Back to Journals » Drug Design, Development and Therapy » Volume 13
Pharmacokinetics and Safety of Esketamine in Chinese Patients Undergoing Painless Gastroscopy in Comparison with Ketamine: A Randomized, Open-Label Clinical Study
Authors Wang J , Huang J, Yang S, Cui C, Ye L, Wang S, Yang G , Pei Q
Received 24 July 2019
Accepted for publication 30 October 2019
Published 6 December 2019 Volume 2019:13 Pages 4135—4144
DOI https://doi.org/10.2147/DDDT.S224553
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Jing Wang,1,2,* Jie Huang,1,3,* Shuang Yang,1,3 Chang Cui,1,3 Ling Ye,1,3 Sai-ying Wang,4 Guo-ping Yang,1,3 Qi Pei1,2
1Center for Clinical Pharmacology, The Third Xiangya Hospital, Central South University, Changsha, Hunan 410013, People’s Republic of China; 2Department of Pharmacy, The Third Xiangya Hospital, Central South University, Changsha, Hunan 410013, People’s Republic of China; 3Clinical Trails Center, The Third Xiangya Hospital, Central South University, Changsha, Hunan 410013, People’s Republic of China; 4Department of Anesthesiology, The Third Xiangya Hospital, Central South University, Changsha, Hunan 410013, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Guo-ping Yang; Qi Pei
The Third Xiangya Hospital, Central South University, Yinpenling Street, Yuelu District, Changsha, Hunan 410013, People’s Republic of China
Tel +86 131 7041 9804
Fax +86 731 8861 8326
Email [email protected]; [email protected]
Purpose: To assess the pharmacokinetics and safety of pure S-ketamine (esketamine) in Chinese patients undergoing painless gastroscopy and evaluate the potential advantage of esketamine in clinical treatment compared with racemate ketamine hydrochloride injection.
Patients and methods: A randomized, open-label, parallel-controlled, Phase I study was performed with 32 patients undergoing painless gastroscopy. Patients received a single dose of esketamine (0.5 mg/kg) or racemic ketamine (1 mg/kg, esketamine:R-ketamine=1:1), injected in 10 s. Blood samples were collected for pharmacokinetic analysis. The concentrations of esketamine, R-ketamine, S-norketamine, and R-norketamine were measured with a validated liquid chromatography with tandem mass spectrometry (LC-MS/MS) method.
Results: After administering a single dose of esketamine and racemate ketamine, the pharmacokinetics parameters of esketamine and S-norketamine are both similar in treatment groups. The clearance of esketamine in two groups was 18.1±3.2 and 18.4±3.4 mL/min•kg, respectively. However, in the ketamine group, esketamine has a larger clearance than R-ketamine (18.4±3.4 mL/min·kg vs 15.8±3.1 mL/min·kg, P<0.001). Further analysis showed that gender did not affect the pharmacokinetics of esketamine and racemate ketamine. Regarding the safety of esketamine and racemate ketamine, no serious adverse events were observed during treatment, and the incidences of adverse events were 75.0% (esketamine) and 87.5% (racemate ketamine). The main adverse reactions were dizziness, agitation, nausea, vomiting, headache, and fatigue. However, compared with racemic ketamine, esketamine offers a shorter recovery time (9 mins vs. 13 mins, P<0.05) and orientation recovery time (11.5 mins vs. 17 mins, P<0.05) after short anesthesia.
Conclusion: Esketamine administration as a single dose of 0.5 mg/kg was generally safe and tolerated in patients undergoing painless gastroscopy. In terms of anesthesia, a relatively small dose of esketamine can be used instead of racemate ketamine for routine treatment without consideration of gender differences.
Keywords: pharmacokinetics, esketamine, racemate ketamine, anesthesia, Chinese patients
Introduction
Ketamine is an antagonist of the N-methyl-d-aspartate receptor which has been used as an anesthetic analgesic for clinical treatment since the 1960s.1 It derived from phencyclidine (PCP) and is designed to alleviate the serious psychosis/psychotic side effects and potential abuse of maternal drugs.2 In recent years, ketamine has been found to have potential therapeutic uses in pain management, neurology, and psychiatry.3–6 Low-dose intravenous ketamine can reduce perioperative opioid consumption and can provide chronic postsurgical pain relief for patients.7,8 High dose ketamine produces anesthetic and analgesic effects.9
Ketamine is a racemic mixture containing two optical isomers, S(+)-ketamine (Esketamine) and R(-)-ketamine.10 Esketamine, a right-handed split of ketamine, entered the German market in 1997 and then was listed in several European countries. Its anesthetic effect is twice as potent as a racemic mixture, and its potency is approximately three times higher than (R)-ketamine.11–14 Because of the dose-dependent side effects of ketamine, low-dose esketamine can reduce the incidence of anesthetic side reactions.15,16 At the equivalent dose of analgesia in healthy volunteers, esketamine has a lower incidence of psychotropic side effects than racemic ketamine, resulting in less impairment in concentration capacity and primary memory and fast recovery.17,18
At present, several studies have reported the pharmacokinetic and pharmacodynamic characteristics of ketamine and esketamine, but the results are not consistent. Geisslinger conducted a study on the stereoselective pharmacokinetics and pharmacodynamics of ketamine in surgical patients after intravenous injection of esketamine (1 mg/kg) or racemic ketamine (2 mg/kg).19 It was found that esketamine was not converted to R(-)-ketamine, and the clearance of esketamine in the racemate ketamine was larger than esketamine, whereas Ihmsen performed a study of continuous pumping of esketamine and racemic ketamine in healthy volunteers, in which the clearance of esketamine in the racemate ketamine was smaller than that of esketamine alone.20
Esketamine has not been listed in China, and the pharmacokinetics and pharmacodynamics of esketamine in the Chinese require further study. Recently, Hengrui Medicine Co., Ltd. completed the preclinical study of esketamine and obtained the clinical research approval from SFDA. This study intended to use racemate ketamine hydrochloride as a reference drug to explore and compare the pharmacokinetics, efficacy, and safety of esketamine and racemate ketamine for the first time in Chinese patients and to provide information for the clinical treatment of esketamine.
Materials and Methods
Participants
A total of 32 volunteers who underwent conventional gastroscopy were included in the study (16 males and 16 females). None of them had general anesthesia contraindications. Patients who are allergic to ketamine hydrochloride, lidocaine, esketamine, propofol, opioids, tropisetron, and neostigmine, and have intolerance to belladonna alkaloids were excluded. All the volunteers provided written informed consent. They were randomized to receive esketamine (0.5 mg/kg) or racemate ketamine (1 mg/kg). In the esketamine group, volunteers were aged 23 to 44 years old, with BMIs of 19.02–25.78 kg/m2. In the ketamine group, volunteers were aged 27 to 58 years old, and the BMI was 19.48–28.93 kg/m2. All volunteers had ASA grades of I–II. Thirty-two volunteers completed the study, and no volunteers withdrew from the study. Table 1 summarizes the baseline demographic information.
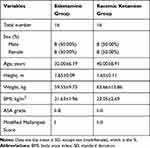 |
Table 1 Demographic Variables of Volunteers Who Underwent Painless Gastroscopy |
Study Design
This was a randomized,open-label, parallel-control, single-center study based on a flowchart (Figure 1). The aim of this study was to evaluate the pharmacokinetics and safety profile of esketamine and racemate ketamine in patients and compare the pharmacokinetics and safety to provide a reference for clinical use. The study was approved by the Medical Ethics Committee of the Third Xiangya Hospital of Central South University. It was conducted in accordance with the principles of the Declaration of Helsinki, Good Clinical Practice Guidelines, and the Guideline for Good Clinical Principles recommended by the SFDA. This study was registered at Chinese Clinical Trial Registry (http://www.chictr.org, number: ChiCTR-IIR-17013178).21
 |
Figure 1 Flowchart of the study design. Blank indicating that randomized, open-label, parallel-control trail until Pharmacokinetics and safety analysis. |
Volunteers were asked for information about previous medical history and history of drug use. Then, patients underwent physical and laboratory tests, baseline vital signs, height, weight were recorded, and body mass index was calculated. Before the examination, 2 mL of 2% lidocaine cement was used for local anesthesia in the throat, in addition to subcutaneous injection of 2% lidocaine 0.2–0.5 mL. When painless gastroscopic anesthesia was performed, according to the weight of the patients, the appropriate volume of racemate ketamine hydrochloride solution (10 mg/mL) or esketamine hydrochloride solution (5 mg/mL) was intravenously administered to the subject (injection within 10 s). Immediate intravenous injection of propofol 0.6 mg/kg was administered and an endoscopic examination was started when the eyelash reflex disappeared, and no obvious limb activity occurred. Simultaneously, propofol was continuously pumped at a rate of 0.25 mg/kg/hr for 15 mins. If there was obvious body motion or swallowing reflex, 0.5 mg/kg propofol was added. In this study, it takes approximately 19±6 mins to do a gastroscopic examination,and only the oral dilator was used to dilate the oral cavity, no additional muscle relaxants or endotracheal intubation was used.
Blood Collection and Bioanalysis
Arterial blood samples (3 mL each) were collected before dose administration (0 hr), immediately after the end of injection, 1 min, 2 mins, 4 mins, 6 mins, 10 mins, 20 mins, 30 mins, 1 hr, 1.5 hrs, and 2 hrs after dosing. Venous blood samples were collected at 4 hrs, 6 hrs, 8 hrs, 12 hrs, and 24 hrs after dosing. Plasma samples were frozen at 20°C before analysis.
Pharmacokinetic measurements of esketamine, R-ketamine, R-norketamine, and S-norketamine in plasma were performed using liquid chromatography with tandem mass spectrometry (LC-MS/MS) after liquid-liquid extraction. Gradient elution was performed on a CHIRALPAK® AGP 5 μm, 150 x 4.0 mm column with trimethoprim as an internal standard. Using positive ion mode, MRM, the ion pairs of esketamine, R-ketamine, R-norketamine and S-norketamine, and internal standard are 238.800→125.100, 238.801→125.101, 224.001→163.001, 224.000→163.000 and 291.600→230.100, respectively. The calibrated range of the method was 2.50 to 1250 ng/mL for all analytes. The intra- and inter-day precisions (CV, %) were <15%, and the accuracies (%) were within the range of 85.0–115.0%.
Pharmacokinetic Analysis
Pharmacokinetic analysis was made from both arterial blood and venous blood and performed with a non-compartmental method using the Pheonix WinNonlin 7.0 (Pharsight Corporation, CA, USA). Actual blood sampling time of each patient was used for analysis. Maximum observed drug concentrations (Cmax) and time of maximum observed drug concentration (Tmax) were obtained directly from the plasma concentration–time curves of esketamine and racemate ketamine. The area under the plasma concentration–time curve from time 0 to the last measurement (AUC0–t) was calculated using the linear trapezoidal method for ascending concentrations and the log trapezoidal method for descending concentrations. The AUC0-∝ was calculated as AUC0–t+ Ct/λz, where Ct is the last measured concentration, and λz is the elimination rate constant calculated using linear regression of the log-linear portion of the plasma concentration–time curve. Clearance (CL) was estimated as dose divided by AUC0-∝, and the volume of distribution (V) was determined by dividing the apparent CL by λz.
Safety Assessment
Safety was assessed according to interviews and adverse event (AE) monitoring. All adverse events that occurred during the clinical study were required to be reported, including abnormalities in clinical symptoms, vital signs, and laboratory tests, and the clinical significance was determined by the monitoring physician. The occurrence, severity, and frequency of AE and/or adverse drug reactions were compared between two treatment groups. If clinical abnormalities were present, further follow-ups were required.
Anesthesia Recovery Index
The anesthesia recovery time and orientation recovery time were recorded. Patient’s recovery time was defined as the time between the administration of the drug and patient’s modified OAA/S (Observer’s Assessment of Alertness/Sedation Scale) score of 5. The recovery orientation was assessed by the “orientation test”. Patients were asked for 10 questions (1 score per question, total of 10 scores); the number of correctly answered questions was counted for each patient. The time from the administration of the drug to the patient’s orientation score of 10 was determined as the orientation recovery time. Sedation scores were used to assess the degree of sedation at 15 mins, 30 mins, and 60 mins after palinesthesia.
Statistical Analyses
This study mainly focuses on PK properties, anesthetic outcomes are secondary findings, the sample size calculations are performed based on clinical pharmacokinetics study guidance. According to the Guidelines for the Study of Clinical Pharmacokinetics of Chemical Drugs recommended by the SFDA,22 pharmacokinetic studies require 8–12 volunteers per group. And Guidance for Industry of Statistical Approaches to Establishing Bioequivalence by FDA suggests that a minimum number of 12 evaluable subjects should be included in any BE study.23 Given the 20% drop-out rate, 16 patients were enrolled in the esketamine group and the ketamine group, respectively.
Statistical analysis was conducted using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA). Student’s t-test and paired t-test were performed on the logarithmically transformed pharmacokinetic parameters (except Tmax) to assess the statistical significance of the two groups. The differences of Tmax, recovery time, and orientation recovery time were further confirmed by the Wilcoxon Mann–Whitney rank-sum test. Sedation score was analyzed using a Cochran Mantel–Haenszel (CMH) chi-square test. P<0.05 was considered to be significant.
Results
Pharmacokinetics
The mean plasma concentration versus time profiles for esketamine and R-ketamine after administering a single dose of esketamine (0.5 mg/kg, test group) and racemate ketamine (1 mg/kg, esketamine:R-ketamine=1:1, reference group) are shown in Figure 2. The pharmacokinetic parameters and statistics of esketamine and R-ketamine are shown in Table 2. The pharmacokinetic parameters of esketamine in pure isomers were close to those obtained in the racemate. After administration of racemate ketamine, the comparison of variables revealed a significant difference of esketamine and R-ketamine within groups (except Tmax and Vz)
 |
Table 2 The Pharmacokinetic Parameters of Esketamine, R-Ketamine, S-Norketamine, and R-Norketamine in the Esketamine Group and the Ketamine Group |
For the main metabolite S-norketamine, the pharmacokinetic variables of S-norketamine following administration of the single esketamine were similar to the administration of the racemate ketamine. The main pharmacokinetic parameters of the metabolites S-norketamine and R-norketamine are shown in Table 2 and mean plasma concentration–time curves of S-norketamine and R-norketamine are shown in Figure 3.
This study also compared sex differences in the kinetic profile of esketamine and its major metabolite. As shown in Table 3, gender had no significant effect on the pharmacokinetics of racemate ketamine and esketamine.
 |
Table 3 The Pharmacokinetic Parameters of Esketamine and S-Norketamine in Different Genders (Esketamine Group) |
Safety
Twenty-three AEs occurred in 12 volunteers who were administered at least one dose of esketamine. The incidence of AE was 75.0%, of which 16 were considered as related to treatment. There were 31 AEs in the ketamine group, and the AE incidence rate was 87.5%, with 25 cases related to treatment. The main adverse reactions were dizziness, agitation, nausea, vomiting, headache, fatigue, high blood pressure, cramps, tremors, hypertonia, and cold sweat. There were no serious AEs and serious adverse reactions reported, and none of the volunteers withdrew from the study due to AEs. The distribution and incidence of AEs related to the study drugs in each group are shown in Table 4.
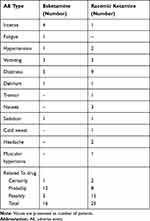 |
Table 4 Summary of the Number and Types of Adverse Events That Related to the Drugs in Each Group |
Anesthesia Recovery Index
The results are shown in Table 5. Under the lower maintenance dose regimen of propofol (0.25mg/kg/hr,continuous pumping for 15 mins) in this trial, 9 patients woke up approximately 4.9±2.4 mins before stopping propofol dosing, and 7 patients woke up approximately 3.0±3.5 mins after stopping propofol dosing in ketamine group, and all the patients woke up 7.0±3.1 mins before stopping esketamine dosing. The sedation score in the esketamine and ketamine groups was similar at each time point after palinesthesia (P>0.05). The recovery time and orientation recovery time of the two groups were significantly different, and esketamine offers a shorter recovery time (9 mins vs 13 mins, P<0.05) and orientation recovery time (11.5mins vs 17mins, P<0.05) after short anesthesia compared to racemic ketamine. The effects of propofol on the recovery time were comparable in two groups, due to the same dosage regimen for propofol in the whole study. However, the anesthesia recovery index did not show statistical differences between males and females (Table 6).
 |
Table 5 Evaluation of Anesthesia Recovery Index After Palinesthesia |
 |
Table 6 Evaluation of Anesthesia Recovery Index After Palinesthesia in Different Genders (Esketamine Group) |
Discussion
The purpose of this study was to evaluate and compare the pharmacokinetics, efficacy, and safety of esketamine with those of racemate ketamine in Chinese patients. The pharmacokinetic parameters of esketamine and S-norketamine are both similar in the pure isomer and the racemate. The results of safety showed that a single dose of 0.5 mg/kg of esketamine and 1 mg/kg of ketamine were both safe and well tolerated in Chinese patients with no serious adverse events. Also, there were no sex differences in the pharmacokinetics of esketamine and S-norketamine in the pure isomer. However, compared with racemate ketamine, esketamine had a shorter recovery time and orientation recovery time, which present potential clinical advantages.
In the current study, the pharmacokinetic parameters of esketamine and S-norketamine in the esketamine and racemate ketamine groups are both comparable. A similar phenomenon was also observed in the study of Geisslinger.19 However, Ihmsen found that esketamine had a higher clearance in the pure isomer than for the racemate.20 The modes of administration and pharmacokinetic interactions may partly account for the inconsistencies, which was also mentioned by Ihmsen. The continuous infusion for esketamine and ketamine was used in the study of Ihmsen, but the short injection was adopted in the current study and Geisslinger’s study. Since the volunteers were patients, other drugs were administrated with esketamine and ketamine in our study (propofol and lidocaine) and in Geisslinger’s study (midazolam). Hence, the possible pharmacokinetic interactions may induce inconsistent results. Though patients in both esketamine and ketamine groups were combined with propofol during anesthesia process, the dose regimen of propofol of two groups was identical (0.6mg/kg for the loading dose; 0.25mg/kg/hr for the maintenance dose). Therefore, the effect of propofol was considered to be balanced in two groups. In addition, Ihmsen also pointed out the difference between arterial and venous blood samples, arterial blood samples are more appropriate to estimate the pharmacokinetic profile, but only during the first minutes of or after administration venous concentrations tend to be smaller than the arterial concentrations. In the present study, in order to consider the convenience of clinical operation and safety issues (collection arterial blood samples are relative easier under anesthesia), arterial blood samples were collected in the first 2 hrs in the operating room, and venous blood samples were collected afterwards (patients returned to the Phase I ward). Therefore, the type of blood samples was not a possible reason for the inconsistencies.
Marnix found that the clearance of esketamine and S-norketamine was 20% greater in women, with higher drug plasma concentrations in men.24 However, no significant difference for pharmacokinetics of esketamine and S-norketamine between genders was observed in the current study (Table 3) and anesthesia recovery index is comparable between gender (Table 6). In our study, the P value for gender difference for AUC of esketamine might be change (e.g., less than 0.05), if the sample size is expanded. However, the relative change of AUC between genders was less than 20%, which was considered clinically irrelevant. In addition, no significant gender difference was observed in the efficacy of the esketamine (Table 6). Based on our pharmacokinetic data and anesthesia recovery index result, we consider that Chinese patients with different genders may not require dose adjustment in the administration of esketamine and ketamine.
The post-awake sedation score observed in the study showed no significant difference between the esketamine and racemic ketamine groups, but the recovery time and orientation recovery time were shorter in the esketamine group (P < 0.05). The probable reason is that both esketamine and R-ketamine act on the N-methyl-D-aspartate receptor and then produce anesthetic and analgesic effects.11 Therefore, esketamine can better meet the requirements of outpatient gastroscopy, which could make patients wake up and return to normal faster than racemic ketamine.
The limitation of this study was the lack of evaluation of cognitive impairment after recovery, such as mood and clustered psychological effects and concentration capacity. Further studies on the relationship between pharmacokinetics and perceptual/cognitive effects of esketamine are needed.
Conclusion
This is the first study to investigate the pharmacokinetic properties (parent and metabolites and gender difference) as well as pharmacodynamic response (e.g., recovery time, sedation score) of esketamine in Chinese population. Our results regarding pharmacokinetics and pharmacodynamics parameters do not differ from previous studies conducted in population with different ethnicities. This no-ethnical difference finding of esketamine is important to confirm its efficacy and feasibility for routine surgical anesthesia, and we consider that Chinese patients with different genders may not require dose adjustment in the administration of esketamine and ketamine.
Ethical Approval
This study has been approved by the Medical Ethics Committee of the Third Xiangya Hospital of Central South University and has been performed according to the ethical standards laid down in the 1964 Declaration of Helsinki.
Data Sharing Statement
The raw data of this study will not be shared because of confidentiality.
Informed Consent
Informed consent was written by all individual participants included in the study.
Acknowledgments
This study was supported by the International Science & Technology Cooperation Program of China (No. 2014DFA30900), National Natural Science Foundation of China (No. 81673519) and National Major New Drug Creation Project of China (No. 2020ZX09201010).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Domino EF, Chodoff P, Corssen G. Pharmacologic effects of CI-581, a new dissociative anesthetic, in man. Clin Pharmacol Ther. 1965;6:279–291. doi:10.1002/cpt196563279
2. Mion G, Villevieille T. Ketamine pharmacology: an update (pharmacodynamics and molecular aspects, recent findings). CNS Neurosci Ther. 2013;19(6):370–380. doi:10.1111/cns.12099
3. Salloum NC, Fava M, Freeman MP, et al. Efficacy of intravenous ketamine treatment in anxious versus nonanxious unipolar treatment-resistant depression. Depress Anxiety. 2019;36(3):235–243. doi:10.1002/da.22875
4. Frey TM, Florin TA, Caruso M, Zhang N, Zhang Y, Mittiga MR. Effect of intranasal ketamine vs fentanyl on pain reduction for extremity injuries in children: the PRIME randomized clinical trial. JAMA Pediatr. 2019;173(2):140–146. doi:10.1001/jamapediatrics
5. Kapur J. Role of NMDA receptors in the pathophysiology and treatment of status epilepticus. Epilepsia Open. 2018;3(Suppl 2):165–168. doi:10.1002/epi4.12270
6. Domino EF. Taming the ketamine tiger. Anesthesiology. 2010;113(3):678–684. doi:10.1097/ALN.0b013e3181ed09a2
7. Laskowski K, Stirling A, McKay WP, Lim HJ. A systematic review of intravenous ketamine for postoperative analgesia. Can J Anaesth. 2011;58(10):911–923. doi:10.1007/s12630-011-9560-0
8. Bell RF, Dahl JB, Moore RA, Kalso E. Peri-operative ketamine for acute post-operative pain: a quantitative and qualitative systematic review (Cochrane review). Acta Anaesthesiol Scand. 2005;49(10):1405–1428. doi:10.1111/j.1399-6576.2005.00814.x
9. Peltoniemi MA, Hagelberg NM, Olkkola KT, Saari TI. Ketamine: a review of clinical pharmacokinetics and pharmacodynamics in anesthesia and pain therapy. Clin Pharmacokinet. 2016;55(9):1059–1077. doi:10.1007/s40262-016-0383-6
10. Sinner B, Graf BM. Ketamine. Handb Exp Pharmacol. 2008;182:313–333. doi:10.1007/978-3-540-74806-9_15
11. Zanos P, Moaddel R, Morris PJ, et al. Ketamine and ketamine metabolite pharmacology: insights into therapeutic mechanisms. Pharmacol Rev. 2018;70(3):621–660. doi:10.1124/pr.116.015198err
12. Himmelseher S, Pfenninger E. [The clinical use of S-(+)-ketamine – a determination of its place]. Anasthesiol Intensivmed Notfallmed Schmerzther. 1998;33(12):764–770. doi:10.1055/s-2007-994851
13. Adams HA, Werner C. [From the racemate to the eutomer: (S)-ketamine. Renaissance of a substance?] Anaesthesist. 1997;46(12):1026–1042. doi:10.1007/s001010050503
14. White PF, Schuttler J, Shafer A, Stanski DR, Horai Y, Trevor AJ. Comparative pharmacology of the ketamine isomers. Studies in volunteers. Br J Anaesth. 1985;57(2):197–203. doi:10.1093/bja/57.2.197
15. Zou L, Tian SY, Quan X, Ye TH. [Psychedelic effects of subanesthetic doses of ketamine]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2009;31(1):68–72.
16. Bowdle TA, Radant AD, Cowley DS, Kharasch ED, Strassman RJ, Roy-Byrne PP. Psychedelic effects of ketamine in healthy volunteers: relationship to steady-state plasma concentrations. Anesthesiology. 1998;88(1):82–88. doi:10.1097/00000542-199801000-00015
17. Pfenninger EG, Durieux ME, Himmelseher S. Cognitive impairment after small-dose ketamine isomers in comparison to equianalgesic racemic ketamine in human volunteers. Anesthesiology. 2002;96(2):357–366. doi:10.1097/00000542-200202000-00022
18. Engelhardt W, Stahl K, Marouche A, Hartung E. [Recovery time after (S)-ketamine or ketamine racemate. Recovery time after short anesthesia in volunteers]. Anaesthesist. 1998;47(3):184–192. doi:10.1007/s001010050546
19. Geisslinger G, Hering W, Thomann P, Knoll R, Kamp HD, Brune K. Pharmacokinetics and pharmacodynamics of ketamine enantiomers in surgical patients using a stereoselective analytical method. Br J Anaesth. 1993;70(6):666–671. doi:10.1093/bja/70.6.666
20. Ihmsen H, Geisslinger G, Schuttler J. Stereoselective pharmacokinetics of ketamine: R(-)-ketamine inhibits the elimination of S(+)-ketamine. Clin Pharmacol Ther. 2001;70(5):431–438. doi:10.1067/mcp.2001.119722
21. State Food and Drug Administration. Good clinical practice guideline. Available from: http://www.sda.gov.cn/WS01/CL0053/24473.html.
22. State Food and Drug Administration. Guidelines for the study of clinical pharmacokinetics of chemical drugs. Available from: http://samr.cfda.gov.cn/WS01/CL1616/83420.html.
23. US Food and Drug Administration. Guidance for industry of statistical approaches to establishing bioequivalence. Available from: https://www.fda.gov/media/70958/download.
24. Sigtermans M, Dahan A, Mooren R, et al. S(+)-ketamine effect on experimental pain and cardiac output: a population pharmacokinetic-pharmacodynamic modeling study in healthy volunteers. Anesthesiology. 2009;111(4):892–903. doi:10.1097/ALN.0b013e3181b437b1
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


