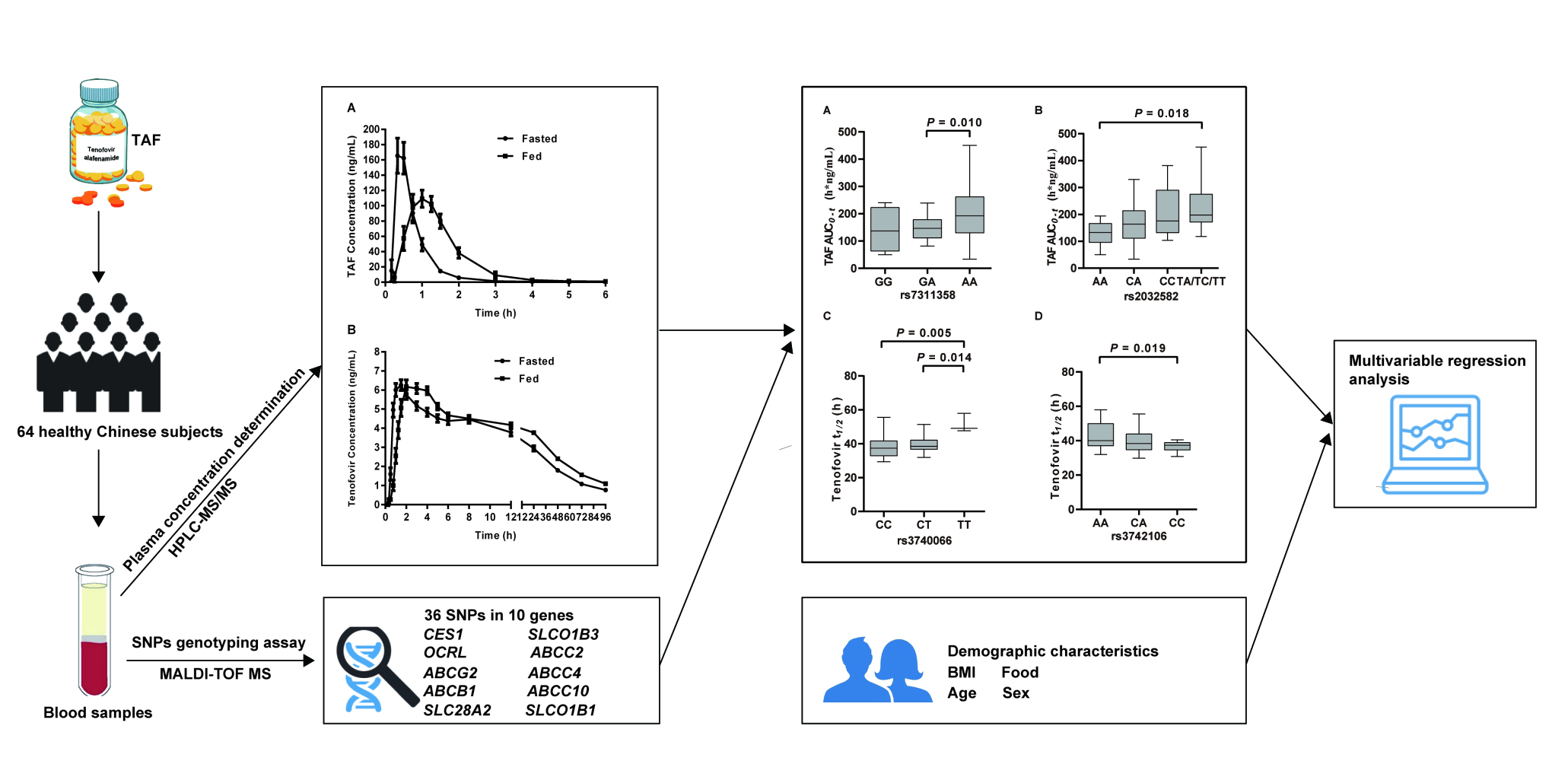
The geometric mean concentration–time curves of TAF and tenofovir in human plasma are presented in Figure 3, and the related pharmacokinetic parameters are summarized in Table 2. After oral administration in fasting subjects, we found TAF was rapidly absorbed with the Cmax (218.74 ng/mL) achieved at 0.33 h with an AUC0-t of 132.10 h·ng/mL. In contrast, these parameters were altered after HFHC diet, with a 20.7% lower Cmax (173.37 ng/mL) occurring at 1.00 h with a 60.4% increase in AUC0-t (211.84 h·ng/mL) compared to the fasted group. The appearance of the major metabolite tenofovir in plasma was accompanied by a rapid decline in circulating TAF, and the concentration was much lower than its parent drug, but the half-life was significantly prolonged (tenofovir versus TAF ~ 40 h versus 0.4 h). Although the Cmax of tenofovir was lower than 10 ng/mL, the AUC0-t in the fed group was 31.2% higher than for fasted group. In addition, taking TAF after HFHC diet delayed the Tmax of TAF and tenofovir.
 |
Table 2 Pharmacokinetic Parameters of TAF and Tenofovir Following an Oral Administration of 25 Mg TAF Under Fasted and Fed Conditions |
Genotypes
The genotypes and allele frequencies of variants in the 64 subjects are shown in Table 3. We found the minor allele frequencies observed for the enzyme/transporter variants were in general agreement with reported dbSNP values (https://www.ncbi.nlm.nih.gov/snp/). All observed genotype frequencies were consistent with expected values according to the HWE test, except for SNPs at rs7057639, rs11597282, rs2231137, rs11568658, rs11854484, and rs4149056 (P < 0.05). Moreover, only homozygous wild-type alleles were observed in 9 SNPs (rs121912777, rs79174032, rs17222723, rs2274406, rs2229109, 1559 A > C, 1564 G > T, 1679 T > C, 1748 G > A). Accordingly, these variants were not considered for further statistical analysis. After these considerations, 21 SNPs were entered into the ANOVA analysis.
 |  |  |
Table 3 Genotypes and Allele Frequencies of Variants in the Participants |
Associations of Nongenetic Factors and Genetic Variants with TAF Pharmacokinetics
Univariate analysis was first employed to examine associations between demographic factors and genetic variants of the selected genes with the pharmacokinetics of TAF and tenofovir. This analysis revealed a strong association between HFHC diet and the pharmacokinetics of TAF (Cmax, Tmax, AUC0-t) and tenofovir (Tmax, t1/2, AUC0-t) (Table 4). Moreover, BMI was found to be associated with the AUC0-t of TAF and Tmax of tenofovir, while a weak association was observed between age and t1/2 of tenofovir. No significant association was observed between sex and pharmacokinetic parameters of TAF and tenofovir.
 |
Table 4 Associations Between Demographic Factors and Pharmacokinetics of TAF and Tenofovir |
For the ANOVA analysis (Table 5), polymorphisms of rs7311358, rs2032582, rs3740066 and rs3742106 were significantly associated with pharmacokinetics of TAF and tenofovir (P < 0.05). Multiple comparison findings (Figure 4A and B) indicated that subjects with the AA genotype in rs7311358 and the T allele in rs2032582 had a significantly higher TAF AUC0-t than the rs7311358 GA genotype and rs2032582 AA variants, respectively (power > 80%). Although we observed a trend for higher AUC values in the rs7311358 AA versus GG variants, this difference did not reach statistical significance (P > 0.05). In addition, participants with the TT genotype in rs3740066 (n = 3, power = 70%) and AA genotype (power > 80%) in rs3742106 had significantly longer tenofovir half-life values than the rs3740066 CC and rs3742106 CC genotypes (Figure 4C and D).
 |
Table 5 Associations Between Genotypes and Pharmacokinetics of TAF and Tenofovir |
Given that multiple factors likely contribute to pharmacokinetic variability, we conducted a multivariate analysis using the stepwise method incorporating independent variables shown to be associated with pharmacokinetic parameters in the preceding univariate analyses, including HFHC diet, BMI, age as well as genotypes involving rs7311358, rs2032582, rs3740066 and rs3742106. The results from regression analyses for TAF and tenofovir (P < 0.05) were shown in Tables 6 and 7, respectively. As shown in Table 6, both HFHC diet and rs7311358 genotypes were independently associated with AUC0-t of TAF. The subjects with the AA genotype in rs7311358 had significantly higher TAF AUC0-t values (1.15 (e0.14) times) than those with a G allele. Table 7 shows HFHC diet, age and rs3740066 variants were predictive of the t1/2 of tenofovir. For every 1% increase in age, the t1/2 of tenofovir increased by about 1.08%. Compared with the subjects with a CC genotype in rs3740066, the participants with the TT genotype had a significantly longer t1/2 (1.23 (e0.21) times) of tenofovir. Although a higher TAF AUC value and shorter tenofovir t1/2 were observed for the rs2032582 T allele and rs3742106 CC variant, respectively, the data did not reach statistical significance in the multiple linear regression analysis.
 |
Table 6 Multivariable Regression Analysis Evaluating the Contributions of Non-Genetic Factors and Genetic Variations to the AUC0-t of TAF |
 |
Table 7 Multivariable Regression Analysis Evaluating the Contributions of Non-Genetic Factors and Genetic Variations to the T1/2 of Tenofovir |
Discussion
Tenofovir alafenamide, a prodrug of tenofovir, is primarily eliminated through conversion to the parent form tenofovir, which is then primarily eliminated through renal excretion.19 Prior evidence suggests an association between the plasma concentration of tenofovir and renal toxicity.20 When administered as TAF, plasma concentrations of tenofovir are much lower, allowing TAF to be better tolerated than other nucleotide reverse transcriptase inhibitors. However, TAF metabolism displays high variability within and between different populations. Indeed, here we found amongst the Cmax of TAF varied up to tenfold in 64 healthy Chinese participants, while the AUC0-t varied up to 15-fold in fasted subjects. Race/ethnicity was previously reported to be associated with the pharmacokinetics of TAF. A pharmacokinetic study performed in 20 Japanese and non-Japanese/Asian subjects showed TAF and tenofovir AUCs were both ~30% higher, and the Cmax values were 32% and 45% higher, respectively, in Japanese versus non-Japanese subjects.21 Compared with these reported values, we found the AUCs of TAF and tenofovir in our study were 12% and 28% higher than Japanese subjects, respectively. This suggests the pharmacokinetics of TAF may vary between Chinese and other Asian descent ethnic groups. However, to substantiate this conclusion, adequately powered clinical pharmacokinetic studies of different races need to be performed in parallel.
Both genetic and non-genetic factors likely contribute to the variations in TAF metabolism observed between individuals. Here we evaluated the effects of HFHC diet, BMI, age and sex as well as polymorphic variations in candidate genes on TAF pharmacokinetics. Previous studies showed that increasing age and decreasing BMI were associated with tenofovir toxicity, especially the risk of kidney damage.22,23 However, we found that BMI was not significantly associated with TAF metabolism in multivariate analysis, although we cannot rule out other confounding effects such as the restricted BMI 19~26 kg/m2 range of our participants and very different PK characteristics and elimination pathways between TAF and other prodrugs of tenofovir. Nonetheless, a strong association was observed between HFHC diet and the pharmacokinetics of TAF and tenofovir. Relative to fasting, the mean TAF AUC0-t was ~50% higher in fed subjects. This finding was consistent with a previous clinical study (40 subjects, predominantly non-Asian subjects) where the mean TAF AUCs were 40% lower in the fasted state.21
Among the targeted polymorphisms chosen for analysis, we included SNPs located in OATP1B3 (SLCO1B3) and P-gp (ABCB1) which are involved in the cellular transport of TAF.14,24 OATP1B3 facilitates the hepatic uptake of TAF while P-gp functions as an efflux pump to restrict the absorption of various drugs. This study is, to our best knowledge, the first to provide evidence of a link between polymorphisms of rs7311358 (SLCO1B3) and rs2032582 (ABCB1), and the pharmacokinetics of TAF in Chinese subjects. Through ANOVA analysis we found the rs7311358 AA genotype and rs2032582 T allele were significantly associated with the increased AUC of TAF. This result was supported by previous studies showing that the 699AA genotype (rs7311358) and 2677T variant (rs2032582) were associated with increased OATP1B3 uptake activity and reduced P-gp efflux activity, respectively.25,26 Intriguingly, a similar finding was reported in Japanese renal transplant recipients, where higher mycophenolic acid (MPA, a substrate of OATP1B3) AUC was observed in subjects with the rs7311358 AA genotype.27 In multivariate analysis, the rs7311358 genotypes were independently associated with AUC0-t of TAF, whereas the association between rs2032582 variants and TAF was not significant after accounting for other factors (P = 0.08). The rs2032582 is a tri-allelic non-synonymous variation that causes substitution of alanine (Ala) with threonine (Thr) or serine (Ser) at the 893 position, and notably, the 893Ser variant (c.2677T) reduces the efflux activity of P-gp compared to the other genetic variants.25 In the current study, we measured higher TAF AUC values for the rs2032582 T allele versus other genetic variations, suggesting changes in P-gp activity are associated with significant pharmacokinetic alterations in TAF. However, these studies need to be expanded to a larger population size to better substantiate the links between polymorphisms of rs2032582 and TAF metabolism.
Tenofovir is the parent form of TAF and is eliminated via the kidney by the combination of glomerular filtration and active tubular secretion. The efflux transporter MRP2 and MRP4 (encoded by ABCC2 and ABCC4, respectively) have been implicated in tenofovir efflux and therefore may confer susceptibility to kidney tubular dysfunction.28,29 Genetic polymorphisms of these transporters have been reported to be associated with higher levels of tenofovir exposure.28,30 In our study, we observed longer half-life time values of plasma tenofovir for the rs3740066 TT and rs3742106 AA genotypes, respectively. However, due to low numbers in rs3740066 TT genotype (n = 3), the clinical relevance between ABCC2 and TFV elimination needs further investigation. In addition, no other factors investigated were associated with plasma concentrations of tenofovir. We speculate that when administered as TAF, the plasma concentrations of tenofovir are low (<10 ng/mL) while the half-time was long (~40 h), resulting from TAF not being converted to tenofovir until entry into the target cells, therefore, other than half-life, other potentially influencing factors were not related to tenofovir plasma concentrations.
Finally, we must acknowledge some of the limitations of our study. First, in terms of certain genetic polymorphisms which can occur at low population frequencies, our sample size was not big enough (32 vs 32) to properly evaluate their associations with TAF and tenofovir pharmacokinetics. Therefore, the uncertainty in these data must be taken into account, and future studies would more comprehensively investigate such polymorphisms, although in the broader scheme, alleles with extremely low frequencies are less likely to be encountered in patient management scenarios. Second, our study was restricted to a select number of enzyme and transporter polymorphisms and a more global approach such as whole-genome sequencing should be considered in future studies. Third, functional studies of genetic variants are warranted to verify the relationships between the gene polymorphisms and the activities of drug transporters or enzymes. Last, due to our study design consisting of only healthy participants, an association between a higher TAF or tenofovir plasma concentrations and adverse effects in patients with compromised health cannot be ruled out. In addition, a crossover study design, which could better understand the changes within subjects, would be appropriate for the similar or relevant studies in the future. Despite these limitations, this study provides new evidence to suggest a critical link between both genetic and non-genetic factors and TAF pharmacokinetics.
Conclusion
In conclusion, this study described the pharmacokinetic characteristics of TAF in Han Chinese, investigating relationships between non-genetic and genetic factors and TAF metabolism. These findings will provide valuable information for the rational application of TAF in clinical practice.
Abbreviations
AUC0-t, area under the plasma concentration–time curve (AUC) from time zero to the last measurable concentration; CHB, chronic hepatitis B virus; Cmax, maximum plasma concentrations; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HWE, Hardy–Weinberg equilibrium; SNPs, single nucleotide polymorphisms; TAF, tenofovir alafenamide fumarate; TDF, tenofovir disoproxil fumarate; t1/2, terminal half-life; Tmax, time to maximum concentration.
Data Sharing Statement
The data are available on reasonable request to the correspondence authors ([email protected]; [email protected]). The raw data, which has been submitted to the medical research public management platform of China, can be downloaded from Resman (Research management, http://www.medresman.org) after data review are completed.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 81803825), Shanghai Shenkang Project (SHDC2020CR4095), LongHua Hospital research projects (RC-2020-02-04 and YM2021016), National Science and Technology Major Projects for “Major New Drugs Innovation and Development” (2017ZX09304001), and Evidence-based capacity building project of traditional Chinese Medicine (1749). The authors report no conflicts of interest.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Liu J, Liang W, Jing W, Liu M. Countdown to 2030: eliminating hepatitis B disease, China. Bull World Health Organ. 2019;97(3):230–238. doi:10.2471/BLT.18.219469
2. Zhang S, Wang F, Zhang Z. Current advances in the elimination of hepatitis B in China by 2030. Front Med. 2017;11(4):490–501. doi:10.1007/s11684-017-0598-4
3. Trinh S, Le AK, Chang ET, et al. Changes in renal function in patients with chronic HBV infection treated with tenofovir disoproxil fumarate vs entecavir. Clin Gastroenterol Hepatol. 2019;17(5):948–956.e1. doi:10.1016/j.cgh.2018.08.037
4. Chung TL, Chen NC, Chen CL. Severe hypophosphatemia induced by denosumab in a patient with osteomalacia and tenofovir disoproxil fumarate-related acquired Fanconi syndrome. Osteoporos Int. 2019;30(2):519–523. doi:10.1007/s00198-018-4679-2
5. Quesada PR, Esteban LL, Garcia JR, et al. Incidence and risk factors for tenofovir-associated renal toxicity in HIV-infected patients. Int J Clin Pharm. 2015;37(5):865–872. doi:10.1007/s11096-015-0132-1
6. Ray AS, Fordyce MW, Hitchcock MJ. Tenofovir alafenamide: a novel prodrug of tenofovir for the treatment of human immunodeficiency virus. Antiviral Res. 2016;125:63–70. doi:10.1016/j.antiviral.2015.11.009
7. Zhao L, Li Z, Zhou Z, et al. Simultaneous determination of tenofovir alafenamide and tenofovir in human plasma by LC-MS/MS and its application to pharmacokinetics study in clinic. J Chromatogr B Analyt Technol Biomed Life Sci. 2019;1117:148–157. doi:10.1016/j.jchromb.2019.04.011
8. Tao X, Lu Y, Zhou Y, Zhang L, Chen Y. Efficacy and safety of the regimens containing tenofovir alafenamide versus tenofovir disoproxil fumarate in fixed-dose single-tablet regimens for initial treatment of HIV-1 infection: a meta-analysis of randomized controlled trials. Int J Infect Dis. 2020;93:108–117. doi:10.1016/j.ijid.2020.01.035
9. Chan HL, Fung S, Seto WK, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAg-positive chronic hepatitis B virus infection: a randomised, double-blind, Phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1(3):185–195. doi:10.1016/S2468-1253(16)30024-3
10. Serota DP, Franch HA, Cartwright EJ. Acute kidney injury in a patient on tenofovir alafenamide fumarate after initiation of treatment for hepatitis C virus infection. Open Forum Infect Dis. 2018;5(8):ofy189. doi:10.1093/ofid/ofy189
11. Novick TK, Choi MJ, Rosenberg AZ, McMahon BA, Fine D, Atta MG. Tenofovir alafenamide nephrotoxicity in an HIV-positive patient: a case report. Medicine (Baltimore). 2017;96(36):e8046. doi:10.1097/MD.0000000000008046
12. Giacomini KM, Yee SW, Ratain MJ, Weinshilboum RM, Kamatani N, Nakamura Y. Pharmacogenomics and patient care: one size does not fit all. Sci Transl Med. 2012;4(153):153ps118. doi:10.1126/scitranslmed.3003471
13. Mirsadeghi S, Larijani B. Personalized medicine: pharmacogenomics and drug development. Acta Med Iran. 2017;55(3):150–165.
14. Murakami E, Wang T, Park Y, et al. Implications of efficient hepatic delivery by tenofovir alafenamide (GS-7340) for hepatitis B virus therapy. Antimicrob Agents Chemother. 2015;59(6):3563–3569. doi:10.1128/AAC.00128-15
15. Ray AS, Cihlar T, Robinson KL, et al. Mechanism of active renal tubular efflux of tenofovir. Antimicrob Agents Chemother. 2006;50(10):3297–3304. doi:10.1128/AAC.00251-06
16. Bam RA, Birkus G, Babusis D, Cihlar T, Yant SR. Metabolism and antiretroviral activity of tenofovir alafenamide in CD4+ T-cells and macrophages from demographically diverse donors. Antivir Ther. 2014;19(7):669–677. doi:10.3851/IMP2767
17. Byrne R, Carey I, Agarwal K. Tenofovir alafenamide in the treatment of chronic hepatitis B virus infection: rationale and clinical trial evidence. Therap Adv Gastroenterol. 2018;11:1756284818786108. doi:10.1177/1756284818786108
18. Bednasz CJ, Venuto CS, Ma Q, et al. Race/ethnicity and protease inhibitor use influence plasma tenofovir exposure in adults living with HIV-1 in AIDS clinical trials group study A5202. Antimicrob Agents Chemother. 2019;63(4):e01638–18. doi:10.1128/AAC.01638-18
19. Moss DM, Neary M, Owen A. The role of drug transporters in the kidney: lessons from tenofovir. Front Pharmacol. 2014;5:248. doi:10.3389/fphar.2014.00248
20. Ezinga M, Wetzels JF, Bosch ME, van der Ven AJ, Burger DM. Long-term treatment with tenofovir: prevalence of kidney tubular dysfunction and its association with tenofovir plasma concentration. Antivir Ther. 2014;19(8):765–771. doi:10.3851/IMP2761
21. Food and Drug Administration (FDA), Center for Drug Evaluation and Research [webpage on the Internet]. Number: 208464Orig1s000: clinical pharmacology and biopharmaceutics review(s); 2016. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208464Orig1s000ClinPharmR.pdf.
22. Baxi SM, Greenblatt RM, Bacchetti P, et al. Common clinical conditions - age, low BMI, ritonavir use, mild renal impairment - affect tenofovir pharmacokinetics in a large cohort of HIV-infected women. AIDS. 2014;28(1):59–66. doi:10.1097/QAD.0000000000000033
23. Kumarasamy N, Sundaram S, Poongulali S, Ezhilarasi C, Pradeep A, Chitra D. Prevalence and factors associated with renal dysfunction in patients on tenofovir disoproxil fumarate-based antiretroviral regimens for HIV infection in Southern India. J Virus Erad. 2018;4(1):37–40. doi:10.1016/S2055-6640(20)30245-4
24. Lepist EI, Phan TK, Roy A, et al. Cobicistat boosts the intestinal absorption of transport substrates, including HIV protease inhibitors and GS-7340, in vitro. Antimicrob Agents Chemother. 2012;56(10):5409–5413. doi:10.1128/AAC.01089-12
25. Kim SW, Lee JH, Lee SH, Hong HJ, Lee MG, Yook KH. ABCB1 c.2677G>T variation is associated with adverse reactions of OROS-methylphenidate in children and adolescents with ADHD. J Clin Psychopharmacol. 2013;33(4):491–498. doi:10.1097/JCP.0b013e3182905a8d
26. Tague LK, Byers DE, Hachem R, et al. Impact of SLCO1B3 polymorphisms on clinical outcomes in lung allograft recipients receiving mycophenolic acid. Pharmacogenomics J. 2020;20(1):69–79. doi:10.1038/s41397-019-0086-0
27. Miura M, Satoh S, Inoue K, et al. Influence of SLCO1B1, 1B3, 2B1 and ABCC2 genetic polymorphisms on mycophenolic acid pharmacokinetics in Japanese renal transplant recipients. Eur J Clin Pharmacol. 2007;63(12):1161–1169. doi:10.1007/s00228-007-0380-7
28. Rungtivasuwan K, Avihingsanon A, Thammajaruk N, et al. Influence of ABCC2 and ABCC4 polymorphisms on tenofovir plasma concentrations in Thai HIV-infected patients. Antimicrob Agents Chemother. 2015;59(6):3240–3245. doi:10.1128/AAC.04930-14
29. Likanonsakul S, Suntisuklappon B, Nitiyanontakij R, et al. A single-nucleotide polymorphism in ABCC4 is associated with tenofovir-related Beta2-microglobulinuria in Thai patients with HIV-1 infection. PLoS One. 2016;11(1):e0147724. doi:10.1371/journal.pone.0147724
30. Manosuthi W, Sukasem C, Thongyen S, Nilkamhang S, Sungkanuparph S. ABCC2*1C and plasma tenofovir concentration are correlated to decreased glomerular filtration rate in patients receiving a tenofovir-containing antiretroviral regimen. J Antimicrob Chemother. 2014;69(8):2195–2201. doi:10.1093/jac/dku129
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.