Back to Journals » Drug Design, Development and Therapy » Volume 15
Pharmacokinetics, Bioequivalence and Safety Evaluation of Two Ticagrelor Tablets Under Fasting and Fed Conditions in Healthy Chinese Subjects
Authors Wang J, Zhang H, Wang R, Cai Y
Received 17 December 2020
Accepted for publication 1 March 2021
Published 15 March 2021 Volume 2021:15 Pages 1181—1193
DOI https://doi.org/10.2147/DDDT.S297918
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Qiongyu Guo
Jin Wang,* Huan Zhang,* Rui Wang, Yun Cai
Center of Medicine Clinical Research, Department of Pharmacy, PLA General Hospital, Beijing, 100853, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yun Cai
Center of Medicine Clinical Research, Department of Pharmacy, PLA General Hospital, 28 Fu Xing Road, Beijing, 100853, People’s Republic of China
Tel +86-10-6693-7166
Fax +86-10-8821-4425
Email [email protected]
Purpose: To evaluate the pharmacokinetics (PK), bioequivalence and safety profiles of test drug and reference drug of 90 mg ticagrelor tablets and their main active metabolite AR-C124910XX under fasting and fed conditions.
Methods: This was a randomized, open-label, single-dose, two-period, two-sequence, and two-treatment crossover study. Subjects were randomized and evenly administered with a single dose of test drug or reference drug of 90 mg ticagrelor tablets orally under fasting or fed conditions with a 7-day washout period. The primary PK parameters were calculated with non-compartmental model, including peak concentration (Cmax), area under the curve (AUC) from zero to last quantifiable concentration (AUC0-t), and AUC from zero to infinity (AUC0-∞). Bioequivalence was judged by whether the 90% confidence intervals (CIs) of the geometric mean ratio (GMR) of the test/reference drugs were within the predefined range of 80– 125%. Adverse events (AEs) were assessed as safety endpoints.
Results: Eighty healthy Chinese subjects (fasting condition: n=40; fed condition: n=40) were enrolled, but two withdrew for personal reasons. As for PK parameters, there was no statistical difference (P> 0.05) between the test and reference drugs under both conditions. As for bioequivalence, the 90% CIs of GMR for Cmax, AUC0-t and AUC0-∞ all fell within 80%-125% regardless of food intake or not. No severe adverse events were observed in the study. Chinese clinical trial registration number is ChiCTR1800015091 (http://www.chictr.org.cn).
Conclusion: Our results demonstrated that the test drug and the reference drug of ticagrelor tablets were bioequivalent. The PK and safety profiles were also similar regardless of food intake or not in healthy Chinese subjects.
Keywords: ticagrelor, bioequivalence, pharmacokinetics, safety
Introduction
Acute coronary syndromes (ACS) are a set of progressive clinical syndromes caused mainly by thrombus formation due to coronary plaque instability and rupture, leading to myocardial ischemia, hypoxia and even myocardial necrosis.1 ACS remains to be a threatening health problem associated with high risk of morbidity and mortality, which results in more than 1,045,000 hospitalizations annually in the United States.2 Dual antiplatelet therapy (DAPT) has been recommended by the American College of Cardiology Foundation/American Heart Association (ACCF/AHA) in the management of ACS,3 including acute treatment and early secondary prevention.4 In previous studies, the use of DAPT with aspirin plus P2Y12 inhibitors (clopidogrel, prasugrel or ticagrelor) has been shown to be associated with the reduced risk of recurrence of cardiovascular events and death.5 However, there are some limitations in the use of clopidogrel, one of the available P2Y12 inhibitors. For instance, it is an inactive prodrug requiring metabolic activation by hepatic cytochrome P450 enzymes (CYP450), such as CYP2C19 with gene polymorphisms, which results in a delayed onset of effect and significant individual differences,6 as well as high platelet reactivity in clinical settings.7 Therefore, there are increasing interests in new P2Y12 inhibitors, such as ticagrelor.
Ticagrelor is a new cyclopentyl-triazolo-pyrimidine antiplatelet agent, which is the first reversely-binding and noncompetitive P2Y12 inhibitor approved for preventing thrombotic events in ACS by European Medical Commission and United States Food and Drug Administration (FDA) in 2010 and 2011, respectively.8 It is not a prodrug and thus does not require metabolic activation, which shows rapid antiplatelet activity and little individual differences.9 As the major metabolite of ticagrelor metabolized by CYP3A4/5, AR-C124910XX also shows a similar potency to ticagrelor in platelet inhibition.10 They are both excreted mainly by bile and partly by the kidney.11 It is therefore obvious that ticagrelor can be applied to patients with kidney dysfunction. Besides, compared with clopidogrel, ticagrelor shows a quicker and higher inhibition of platelet aggregation (IPA), which is not affected by the gene polymorphisms of CYP2C19, and also behaves well in patients with high platelet reactivity to clopidogrel.12 In previous clinical trials,13–15 it has been shown that ticagrelor exhibits a higher reduction rate in the occurrence of vascular death, myocardial infarction and stroke than clopidogrel, with an acceptable risk of bleeding. Therefore, many guidelines have recommended ticagrelor as the first-line treatment in DAPT for ACS patients with high platelet reactivity, chronic kidney disease, complex coronary artery disease and so on.16–18
Although the branded drug of ticagrelor19 has been on the market for many years, it is not affordable to be prescribed for patients with ACS in developing countries, especially when related to the long secondary prevention. Therefore, we conducted bioequivalence evaluation of a generic version of ticagrelor to support the marketing application. The approval of the generic product will reduce cost and thus improve access to the medicine. According to the bioequivalence study guidelines,20,21 the bioequivalence evaluation for an orally administered, immediate-release tablet, such as ticagrelor tablet, should be conducted under both fasting and fed conditions, except when labeled to be taken only on an empty stomach. In addition, because of the approximately similar potency to ticagrelor in platelet inhabitation, which contributes significantly to efficacy and safety, the metabolite AR-C124910XX data are also required to provide supportive evidence of a comparable therapeutic outcome.20 Therefore, this study was designed to evaluate the pharmacokinetics, bioequivalence and safety of the test drug and reference drug of ticagrelor in healthy Chinese subjects under both fasting and fed conditions.
Materials and Methods
Formulations
The test drug (T) of ticagrelor of 90 mg/tablet (batch number: 170503; production date: May 2017; expiration date: April 2019) was produced by Jiangsu FEIMA Pharmaceutical Co., Ltd. (Jiangsu, China). The reference drug (R) of ticagrelor of 90 mg/tablet (batch number: RAMX, batch packaging record: 1705151; production date: March 2017; expiration date: February 2019) was purchased from AstraZeneca Pharmaceutical Co., Ltd. (London, UK).
Subjects
Eligible healthy subjects were at least 18 years of age, with a minimum weight of 45 kg for women or 50 kg for men and a body mass index (BMI) in the range of 19.0–26.0 kg/m2. All subjects were assessed to be healthy based on vital signs, physical examinations, clinical laboratory tests and electrocardiogram (ECG) and agreed to practice contraception throughout the study period and within 3 months after the end of the study. No other medicines were taken within 14 days before the trial.
Subjects were excluded if any of the following conditions were present: 1) unqualified for the physical examination judged by physicians; 2) pregnancy, lactation or menstruation (including the predicted menstrual period falling in 0–9 days before the study); 3) history and/or presence of intracranial hemorrhage, hemophilia, coagulation disorders, atrioventricular block, sinus bradycardia, sinus tachycardia or other conditions increasing the propensity for bleeding; 4) donation of blood in the past 30 days; 5) history and/or presence of gastrointestinal, renal, hepatic, cardiovascular, hematological, respiratory, nervous or psychological disease; 6) allergy to any component of the test or reference drugs; 7) heavy cigarette smoking (>5 cigarettes per day), alcohol or drug abuse currently or within 1 year before study; 8) ingestion of drugs and food affecting CYP3A4/5 in the past 30 days; 9) participation in another clinical trial within 3 months before the study.
Ethics
The bioequivalence study has registered with the Chinese Clinical Trial Registry (http://www.chictr.org.cn, ChiCTR1800015091) in 2018 and been approved by the Independent Ethics Committee (IEC) of People’s Liberation Army (PLA) General Hospital (C2017-061-03). The study was conducted in accordance with the Declaration of Helsinki (1989), the Guidelines of Good Clinical Practice (GCP) and local applicable laws and regulations. All subjects have provided written informed consent forms (ICF) prior to their participation in the study.
Study Design
This was a randomized, open-label, single-dose, two-period, two-sequence, and two-treatment crossover bioequivalence study, aimed to evaluate the bioequivalence of two ticagrelor tablets in both fasting and fed conditions. All subjects were randomly assigned to the Test-Reference (T-R) drug group and Reference-Test (R-T) drug group. They were admitted to the hospital and checked one day before the start of planned drug administration and left the center 72 h later (on day 4, 3 days after the drug administration) with related assessments completed. The study flow chart is presented in Figure 1.
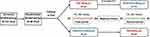 |
Figure 1 The study flow chart under fasting and fed conditions. |
Fasting Bioequivalence Study
Following the screening, eligible healthy subjects were randomized to receiving a single oral dose of 90 mg test or reference tablets with 240 mL warm water following fasting for at least 10 h overnight. The drug was cross-administrated after a 7-day washout period.
Fed Bioequivalence Study
The procedures were the same as the fasting bioequivalence study except that all subjects had a standard high-fat, high-calorie breakfast within half an hour before taking the designed drug.
PK Parameters
Venous blood samples (4 mL) were collected at 1 h predosing and 0.5, 1, 1.25, 1.5, 1.75, 2, 2.5, 3, 3.5, 4, 4.5, 6, 8, 10, 12, 16, 24, 36 and 48 h postdosing into a vacuum anticoagulation tube containing Heparin lithium. The blood samples were centrifuged (1500 g, 10 min) at 4 °C to separate the plasma which was divided into two parts (drug monitoring and backup). All samples were stored at −20 °C within 1 h and transferred to −80 °C in 96 h for storage until analysis.
Plasma concentrations of ticagrelor and its main active metabolite AR-C124910XX were measured by an established and validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) method. The accuracy, intra-day and inter-day precision, and sample stability under different conditions were adequate for the method, which were presented in Supplement Tables S1 and S2. The standard curves of both ticagrelor and AR-C124910XX were linear over the range of 1.00 ng/mL to 600 ng/mL. The lowest limit of quantitation (LLOQ) was 1.00 ng/mL (N=6). Quality control (QC) samples were consisted of low-quality control (LQC), geometric mean-quality control (GMQC), middle-quality control (MQC), and high-quality control (HQC) samples.
The pharmacokinetics (PK) parameters were calculated with non-compartmental model using Phoenix WinNonlin version 6.3. The primary PK endpoints including peak concentration (Cmax), area under the curve (AUC) from zero to t time point (AUC0-t), and AUC from zero to infinity (AUC0-∞). The secondary PK endpoints were rate constant of apparent terminal elimination (λz), terminal elimination half-life (t1/2), time to Cmax (Tmax) and relative bioavailability (F). The Cmax and Tmax were directly obtained from the plasma concentration data. The AUC0-t was calculated by the linear/log trapezoidal method and AUC0-∞ was calculated as the sum of AUC0-t and Ct/λz, in which the λz was obtained by the linear regression during the terminal log-linear phase of the concentration–time curve. The t1/2 was calculated as 0.693/λz, while the F was calculated as follows: F = AUC0-t (test drug)/AUC0-t (reference drug) × 100%.
Safety Assessments
Safety endpoints were evaluated by vital signs (body temperature, blood pressure, pulse, and breathing rate), physical examinations, clinical laboratory tests (routine blood, serum biochemistry, routine urine, routine stool, etc.), 12-lead electrocardiogram, adverse events (AEs) and drug-related AEs.
AEs were coded by the Medical Dictionary for Regulatory Activities (MedDRA, version 20.0) and categorized by System Organ Class (SOC) and Preferred Term (PT). The severities of AEs were graded by Common Terminology Criteria for Adverse Events (CTCAE, version 4.03).
Analysis Sets
- Full Analysis Set (FAS): All randomized subjects were included in FAS to analyze compliance and demographic characteristics.
- Safety Set (SS): All FAS subjects receiving at least one study drug were included in SS.
- Pharmacokinetic Analysis Concentration Set (PKCS): All SS subjects with at least one available concentration data value were included in PKCS to describe the designed drug concentration data.
- Pharmacokinetic Analysis Parameter Set (PKPS): All SS subjects with at least one available PK parameter were included in PKPS for PK analysis.
- Bioequivalence Analysis Set (BES): Subjects who were eligible to evaluate PK parameters in at least one cycle were included in BES for bioequivalence analysis.
Statistical Analysis
Statistical analysis was conducted with the statistical software package SAS Enterprise Guide 9.4 (SAS Institute Inc., USA). All demographic characteristics, including age, sex, ethnicity, height (cm), weight (kg) and BMI (kg/m2), were assessed by descriptive analysis. Student’s t-test and Fisher’s exact test were used to calculate P values between groups of continuous variables and categorical variables, respectively. PK endpoints were calculated with non-compartmental model based on PKPS and also summarized by descriptive analysis. The descriptive statistics included mean, standard deviation, geometric mean, coefficient of variation (%CV), median, minimum and maximum. Student’s t-test was applied to calculate P values of Cmax, AUC and λz between gender groups, with Wilcoxon rank-sum test for t1/2 and tmax. Bioequivalence was evaluated by the Analysis of Variance (ANOVA) for calculating the 90% confidence intervals (CIs) of the geometric mean ratios (Cmax, AUC0-t, and AUC0-∞), with assessing the effects of sequence, period, formulations. When the 90% CIs of the geometric mean ratio (GMR) of the test/reference drug were within the predefined range of 80%-125%, the two drugs were considered bioequivalent. Non-parametric analysis (Wilcoxon signed-rank test) was performed to calculate the median and 90% CIs for non-transformed values of Tmax.
Results
Subjects
As shown in Figure 1, 99 healthy Chinese subjects were screened and 40 of them (12 males and 28 females) were enrolled for the study under fasting condition. Ultimately, 39 subjects completed the study, but one withdrew after the test drug administration for personal reason in T-R drug group. For the study under fed condition, a total of 112 healthy Chinese subjects were screened and 40 of them (12 males and 28 females) were enrolled. Finally, 39 subjects completed the study, with 1 withdrawing after the test drug administration due to advanced menstruation in T-R drug group. The T-R and R-T drug groups were well matched based on demographic characteristics, including age, gender, ethnicity, height and BMI (Table 1).
 |
Table 1 Baseline Demographic Characteristics – FAS |
PK Parameters
All PK parameters were analyzed based on PKCS and PKPS. In the study under fasting condition, one subject was excluded from PKCS and PKPS because the drug plasma concentration was lower than the LLOQ (1.00 ng/mL) in the test drug group, and another one was excluded because of withdrawing during reference drug administration. In the study under fed condition, all 40 randomized subjects were included for PKCS and PKPS in the test drug group, while 39 subjects were included in the reference drug group due to one withdrawing for advanced menstruation. The mean plasma concentration–time curves of test versus reference ticagrelor drugs were superimposable under both fasting and fed conditions (Figures 2 and 3), as well as that of AR-C124910XX (Figures S1 and S2). Primary and secondary PK parameters of ticagrelor tablets under both fasting and fed conditions are shown in Table 2. The data showed that no significant differences were found between the groups receiving the test and reference drug of ticagrelor.
 |
Table 2 Pharmacokinetic Parameters of Ticagrelor Under Fasting and Fed Conditions – PKPS |
 |
Figure 2 Mean plasma concentration–time curves of ticagrelor under fasting condition – Pharmacokinetic Analysis Concentration Set (PKCS). |
 |
Figure 3 Mean plasma concentration–time curves of ticagrelor under fed condition – Pharmacokinetic Analysis Concentration Set (PKCS). |
The PK parameters of ticagrelor and AR-C124910XX by gender classification after administrating a single dose of the test and reference drug under fasting and fed conditions are presented in Table 3. As shown, the effect of gender on the PK parameters was almost identical among the group receiving test drug (Supplement Table S3), the group receiving reference drug (Supplement Table S4) and the combined group. From the pooled results under fasting condition, the AUC0-t, AUC0-∞ and Cmax of ticagrelor were increased by 16.9% (P=0.084), 16.9% (P=0.095) and 43.8% (P<0.05), respectively, in the female subjects comparing with the male subjects. Meanwhile, the AUC0-t, AUC0-∞ and Cmax of AR-C124910XX were also statistically higher in females than in males by 49.6%, 49.3% and 45.2%, respectively (P<0.05). In the pooled analysis under fed condition, a 21.2% increase in AUC0-t and a 22.7% increase in AUC0-∞ of AR-C124910XX (P<0.05) were observed in the female subjects comparing with the male subjects, with only numerically higher AUC0-t (13.4%) and AUC0-∞ (14.3%) found for ticagrelor in the female subjects (P>0.05).
 |
Table 3 Summary of PK Parameters of Ticagrelor and AR-C124910XX in the Male and Female Subjects After Administration of T and R Under Fasting and Fed Conditions – PKPS |
Bioequivalence
All 40 subjects were enrolled in the BES under fasting or fed condition. As shown in Table 4, regarding the AUC0-t, AUC0-∞ and Cmax for ticagrelor and its main active metabolite AR-C124910XX, the 90% CIs for the GMRs ranged from 95.09% to 111.67% and were within the predefined range of 80% to 125% under both fasting and fed conditions. The data indicated that the test drug of ticagrelor was bioequivalent to the reference drug regardless of food intake or not. Based on the ANOVA results, a significant period effect was observed in the AUC of ticagrelor under both fasting and fed conditions (P<0.05), as well as in the AUC0-∞ of its metabolite under fed condition. However, it can be negligible for a fully adequate 7-day washout period. As for bioequivalence analysis of Tmax for ticagrelor and AR-C124910XX, there was also no statistically significant difference between test and reference drug based on Wilcoxon signed-rank test under both conditions (P>0.05) (Table 5).
 |
Table 4 Bioequivalence Statistics for PK Parameters of Ticagrelor and AR-C124910XX Under Fasting and Fed Conditions – BES |
 |
Table 5 The Rank of Tmax of Ticagrelor and AR-C124910XX Under Fasting and Fed Conditions Based on Wilcoxon Signed-Rank Test – BES |
Safety
The overview of AEs is summarized in Table 6. No serious AEs were reported. In the study under fasting condition, a total of 12 AEs (T=6, R=6) were recorded in 9 subjects (9/79, 11.4%), and 6 AEs recorded in 6 subjects were thought to be drug-related (6/79, 7.6%). The drug-related AEs were abnormal T wave (5% vs 2.5%), increased uric acid (0% vs 2.5%), increased serum ALT (0% vs 2.5%), and dizziness (2.5% vs 0%). In the study under fed condition, a total of 10 AEs (T=5, R=5) were reported in 8 subjects (8/79, 10.1%), and 3 AEs occurring in 3 subjects were thought to be drug-related (3/79, 3.8%). The drug-related AEs were abnormal T wave (2.5% vs 2.5%) and increased uric acid (2.5% vs 0%). All the AEs were mild and participants spontaneously recovered without special intervention regardless of food, except one case of increased uric acid under the fasting condition, the outcome of which was unknown. The test drug of ticagrelor showed good tolerance in all subjects, as well as the reference drug.
 |
Table 6 Summary of AE Under Fasting and Fed Conditions – SS |
Discussion
The present bioequivalence study was conducted to compare the PK, bioequivalence and safety profiles between the test generic ticagrelor and the branded reference drug in healthy Chinese subjects, with enrolling forty subjects under fasting condition and another forty under fed condition. The results demonstrated that the PK profiles, including Cmax, AUC0-t, AUC0-∞, Tmax, t1/2 and λz, and safety profiles were similar in the two formulations of ticagrelor. Indeed, bioequivalence was well established based on the AUC (AUC0-t and AUC0-∞) and Cmax under fasting condition as well as under fed condition, suggesting the generic ticagrelor can be used as a replacement of the reference drug to improve access and reduce the drug-related cost.
According to the guidelines20,21 on investigation of bioequivalence, AUC0-t and AUC0-∞, the total drug exposure integrated over time, reflect the extent of absorption, while Cmax and Tmax indicate the rate of absorption, all of which are primary and important parameters in the theory of pharmacokinetics. These PK parameters were calculated by the non-compartmental model to explore the PK characteristics of ticagrelor and its metabolites. When concerned with bioequivalence studies, non-compartmental model would get more consistent PK parameters than compartmental models.
Our results showed that the female subjects were more likely to have higher values in AUC and Cmax of ticagrelor and AR-C124910XX than male subjects under fasting and fed conditions. There are only two reported studies investigating the effect of gender on ticagrelor.22,23 Teng et al22 observe that the AUC0-∞ and Cmax of ticagrelor under fasting condition are increased by 37% and 52% in females vs. males, with 55% and 56% increase in AUC0-∞ and Cmax of AR-C124910XX, respectively. Another study23 also reports 20.2% - 33.8% increase in AUC0-∞ and 30.2–32.7% increase in Cmax of ticagrelor and AR-C124910XX in females. Our results were consistent with that reported by these previous studies. The reason could be attributed to the gender-related physical difference, lower hepatic activity of p-gp (the protein of excretion) and so on.24 However, whether the gender difference in PK parameters has clinical significance remains to be explored. In addition, it would be more convincing if the male-to-female ratio reached 1:1, where males accounted for 70% in our study.
Some studies have investigated the PK profiles of a single 90-mg dose of the reference drug ticagrelor in healthy volunteers under fasting condition.23,25,26 Compared with the study26 (N=36) cited by the label of patented ticagrelor (AstraZeneca), the Cmax, Tmax and t1/2 of both formulations of ticagrelor and AR-C124910XX in our study were approximately consistent with that in the United States, showing rapid absorption and elimination. However, the AUC0-t and AUC0-∞ in the present study were slightly higher, indicating higher exposure and bioavailability. One reason for the subtle difference may be accounted by the ethnic disparity. All subjects in our study were Chinese, while most subjects enrolled in the compared study26 were Caucasians. There exist some physiological differences between ethnics, such as smaller body size and lower quantities of CYP3A enzymes in Chinese when compared with Caucasians, which means higher exposure of ticagrelor.27 Besides, pharmacogenetic differences also contribute to the ethnic disparity, where the functional variants of CYP enzymes are common. For example, CYP3A4, the main metabolic enzyme of ticagrelor, has a loss-of-function variant CYP3A4*20, which has a different prevalence in differing ethnics: Chinese (22%), Caucasians (6%).28 It has been reported that Asians show higher bioavailability of ticagrelor, as reflected by AUC, than Caucasians in many studies.29–31 Meanwhile, the AUC values were in agreement with a study23 (N=25) conducted in Chinese subjects under fasting condition. Another reason for the higher AUC observed in our study may be due to the influence of gender. It has been demonstrated that females have higher AUC of ticagrelor and AR-C124910XX than males in the present and previous study,22,24 with 30% females in our study compared with 22.2% in Teng’s study.26
From the results under fed condition, Tmax of ticagrelor and AR-C124910XX was delayed due to a standard high-fat, high-calorie meal in both test and reference drug, compared with the current results under fasting condition. Meanwhile, food consumption increased the AUC but decreased the Cmax of ticagrelor, while the AUC and Cmax of AR-C124910XX all decreased, regardless of the test or reference drug. It indicated that food could enhance the absorption of ticagrelor, reflected by increased AUC, but slow down the rate of absorption reflected by decreased Tmax and Cmax, which thus influenced the formation of the main metabolite (AR-C124910XX). However, our finding was slightly different from the previous study cited by the label of patented ticagrelor (AstraZeneca).25,32 In the cited study,32 healthy Hispanics (N=19), Blacks (N=5) and Caucasians (N=2) subjects were enrolled and administrated with 3*90 mg ticagrelor tablets to investigate the food–drug interactions. It has been found that food results in a 21% increase in AUC of ticagrelor and a 22% decrease in Cmax of AR-C124910XX, with no effect on Cmax of ticagrelor and AUC of AR-C124910XX. Inter-ethnic difference maybe could account for the variability, with different levels of metabolizing enzymes and transporters, influencing the drug absorption and metabolism.27 Moreover, different study design might also be one of the reasons. The cited study32 is conducted with the same subjects, minimizing the variation between groups under both fasting and fed conditions. However, with the main objective to estimate the bioequivalence rather than food–drug interactions, we conducted the present study in two different groups of subjects, causing an imprecise influence of food on PK profiles of ticagrelor. In conclusion, there existed some subtle deviations in AUC and Cmax compared with the previous study, but it made no difference to the bioequivalence between the test drug and the reference drug.
Safety evaluation indicated all the AEs were mild and the affected participants recovered without special intervention, except one participant with AE lost contact during follow-up. The AEs reported in the our study were abnormal T wave, positive urine albumin and RBC, increased occult blood, uric acid, serum ALT, WBC and neutrophils percentage, dizziness, hemorrhage and hyperchlorhydria, which had been reported in previous studies.25 There was no significant difference in the rate of AEs between the test and reference drug groups under fasting as well as fed condition. The most common AEs reported in the label of ticagrelor are bleeding (11.6%), increased uric acid (22%) and dyspnea (13.8%), which have also occurred in the present study apart from dyspnea. In short, both formulations of ticagrelor showed good tolerance regardless of fast or fed condition in healthy Chinese subjects.
Conclusions
The obtained primary PK parameters (Cmax, AUC0-t, AUC0-∞) in healthy Chinese subjects demonstrated that the test drug of ticagrelor was bioequivalent to the reference drug regardless under fasting or fed condition. The two formulations were pharmacokinetically similar and well tolerated. These findings indicated that the test generic ticagrelor can be used as an alternative medicine to the branded reference drug in China.
Abbreviations
PK, pharmacokinetics; Cmax, peak concentration; AUC, area under the curve; AUC0–t, AUC from zero to last quantifiable concentration; AUC0–∞, AUC from zero to infinity; CIs, confidence intervals; GMR, geometric mean ratio; AEs, adverse events; ACS, acute coronary syndromes; DAPT, dual antiplatelet therapy; ACCF/AHA, American College of Cardiology Foundation/American Heart Association; CYP450, cytochrome P450 enzymes; FDA, United States Food and Drug Administration; IPA, inhibition of platelet aggregation; T, test drug; R, reference drug; BMI, body mass index; ECG, electrocardiogram; IEC, Independent Ethics Committee; PLA, People’s Liberation Army; GCP, the Guidelines of Good Clinical Practice; ICF, informed consent forms; T-R, Test-Reference; R-T, Reference-Test; LC-MS/MS, liquid chromatography-tandem mass spectrometry; QC, quality controls; LQC, low-quality control; GMQC, geometric mean-quality control; MQC, middle-quality control; HQC, high-quality control; λz, rate constant of apparent terminal elimination; t1/2, terminal elimination half-life; Tmax, time to Cmax; F, relative bioavailability; SOC, System Organ Class; PT, Preferred Term; CTCAE, Common Terminology Criteria for Adverse Events; FAS, full analysis set; SS, safety set; PKCS, pharmacokinetic analysis concentration set; PKPS, pharmacokinetic analysis parameter set; BES, bioequivalence analysis set; %CV, coefficient of variation; ANOVA, analysis of variance.
Date Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
The clinical trial was approved by the Independent Ethics Committee of People’s Liberation Army General Hospital (C2017-061-03). The study was conducted in accordance with the Declaration of Helsinki (1989), the Guidelines of Good Clinical Practice and local applicable laws and regulations. All subjects have provided written informed consent forms prior to their participation in the study.
Consent for Publication
All named authors take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Acknowledgment
All authors sincerely acknowledge the subjects who were enrolled for their contributions to the clinical trial.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors declare that there are no conflicts of interest.
References
1. Smith JN, Negrelli JM, Manek MB, Hawes EM, Viera AJ. Diagnosis and management of acute coronary syndrome: an evidence-based update. J Am Board Fam Med. 2015;28(2):283–93. doi:10.3122/jabfm.2015.02.140189
2. Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139–e596.
3. Tersalvi G, Biasco L, Cioffi GM, Pedrazzini G. Acute coronary syndrome, antiplatelet therapy, and bleeding: a clinical perspective. J Clin Med. 2020;9(7):2064. doi:10.3390/jcm9072064
4. Al-Salama ZT, Keating GM, Keam SJ. Ticagrelor: a review in long term secondary prevention of cardiovascular events. Drugs. 2017;77(18):2025–2036. doi:10.1007/s40265-017-0844-8
5. Dobesh PP, Finks SW, Trujillo TC. Dual antiplatelet therapy for long-term secondary prevention of atherosclerotic cardiovascular events. Clin Ther. 2020;42(10):2084–2097. doi:10.1016/j.clinthera.2020.08.003
6. Pereira NL, Rihal CS, So DYF, et al. Clopidogrel pharmacogenetics. Circ Cardiovasc Interv. 2019;12(4):e007811. doi:10.1161/CIRCINTERVENTIONS.119.007811
7. Wang Y, Chen W, Lin Y, et al. Ticagrelor plus aspirin versus clopidogrel plus aspirin for platelet reactivity in patients with minor stroke or transient ischaemic attack: open label, blinded endpoint, randomised controlled Phase II trial. BMJ. 2019;365:l2211. doi:10.1136/bmj.l2211
8. Deeks ED. Ticagrelor: a review of its use in the management of acute coronary syndromes. Drugs. 2011;71(7):909–933. doi:10.2165/11206850-000000000-00000
9. Kubisa MJ, Jezewski MP, Gasecka A, Siller-Matula JM, Postula M. Ticagrelor – toward more efficient platelet inhibition and beyond. Ther Clin Risk Manag. 2018;14:129–140. doi:10.2147/TCRM.S152369
10. Wang H, Qi J, Li Y, et al. Pharmacodynamics and pharmacokinetics of ticagrelor vs. clopidogrel in patients with acute coronary syndromes and chronic kidney disease. Br J Clin Pharmacol. 2018;84(1):88–96. doi:10.1111/bcp.13436
11. Rosa GM, Bianco D, Valbusa A, et al. Pharmacokinetics and pharmacodynamics of ticagrelor in the treatment of cardiac ischemia. Expert Opin Drug Metab Toxicol. 2016;12(12):1491–1502. doi:10.1080/17425255.2016.1244524
12. Gurbel PA, Bliden KP, Butler K, et al. Response to ticagrelor in clopidogrel nonresponders and responders and effect of switching therapies: the RESPOND study. Circulation. 2010;121(10):1188–1199. doi:10.1161/CIRCULATIONAHA.109.919456
13. Mahaffey KW, Held C, Wojdyla DM, et al. Ticagrelor effects on myocardial infarction and the impact of event adjudication in the PLATO (Platelet Inhibition and Patient Outcomes) trial. J Am Coll Cardiol. 2014;63(15):1493–1499. doi:10.1016/j.jacc.2014.01.038
14. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045–1057. doi:10.1056/NEJMoa0904327
15. Andell P, James SK, Cannon CP, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes and chronic obstructive pulmonary disease: an analysis from the platelet inhibition and Patient Outcomes (PLATO) Trial. J Am Heart Assoc. 2015;4(10):e002490. doi:10.1161/JAHA.115.002490
16. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC Guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64(24):e139–e228.
17. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease, 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction, 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes, and 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation. 2016;134(10)e123–55.
18. Collet J-P, Thiele H. The ‘Ten Commandments’ for the 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2020;41(37):3495–3497. doi:10.1093/eurheartj/ehaa624
19. Wijeyeratne YD, Joshi R, Heptinstall S. Ticagrelor: a P2Y12 antagonist for use in acute coronary syndromes. Expert Rev Clin Pharmacol. 2012;5(3):257–69. doi:10.1586/ecp.12.17
20. Center for Drug Evaluation, National Medical Products Administration(NMPA). Guideline on the investigation of bioequivalence. Available from: http://www.cde.org.cn/zdyz.do?method=largePage&id=353342c97683d4fb.
21. FDA. Center for Drug Evaluation and Research (CDER). Guideline for bioequivalence studies with pharmacokinetic endpoints for drugs submitted under an ANDA. Available from: https://www.fda.gov/media/87219/download.
22. Teng R, Mitchell P, Butler K. Effect of age and gender on pharmacokinetics and pharmacodynamics of a single ticagrelor dose in healthy individuals. Eur J Clin Pharmacol. 2012;68(8):1175–1182. doi:10.1007/s00228-012-1227-4
23. Feng W, Liu D, Wang Y, et al. Effects of food and gender on pharmacokinetics of ticagrelor and its main active metabolite AR-C124910XX in healthy Chinese subjects. Int J Clin Pharmacol Ther. 2018;56(8):372–380. doi:10.5414/CP203220
24. Bebawy M, Chetty M. Gender differences in p-glycoprotein expression and function: effects on drug disposition and outcome. Curr Drug Metab. 2009;10(4):322–328. doi:10.2174/138920009788498996
25. FDA. The label on ticagrelor. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/022433s029lbl.pdf.
26. Teng R, Carlson G, Hsia J. An open-label, randomized bioavailability study with alternative methods of administration of crushed ticagrelor tablets in healthy volunteers. Int J Clin Pharmacol Ther. 2015;53(2):182–189. doi:10.5414/CP202202
27. Ramamoorthy A, Pacanowski MA, Bull J, Zhang L. Racial/ethnic differences in drug disposition and response: review of recently approved drugs. Clin Pharmacol Ther. 2015;97(3):263–273. doi:10.1002/cpt.61
28. Westlind-Johnsson A, Hermann R, Huennemeyer A, et al. Identification and characterization of CYP3A4*20, a novel rare CYP3A4 allele without functional activity. Clin Pharmacol Ther. 2006;79(4):339–349. doi:10.1016/j.clpt.2005.11.015
29. Hiasa Y, Teng R, Emanuelsson H. Pharmacodynamics, pharmacokinetics and safety of ticagrelor in Asian patients with stable coronary artery disease. Cardiovasc Interv Ther. 2014;29(4):324–333. doi:10.1007/s12928-014-0277-1
30. Li H, Butler K, Yang L, Yang Z, Teng R. Pharmacokinetics and tolerability of single and multiple doses of ticagrelor in healthy Chinese subjects: an open-label, sequential, two-cohort, single-centre study. Clin Drug Investig. 2012;32(2):87–97. doi:10.2165/11595930-000000000-00000
31. Teng R, Butler K. Pharmacokinetics, pharmacodynamics, and tolerability of single and multiple doses of ticagrelor in Japanese and Caucasian volunteers. Int J Clin Pharmacol Ther. 2014;52(6):478–491. doi:10.5414/CP202017
32. Teng R, Mitchell PD, Butler K. Lack of significant food effect on the pharmacokinetics of ticagrelor in healthy volunteers. J Clin Pharm Ther. 2012;37(4):464–468. doi:10.1111/j.1365-2710.2011.01307.x
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
