Back to Journals » Clinical Pharmacology: Advances and Applications » Volume 9
Pharmacokinetics and outcome of tazobactam/piperacillin in Japanese patients undergoing low-flow continuous renal replacement therapy: dosage considerations
Authors Kohama H, Ide T, Ikawa K, Morikawa N, Nishi S
Received 12 November 2016
Accepted for publication 15 December 2016
Published 24 February 2017 Volume 2017:9 Pages 39—44
DOI https://doi.org/10.2147/CPAA.S127502
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Arthur E. Frankel
Hanako Kohama,1 Takeshi Ide,1 Kazuro Ikawa,2 Norifumi Morikawa,2 Shinichi Nishi1
1Division of Intensive Care Unit, Hyogo College of Medicine, Nishinomiya, 2Department of Clinical Pharmacotherapy, Hiroshima University, Hiroshima, Japan
Background: Tazobactam/piperacillin (TAZ/PIPC), which is often combined with continuous renal replacement therapy (CRRT), induces renal excretion and is thought to have a high component removal rate for blood purification. CRRT procedures vary depending on the country, region, and institution. It is not clear whether the dose of TAZ/PIPC for use in Japan can be determined based on studies conducted in other countries. Therefore, in this study, we examined the suitability of recommended dose in Japan.
Methods: The study subjects consisted of 10 patients who received TAZ/PIPC during CRRT in the intensive care unit of Hyogo College of Medicine, Nishinomiya, Japan. We used a one-compartment model to characterize and parameterize the pharmacokinetics of TAZ/PIPC because their blood levels were eliminated monoexponentially.
Results: Compared with the data of healthy adults, the half-lives (t1/2) of both PIPC and TAZ were prolonged while their clearance rates decreased.
Conclusion: For the continuous hemodiafiltration procedure adopted in Japan, we concluded that the dose and frequency were appropriate because the patients who received PIPC/TAZ 2.25 g twice a day during continuous hemodiafiltration maintained appropriate blood levels of both PIPC and TAZ.
Keywords: hemodiafiltration, antibiotics, dosage regimen
Background
Tazobactam/piperacillin (TAZ/PIPC) is an antibiotic combination commonly used in hospital intensive care unit (ICU) and is often combined with continuous renal replacement therapy (CRRT), introduced for treating sepsis and acute renal failure. According to the package insert of TAZ/PIPC (Zosyn®, Taisho Toyama Pharmaceutical Co., Ltd., Tokyo, Japan), the dosage for patients with sepsis or pneumonia is 4.5 g three to four times per day.1–3 However, the regimen should be adjusted based on decreased renal function rather than the renal excretion of the drug. According to the Sanford guide to antimicrobial therapy 2014, the recommended dosages in renal impairment and during CRRT are 2.25 g three to four times and 2.25 g four times per day, respectively.
Because both PIPC and TAZ are low molecular weight compounds (535.57 and 300.29 Da, respectively) with low protein binding rates (16% and 4%, respectively), the component removal rate during blood purification is high.4 In Europe and the USA, it has been reported that 4.5 g of TAZ/PIPC twice a day, which comprises the regimen for CRRT, is low while 4.5 g thrice a day is suitable.5,6 In particular, it is recommended to administer patients with sepsis TAZ/PIPC 4.5 g four times a day for the first 48 h.7 The procedure adopted for CRRT in the USA was reported to be as follows: blood flow, 150 (120–170) mL.min–1 and the amount of blood purification, which is the sum of the dialysis fluid and filtration flow rates, is commonly 35 mL⋅kg−1⋅h−1.8 In the UK, the following procedure was reported: blood flow, 150–200 mL.min–1 and amount of blood purification, 32 (29–36) mL⋅kg−1⋅h−1.9 In Australia and New Zealand, the following specifications were reported: blood flow, 200 mL.min–1 and the amount of blood purification, 24.3 mL⋅kg−1⋅h−1.10 In Japan, the following procedure was reported: blood flow, 80 mL.min–1 and the median amount of blood purification, 16 mL⋅kg−1⋅h−1.11 These reports indicate that the values vary depending on the country, region, and institution.
The modality setting for CRRT in Japan tends to be lower for both blood flow and blood purification than what is used in Western countries. Therefore, it is not clear whether the dose of drugs used in CRRT in Japan can be determined based on studies performed in other countries. Presently, no study has examined the pharmacokinetics (PK) of TAZ/PIPC during CRRT in Japan. Although the recommended dosage of TAZ/PIPC during CRRT is 2.25 g three to four times per day in Japan, it is suggested that a higher dose regimen may be required in studies in other countries. Therefore, we examine the suitability of the current dose of TAZ/PIPC recommended for use in CRRT procedures in Japan.
By the Shiba’s report,12 which TAZ/PIPC was administered to healthy Japanese male volunteers, in the group of TAZ/PIPC 4.5 g four times per day, the half-lives (t1/2), maximum plasma concentration level (Cmax), the area under the plasma concentration–time curve (AUC), the total body clearance (CLtot), and the volume of distribution (Vd) of PIPC were 0.99 h, 267 mg/L, 300 mg⋅h/L, 13.5 L/h, and 13.7 L, respectively. The t1/2, Cmax, AUC, CLtot, and Vd of TAZ were 0.84 h, 32.5 mg/L, 38.7 mg⋅h/L, 13.0 L/h, and 13.6 L, respectively.
Methods
This study was approved by the Ethics Committee of Hyogo College of Medicine. The study protocol and implications were explained to the participants verbally and in writing. The participants provided their written informed consent prior to participation in this study.
The study subjects consisted of 10 patients who received TAZ/PIPC (Zosyn) for the treatment of infectious disease during CRRT in the ICU of Hyogo College of Medicine, Nishinomiya, Japan between December 2011 and March 2015. Of these 10 patients, 6, 3, and 1 received TAZ/PIPC 2.25 g twice and three times a day and 4.5 g twice a day. For patients who received continuous hemodialysis (CHD) as CRRT, the dialysis fluid flow rate was set at 800 mL/h while those who received continuous hemodiafiltration (CHDF) had both the dialysis fluid and replenisher solution flow rates set at 400 mL/h. The blood and filtrate flow rates were set at 80 mL.min–1 and 800 mL/h, respectively, depending on the patient.
Blood sampling was conducted seven times in total (before initiation and after termination of TAZ/PIPC administration, at 30 min, and 1, 2, and 4 h after the completion of administration and immediately before the following dose administration). For blood sampling, blood samples each were collected through the blood removal and autotransfusion dialysis catheter routes, which were defined as the samples before and after filtration, respectively. We also obtained a 3 mL diafiltrate with the blood sampling, and all the samples were frozen at −20°C after the serum was separated. After the linkable anonymizing was applied to the data to ensure that the patients could not be identified, the samples were transferred to the Department of Clinical Pharmacotherapy, Hiroshima University, to measure TAZ/PIPC levels in the blood and diafiltrate by using high-performance liquid chromatography (HPLC).13 Furthermore, acetonitrile and dichloromethane deproteinization was performed on the sample. The HPLC system included a C18 column while the detection was performed at 220 nm with a mobile phase consisting of phosphate buffer (pH 2.6) with acetonitrile (75:25 and 95:5, PIPC and TAZ, respectively). The determination limit was 0.5 and 1 μg/ mL for PIPC and TAZ, respectively. For intra- and interday measurement, the precision was 0.69%–7.24% (PIPC) and 1.10%–9.49% (TAZ), and the accuracy was 99.6%–107% (PIPC) and 97.5%–112% (TAZ).
We used a one-compartment model to characterize and parameterize the PK of TAZ/PIPC, because semilogarithmic plots showed that their blood levels were eliminated monoexponentially. The t1/2 was calculated using the elimination rate constant (kel) as follows: t1/2 = ln 2/kel. The AUC was calculated using the trapezoid summation after binding the overtime plasma concentration values with a straight line. The CLtot and the Vd were calculated as follows: CLtot = intravenous (IV) dose/AUC and Vd = C0/IV dose.
Results
Table 1 shows the patient characteristics, and the common infections leading to the administration of TAZ/PIPC were pneumonia, bacteremia, peritonitis, and cholecystitis. The pathogenic bacteria, which were acquired during empiric therapy in some patients, were Enterococcus faecium, Klebsiella pneumoniae, Acinetobacter baumannii, and Bacteroides ovatus. TAZ/PIPC was used as the first dose in only one patient in the 2.25 g twice daily group while nine received it 3–7 days later. For the CRRT, the CHDF, and CHD modalities were used in eight and two patients, respectively. The CRRT was introduced based on renal indications associated with septic acute kidney injury in all the patients.
Both the blood urea nitrogen (BUN) and creatinine (Cre) levels were elevated in all the patients (2.25 g twice daily, 2.25 g three times daily, and 4.5 g twice daily groups; BUN and Cre, 59±29 and 3.09±1.36, 48±27 and 2.27±0.68, and 128 and 4.8 mg/dL, respectively). The creatinine clearance rate (Ccr) calculated using the Cockcroft–Gault formula was 18.9±6.9, 23.7±5.6, and 11.5 mL.min–1 in the 2.25 g twice daily, 2.25 g three times daily, and 4.5 g twice daily groups, respectively. Six patients developed liver damage and showed increased aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels (AST/ALT 151±174/156±286, 84±53/132±108, and 23/8 U/L in the 2.25 g twice daily, 2.25 g three times daily, and 4.5 g twice daily groups, respectively).
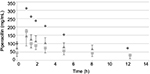
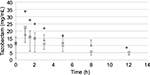
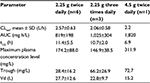
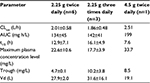
Figures 1 and 2 show the changes in the blood levels of PIPC and TAZ. Tables 2 and 3 show the PK parameters of PIPC and TAZ. The CLtot of PIPC was 2.57±0.63, 2.06±0.58, and 2.2 L/h in the 2.25 g twice daily, 2.25 g three times daily, and 4.5 g twice daily groups, respectively, which indicated that it was reduced by 14%–19% compared to that revealed by the data of the healthy adults. We referred to the data which six Japanese healthy male volunteers received TAZ/PIPC 4.5 g four times a day.12 The CLtot of PIPC in healthy adults was 13.5 L/h. The t1/2 of PIPC was 11.4±5.5, 10.7±2.0, and 6.9 h in the 2.25 g twice daily, 2.25 g three times daily, and 4.5 g twice daily groups, respectively, which indicated that it was prolonged 7–11 times more than that of the healthy adults. The t1/2 of PIPC in the healthy adults was 0.99 h. The CLtot of TAZ was 2.01±0.58, 1.86±0.48, and 2.51 in the 2.25 g twice daily, 2.25 g three times daily, and 4.5 g twice daily groups, respectively, which indicated that the clearance was reduced by 14%–19% compared to that shown by the data of the healthy adults. The CLtot of TAZ in healthy adults was 13.0 L/h. The t1/2 of TAZ was 12.9±7.1, 16.1±4.9, and 7.6 h in the 2.25 g twice daily, 2.25 g three times daily, and 4.5 g twice daily groups, respectively, which indicated that it was prolonged 9–19 times more than that of the healthy adults. The t1/2 of TAZ in the healthy adults was 0.84 h.
Discussion
The drug clearance (CLtot) of PIPC and TAZ was correlated with the blood flow and the amount of blood purification.14 Compared to similar studies performed in other countries, a study by Seyler et al,7 using TAZ/PIPC on a 4.5 g four times daily dosage regimen, reported that the blood flow was set at 150 mL/h, which was higher than that of our study (the blood flow of our study was 80 mL/h). The amount of blood purification was 45 mL.min–1, which was higher than that of our study (the amount of blood purification of our study was 13.3 mL.min–1). In their report, the t1/2 of PIPC was significantly lower (4.16 h) than that of our study (11.4, 10.7, and 7.6 h in the 2.25 g twice daily, 2.25 g three times daily, and 4.5 g twice daily groups, respectively). The CLtot value of PIPC reported by Seyler et al showed a higher value (4.14 L/h) than that of all the groups in our study (2.57, 2.06, and 2.2 L/h in the 2.25 g twice daily, 2.25 g three times daily, and 4.5 g twice daily groups, respectively). The modality setting for CRRT in foreign countries tends to be higher for both blood flow and blood purification than what is used in Japan. Therefore, the t1/2 of the drugs tends to be prolonged and the CLtot tends to be reduced in Japan.
In addition, the theoretical value of the drug clearance rates during CRRT (CLCRRT) can be calculated as follows: CLCRRT = sieving coefficient (Sc) × dialysis waste fluid flow. The CLCRRT of PIPC was determined to be 0.77±0.23, 0.63±0.08, and 0.59 L/h in the 2.25 g twice daily, 2.25 g three times daily, and 4.5 g twice daily groups, respectively. Because of the analysis used in our study, these values were relatively lower than the CLtot was; therefore, the rate of removal of the drugs with CRRT was low. Thus, in the CRRT modality used in Japan, we think that increasing the quantity and additional dosage of TAZ/PIPC is not necessary.
TAZ/PIPC is an antibiotic combination consisting of a beta-lactamase inhibitor and penicillin in a 1:8 ratio. TAZ inactivates the beta-lactamase produced by bacteria, which protects PIPC from being degraded by beta-lactamase. Therefore, it exhibits an antibacterial action against PIPC-resistant bacteria. PIPC shows antibacterial activity by inhibiting bacterial cell wall synthesis. For the combination therapy, a ratio of TAZ:PIPC ranging from 1:8 to 2:1 is thought to provide excellent therapeutic effect, and the ratio of the blood level is maintained at 1:8 to 1:9 without remarkable change.15 Thus, no change in efficacy is anticipated by the changed ratio.
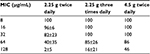
Pseudomonas aeruginosa is considered to exhibit the following characteristics: half-maximal (50%) minimal inhibitory concentration (MIC50) and MIC90 of 8 and 128 μg/mL, respectively.16 The antibacterial effects of beta-lactam derivative antibiotics are known to correlate with the time above MIC (TAM), which suggest that the blood level exceeds the MIC.17 An indication that penicillin antibiotics provide a maximal antibacterial effect is thought to be the achievement of a TAM rate of ≥50%.18 In our study, the TAM% of the bacteria, with an assumed MIC of 8 μg/mL, was 100% in all the groups (2.25 g twice daily, 2.25 g three times daily, and 4.5 g twice daily groups). The MIC, which met the condition requiring that the TAM achieved ≥50%, was 32 μg/mL (Table 4).
A blood level of 2.5–5.0 μg/mL, which is maintained continuously for 1–2 h, is necessary to ensure that TAZ provides beta-lactamase inhibitory effects.15 In our study, all the subjects maintained TAZ blood levels at 2.5 μg/mL. Consequently, even in the 2.25 g twice daily group, which received the lowest drug dose of all the study groups, the beta-lactamase inhibitory effects of TAZ exhibited the maximal antibacterial effect of penicillin antibiotics against bacteria with a MIC value of 32 μg/mL.
Our study revealed that TAZ/PIPC showed a prolonged t1/2 in the 2.25 g twice daily group and a reduced clearance rate that maintained the blood levels. In the pharmacodynamic analysis, the antibacterial effects of the drug with a MIC value of 32 μg/mL can be expected. Therefore, the 2.25 g twice daily regimen can be considered appropriate for the common modality setting of CRRT used in Japan, which consists of a continuous venovenous hemodiafiltration blood flow of 80 mL.min–1 and blood purification amount of 10–16 mL·kg−1·h−1. However, clearance mediated by the residual renal function is thought to occur in patients who underwent CRRT for nonrenal indications, and in such cases, it would be necessary to exercise caution to avoid incidences of under dosing.
Drug metabolism during CRRT is known to be influenced by the percentage liver metabolism exhibited by a patient. In addition, in such situations, the liver metabolism was greatly influenced by various factors, including the liver and residual renal function, tubular secretion,19 and removal of drugs by blood purification of the patient. In our study, the residual renal function was remarkably low, and the rate of drug removal by blood purification was low because the targeted drugs were largely renally excreted, and the criteria for initiating CRRT were renal indication. Therefore, we concluded that the drug concentration was maintained at a lower dose than was expected.
Limitations
We have to state a limitation of this study. The number of investigations was 10. They are very small, and it may be insufficient to assert that 2.25 g twice a day as the dosage of TAZ/PIPC during CRRT is the most appropriate. But we think that just because CRRT is performed does not mean we have to increase the amount and time of dosage of the medicine.
Conclusion
Considering that the common modality setting of TAZ/PIPC administration during CRRT in Japan uses a dosage of 2.25 g twice daily where the blood levels are maintained, we propose that the dose and frequency are appropriate, based on the outcome of this study.
Disclosure
The authors report no conflicts of interest in this work.
References
Watanabe A, Aoki N, Niki Y, Saito A, Kohno S, Shiba K. Clinical pharmacological study of tazobactam/piperacillin in patients with community-acquired pneumonia. Jpn J Chemother. 2010;58:11–28. | ||
Aikawa N, Saito A, Soma K, Watanabe A. Multicenter open-label study of tazobactam/piperacillin in patients with hospital-acquired pneumonia. Jpn J Chemother. 2010;58:50–61. | ||
Shiba K, Ishikawa S, Kawai S, Mikamo H, Yokoyama T. Phase tazobactam/piperacillin (1:8) study in patients with sepsis or infective endocarditis. Jpn J Chemother. 2010;58(S-1):73–87. | ||
Maeda T, Kumuro M, Matsushita H. Pharmacokinetic study of tazobactam/piperacillin in experimental animals. Chemotherapy. 1994;42:206–216. | ||
Valtonen M, Tiula E, Takkunen O, Backman JT, Neuvonen PJ. Elimination of the piperacillin/tazobactam combination during continuous venovenous haemofiltration and haemodiafiltration in patients with acute renal failure. J Antimicrob Chemother. 2001;48(6):881–885. | ||
van der Werf TS, Mulder POM, Zijlstra JG, Uges DR, Stegeman CA. Pharmacokinetics of piperacillin and tazobactam in critically ill patients with renal failure, treated with continuous veno-venous hemofiltration (CVVH). Intensive Care Med. 1997;23(8):873–877. | ||
Seyler L, Cotton F, Taccone FS, et al. Recommended β-lactam regimens are inadequate in septic patients treated with continuous renal replacement therapy. Crit Care. 2011;15(3):R137. | ||
Overberger P, Pesacreta M, Palevsky PM; VA/NIH Acute Renal Failure Trial Network. Management of renal replacement therapy in acute kidney injury: a survey of practitioner prescribing practices. Clin J Am Soc Nephrol. 2007;2(4):623–630. | ||
Gatward JJ, Gibbon GJ, Wrathall G, Padkin A. Renal replacement therapy for acute renal failure: a survey of practice in adult intensive care units in the United Kingdom. Anaesthesia. 2008;63(9):959–966. | ||
The RENAL Study Investigators. Renal replacement therapy for acute kidney injury in Australian and New Zealand intensive care units: a practice survey. Crit Care Resusc. 2008;10(3):225–230. | ||
Hanafusa N. Application of continuous renal replacement therapy: what should we consider based on existing evidence? Blood Purif. 2015;40(4):312–319. | ||
Shiba K. Phase study of tazobactam/piperacillin in healthy volunteers. Jpn J Chemother. 2010;58:1–10. | ||
Connor MJ Jr, Salem C, Bauer SR, et al. Therapeutic drug monitoring of piperacillin-tazobactam using spent dialysate effluent in patients receiving continuous venovenous hemodialysis. Antimicrob Agents Chemother. 2011;55(2):557–560. | ||
Roberts DM, Liu X, Roberts JA, et al. A multicenter study on the effect of continuous hemodiafiltration intensity on antibiotic pharmacokinetics. Crit Care. 2015;19(1):84–92. | ||
Higashitani F, Mitsuhashi S, Inoue M, et al. In vitro and in vivo efficacy of tazobactam and piperacillin combination and their most effective combination ratio. Chemotherapy.1994;42:26–33. | ||
Yamaguchi K, Ishii Y, Tateda K, et al. Nationwide surveillance of parenteral antibiotics containing meropenem activities against clinically isolated strains in 2012. Jpn J Antibiot. 2014;67(2):73–107. Japanese. | ||
Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26(1):1–12. | ||
Drusano GL. Prevention of resistance: a goal for dose selection for antimicrobial agents. Clin Infect Dis.2003;36(Suppl 1):42–50. | ||
Kumuro M, Maeda T, Kakuo H, Matsushita H, Shimada J. Inhibition of the renal excretion of tazobactam by piperacillin. J Antimicrob Chemother.1994;34(4):555–564. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.