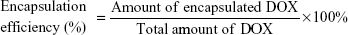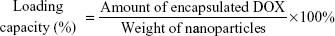Back to Journals » International Journal of Nanomedicine » Volume 10 » Issue 1
pH-responsive glycol chitosan-cross-linked carboxymethyl-β-cyclodextrin nanoparticles for controlled release of anticancer drugs
Authors Wang Y, Qin F, Tan H, Zhang Y, Jiang M, Lu M, Yao X
Received 7 July 2015
Accepted for publication 2 October 2015
Published 8 December 2015 Volume 2015:10(1) Pages 7359—7370
DOI https://doi.org/10.2147/IJN.S91906
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lei Yang
Yiwen Wang,* Fei Qin,* Haina Tan, Yan Zhang, Miao Jiang, Mei Lu, Xin Yao
School of Chemistry and Chemical Engineering, University of Chinese Academy of Sciences, Beijing, People’s Republic of China
*These authors contributed equally to this work
Abstract: Carboxymethyl-β-cyclodextrin (CMβ-CD)-modified glycol chitosan (GCS) nanoparticles (GCS-CMβ-CD NPs) were synthesized, and their pH-sensitive drug-release properties were investigated. GCS-CMβ-CD NPs could encapsulate doxorubicin hydrochloride (DOX), and the encapsulation efficiency and loading capacity increased with the amount of CMβ-CD. Drug-release studies indicate that DOX released was greater in acidic medium (pH 5.0) than in weakly basic medium (pH 7.4). The mechanism underlying the pH-sensitive properties of the carrier was analyzed. Finally, the MCF-7 (human breast cancer) and SW480 cell lines (human colon cancer) were used to evaluate the cytotoxicity of the NPs. The drug-loaded carriers show good inhibition of the growth of cancer cells compared with free DOX, and the carriers have good biocompatibility. In addition, the drug-loaded NPs have sustained drug-release properties. All these properties of the newly synthesized GCS-CMβ-CD NPs suggest a promising potential as an effective anticancer drug-delivery system for controlled drug release.
Keywords: MCF-7, SW480, surface plasmon resonance, encapsulation efficiency, loading capacity, cell viability
Introduction
Chemotherapy is one of the most effective methods of cancer therapy. However, many conventional nonspecific antitumor drugs have significant side effects.1 To reduce the cytotoxicity and improve the therapeutic efficacy of these drugs, several environmentally responsive nanoparticle (NP)-based drug-delivery systems2–4 have been explored in recent decades. Among them, pH-responsive NPs have been extensively investigated2 because of the slightly lower pH (~4.0–6.8)4–6 of extracellular fluids and intracellular vesicles of tumors. For instance, She et al7 made pH-responsive NPs of polyethylene glycosylated peptide dendron–doxorubicin conjugates for cancer therapy. Thomas et al8 fabricated pH-responsive chitosan (CS)/heparin nanocapsules to explore the intracellular delivery of doxorubicin. Polymers are being exploited in pH-responsive drug-delivery systems because of the ease of adjusting their properties to elicit these responses.9 With regard to the safety of the vehicle, natural polysaccharides have received increasing attention as drug-carrier candidates in these studies.
In recent years, NP drug-delivery systems based on CS and its derivatives have been widely investigated for various pharmaceutical applications.10–13 This interest is because CS and its derivatives have many merits as drug carriers, such as nontoxicity, high biocompatibility, biodegradability, and easy chemical modification. Furthermore, they are mucoadhesive and are able to open the tight junctions between the epithelial cells.10 However, many anticancer agents are hydrophobic drugs.14,15 Due to the hydrophilic nature of CS, it is difficult to entrap hydrophobic anticancer drugs in CS NPs.16–18
The introduction of cyclodextrins (CDs) is a good method to successfully overcome these deficiencies. CDs have hydrophilic cavity exteriors and hydrophobic cavity interiors, giving them the ability to encapsulate hydrophobic moieties within their cavities19 and improve the solubility, stability, and bioavailability of hydrophobic drugs. New polymers combining CS and CDs have been investigated, but the reported procedures were complicated, and the production of poisonous dissolvent was inevitable.14,20 Wang et al21 used β-CD-modified CS-poly(acrylic acid) NPs to encapsulate the anticancer drug paclitaxel. These NPs showed enhanced paclitaxel solubility in aqueous solution. However, there was no pH-sensitive release property for the encapsulated drug in vitro. Trapani et al22 reported CS/sulfobutyl ether-β-CD NPs for oral administration of the small peptide glutathione. Although glutathione could be encapsulated into the cores of these NPs, it could not be released from the carrier in an in vitro release test performed in simulated gastric and intestinal media without enzymes. Yuan et al23 developed CS grafted with mono-6-deoxy-6-(p-toluenesulfonyl)-β-CD NPs for the controlled release of the poorly water-soluble drugs such as ketoprofen. However, the drug was released more slowly in pH 4.0 medium than in pH 6.8 medium. There is also a report on the release of ketoprofen by hydroxypropyl CS-graft-carboxymethyl β-CD microparticles, which was not different in pH 1.4 and pH 7.4 media.24 In conclusion, by combining the mucoadhesive property of CH and the hydrophobic property of β-CD cavities, CS-β-CD derivatives have attracted many researchers’ attention and improved the loading ability of hydrophobic drugs, but further investigation is still needed to improve their controlled-release ability before CS-β-CD derivatives can be used as clinical cancer drug carriers.
In this study, we attempt to synthesize a novel pH-sensitive CS-β-CD derivative by covalently conjugating carboxymethyl-β-CD (CMβ-CD) onto the main chains of glycol chitosan (GCS). The anticancer drug doxorubicin hydrochloride (DOX) was used as a model drug to prepare self-assembled drug-loaded GCS-CMβ-CD NPs in water. The obtained GCS-CMβ-CD NPs are expected to not only bind DOX but also specifically release it via the changing dissociation status of carboxylic acid groups at different pH values. The release properties of the NPs were investigated under simulated physiological conditions in vitro and by surface plasmon resonance (SPR) in real time. Their biosafety and antitumor efficacy were also evaluated.
Materials and methods
Materials
GCS (degree of polymerization ≥400) and β-CD were purchased from Sigma-Aldrich Co (St Louis, MO, USA). DOX was obtained from the National Institute for Food and Drug Control (NIFDC; Beijing, People’s Republic of China). Fetal bovine serum, penicillin–streptomycin, and trypsin were bought from Thermo Fisher Scientific (Waltham, MA, USA). Minimum essential medium was purchased from HyClone. 1-Octanethiol (1-OT) was obtained from Acros. Mucin from porcine stomach (PGM) was purchased from Sigma-Aldrich Co. Trypan blue was obtained from Thermo Fisher Scientific. Monochloroacetic acid, sodium polyphosphate (tripolyphosphate), and other reagents were all analytical grade and purchased from Beijing Chemical Reagents Co (Beijing, People’s Republic of China). Millipore Milli Q (18 KΩ cm) water was used in all experiments.
Preparation of blank and drug-loaded GCS-CMβ-CD NPs
CMβ-CD and GCS-CMβ-CD were synthesized as previously described25,26 with slight modifications (Supplementary material. The characteristics and quantitative of CMβ-CD are shown in Figure S1 to S5.). For the obtained CMβ-CDs, β-CD-(CH2COOH)4 through β-CD-(CH2COOH)7 are the main CMβ-CD components. Three different GCS-CMβ-CDs (GCS7.4-CMβ-CD, GCS13.0-CMβ-CD, and GCS23.3-CMβ-CD) were obtained by adjusting the concentrations of NaCl during the reaction of CMβ-CD and GCS (Table S1).
GCS-CMβ-CD NPs were prepared by ionic gelation as described in the literature27 with slight modifications. In brief, GCS-CMβ-CD (20 mg) was dissolved in 10 mL phosphate-buffered saline (PBS) (1 mM) at pH 6.0, and 0.57 mL tripolyphosphate aqueous solution (1 mg/mL) was added to GCS-CMβ-CD solution under magnetic stirring at room temperature, leading to the spontaneous formation of NPs. The resultant product was dialyzed (Mw cutoff 8,000) against 1 mM PBS at pH 6.0 and distilled water over a total of 4 hours and then freeze-dried. For the preparation of DOX-loaded GCS-CMβ-CD (DOX-GCS-CMβ-CD NPs) NPs, DOX and GCS-CMβ-CD (nDOX:nCMβ-CD =1.5/1) were mixed under magnetic stirring in pH 6.0 PBS (1 mM) for 4 hours to allow the complete combination of the reactants. Then the follow-up procedure was the same as blank GCS-CMβ-CD NPs. The scheme of GCS-CMβ-CD NP/DOX-GCS-CMβ-CD NP was shown in Figure S6.
Physicochemical characterization of blank and DOX-loaded GCS-CMβ-CD NPs
The size and zeta potential of the NPs were measured with a Delsa Nano Cerious Particle Analyzer (Beckman Coulter, USA). The morphologies of prepared samples were characterized by scanning electron microscope (SEM) (S-4800, Japan). The encapsulation efficiency (EE) (the accuracy of the encapsulation efficiency is shown in Figure S7 and S8) of DOX by the NPs was directly determined by dissolving 1 mg drug-loaded NPs in 4 mL PBS at pH 5.0 and keeping the solution for 72 hours to determine decomposition. After 72 hours in the dark, the amount of free DOX was determined by fluorescence spectrophotometry with the excitation and emission wavelengths set at 498 and 589 nm, respectively.
The EE and loading capacity (LE) of the NPs were calculated using the following equations:28
|
|
|
|
SPR online detection of drug release
The mechanism of drug release at different acidities was determined by SPR. The SPR substrate was prepared as previously described. First, a 1-OT self-assembled monolayer was affixed to pretreated Au film through overnight immersion at 4°C in 1 mM 1-OT ethanol solution. The 1-OT modified Au film was washed with excess ethanol and then with water. After drying with nitrogen, 50 μL of 100 μg/mL PGM was dropped onto the modified film and kept for 1 hour. The film was washed with Tris–HCl (pH 3.0) and water and then dried with nitrogen. A 40 μL NP solution (dissolved in pH 6.0 PBS) was dropped onto the film and incubated for 30 minutes. In solutions of pH 5.0–7.4, some negatively charged PGM hydrophilic chains existed on the surface, so the positively charged GCS-CMβ-CD or DOX-GCS-CMβ-CD NPs could be immobilized on the PGM by electrostatic interactions. Finally, the film was rinsed thoroughly with 10 mM PBS and then with water. After drying with N2, the modified Au film was used for experiments. PBS (pH 6.0) was used as a carrier fluid; after a constant baseline was obtained, pH 5.0 or 7.4 PBS was injected into the NPs assembled flow cell. The release of drug or the change of NP structure caused the change of the intensity of reflected light on fixed angle that was recorded in real time.
In vitro drug-release studies
In vitro drug-release studies were performed in phosphate buffer (pH 5.0 and 7.4 PBS) with dialysis membrane (Mw cutoff 8,000) for 60 hours. Typically, the drug-loaded NPs (2.5 mg) and 2.5 mL release medium were placed in a dialysis membrane. The dialysis membrane was immersed in 10 mL release medium with continuous stirring at 150 rpm. At different time intervals, a 4 mL sample of the dialysis medium was taken and replaced with fresh release medium (4 mL). The amount of free DOX was determined by fluorescence spectrophotometer.18 The amount of DOX already removed was taken into consideration when calculating the amount of DOX in each sample. The analysis was performed in triplicate for each sample.
Cell viability study
DOX-loaded NPs and blank NPs were assessed for in vitro toxicity by CellTiter-Glo (CTG) assays29 against the MCF-7 and SW480 cell lines. The cells were cultivated in minimum essential medium supplemented with 0.01 mg/mL human recombinant insulin, 10% fetal bovine serum, and antibiotics (50 units/mL penicillin and 50 units/mL streptomycin) at 37°C in a humidified atmosphere containing 5% CO2 and seeded in a 96-well plate at the proper density. After 24 hours incubation, 100 μL serial dilutions of NPs or doxorubicin medium were added, and the cells were cultured for another 72 hours. During the incubation process, cells were counted at 24, 48, and 72 hours. The CTG assay protocol is as follows: 80 μL medium was removed from each well, and the plates were kept at room temperature for 30 minutes. After adding 60 μL reagent (CTG assay kit) to each well and shaking plates (avoiding light) for 2 minutes on a plate shaker, the plates were incubated in the dark at room temperature for 10 minutes. Finally, the luminescence was recorded by a multiplate reader.
Fluorescent image experiment was carried by a conventional fluorescent microscope (Olympus IX 73; Olympus America, Inc., Melville, NY, USA). After 24 hours incubation of MCF-7 cell lines, 800 μL serial dilutions of NPs were added, and the cells were cultured for another 0, 2, 4, 6, 8, and 12 hours, and then the fluorescence image of the DOX-GCS-CMβ-CD NP in the cells was recorded.
Results and discussion
Characterization of NPs
GCS-CMβ-CD NPs and DOX-GCS-CMβ-CD NPs were both prepared by the ionic gelation method. The typical morphology of these NPs was measured by SEM (Figure 1). The NPs were spherical, with a narrow size distribution. The diameter of the three types of blank NPs was all ~100–180 nm, and the corresponding drug-loaded NPs were ~300–500 nm. In addition to the volume of DOX, the positive charge of DOX is another cause of the increase in the size of the drug-loaded particles. However, there was no obvious difference in size among the three types of drug-loaded NPs or blank NPs made with three types of polymers. That is because every seven or more GCS units only have one CMβ-CD. Taking into account the large degree of polymerization of GCS (≥400), the NCMβ-CD/NGCS ratio is relatively low and has a minimal effect on the size of the NPs.
The size and zeta potential of the NPs were also characterized by dynamic light scattering (DLS), and the results are shown in Table 1. The size of the NPs observed by DLS was much larger than that from SEM observation (the size of GCS7.4-CMβ-CD NPs is 102 nm by SEM and 505 nm by DLS measurement), which is due to the existence of a hydrated radius30 and aggregation of NPs in solution28 when measured by DLS. The zeta potential of the three types of drug-loaded NPs ranged from +8.82 to +14.95 mV. The positive zeta potential is likely due to the free positively charged GCS amino groups.31 Compared with blank NPs, the zeta potential of drug-loaded NPs increased for all three polymer NPs (Table 1). There may be two reasons for these phenomena. One is the positive charge of the DOX in the NPs, and another is more positively charged amino groups on the surface of NPs because of the increased surface area of the drug-loaded NPs. In addition, positive potential promotes electrostatic interaction with the overall negative charge of the cell membrane.28
Determination of DOX EE and LC
DOX is an anthraquinone compound with maximum fluorescence at 589 nm. Therefore, the amount of DOX in DOX-GCS-CMβ-CD NPs was investigated using fluorescence spectrophotometry. The fluorescence spectroscopy of the three drug-loaded NPs is shown in Figure 2 (curves a, b, and c), and the inset is the calibration curve of DOX. The amount of drug in the three drug-loaded NPs could be determined through the calibration curve of DOX, and the results are listed in Table 1.
Table 1 also shows that the EE and LC of these NPs increased with increasing CMβ-CD. The LC of DOX-GCS7.4-CMβ-CD is 8.42%, which is almost triple the 3.21% of DOX-GCS23.3-CMβ-CD. The EE also increased from 12.44% for DOX-GCS23.3-CMβ-CD to 31.03% for DOX-GCS7.4-CMβ-CD. The results are probably due to two main interactions between DOX and CMβ-CD. On one hand, CMβ-CD could effectively load poorly water-soluble drugs into the cavity to increase the drug EE. On the other hand, there are electrostatic effects between DOX and CMβ-CD. DOX is positively charged (pKa =8.6) at pH 6.0, while most carboxylic acid groups (pKa =4.7) dissociate into negatively charged carboxylates. As a result, the strong electrostatic interactions between DOX and CMβ-CD could attract more DOX to the cavities of CMβ-CD, which enhance the interaction between CMβ-CD and DOX. With increased CMβ-CD content, more DOX is then encapsulated into the particles. Taking into account of the amount of loaded drug, DOX-GCS7.4-CMβ-CD and DOX-GCS13.0-CMβ-CD were used in the following investigation.
The local tumor environment (tumor extracellular pH 6.5–7.2, endosomal/lysosomal pH 4.5–6) is usually lower than that of the blood plasma (pH 7.4).6,32 If the carrier can release the drug in an acidic environment but retain it under physiological conditions, it will improve the drug-targeting action and facilitate drug release, decreasing side effects and increasing drug bioavailability.
The cumulative release of DOX from NPs was carried out in simulated physiological conditions (PBS, pH 7.4) and simulated tumor microenvironment (PBS, pH 5.0) for 60 hours. The release profiles of DOX-GCS7.4-CMβ-CD NPs and DOX-GCS13.0-CMβ-CD NPs are shown in Figure 3. The drug released rapidly in the beginning, which was mainly due to desorption of the surface-bound or adsorbed drug.23 Figure 3A shows that for DOX-GCS7.4-CMβ-CD NPs, only 50% of the drug was released at pH 7.4 after 60 hours incubation. However, at pH 5.0, it was >80%, suggesting that the release of DOX could be adjusted by pH value.
The synthesized CMβ-CDs were composed of β-CD-(CH2COOH)2, β-CD-(CH2COOH)3, β-CD-(CH2COOH)4, β-CD-(CH2COOH)5, β-CD-(CH2COOH)6, and β-CD-(CH2COOH)7 indicating that more than one carboxymethyl combined with β-CD. One reacted with GCS, but there were also many free carboxymethyl groups around the CMβ-CD cavity. The carboxymethyl groups and amino groups in the GCS have different protonation states in different pH environments. The pKa of DOX is ~8.6,33 and its charge state is affected by pH. This property makes it possible for NPs to release drugs by changing their ionization state to change the electrostatic force of the NPs depending on the pH.9 At pH 7.4, the interaction between the positively charged DOX (pKa =8.6) and the negatively charged carboxylic acid groups (pKa =4.7)34 is strong, so DOX is hard to remove from the NPs. In the medium at pH 5.0, the pH is close to the pKa of carboxylic acid groups, so the negative charge of CMβ-CD decreased, weakening the electrostatic interaction between CMβ-CD and DOX. For this reason, it becomes easy for DOX to diffuse out of the NPs. The DOX released faster in medium at pH 5.0 than that at pH 7.4, which further indicated that the release behavior of DOX is sensitive to pH.
Because swelling, erosion, or degradation of NP matrix23 occurs in all release media, drug release was observed in solutions at both pH 5.0 and 7.4. However, the anticancer drug release was much greater and faster in acidic conditions than in weakly basic environment, reducing the danger to normal cells from the anticancer drug. Thus, the newly synthesized DOX-GCS-CMβ-CD is appropriate for use as a potential carrier for anticancer drug delivery in tumor therapy.
The DOX-GCS13.0-CMβ-CD NPs displayed in Figure 3B showed the same release property as DOX-GCS7.4-CMβ-CD NPs. It exhibited rapid release over the first few hours, followed by a slow release. The release rate and amount were faster at pH 5.0 than that at pH 7.4. These results indicated that although the amount of grafted CMβ-CD in DOX-GCS7.4-CMβ-CD NPs and DOX-GCS13.0-CMβ-CD NPs is different, these two drug carriers are both pH sensitive, which further verifies the function of multiple carboxymethyl groups and the ability to load carriers with anticancer drugs followed by targeted release.
At pH 7.4, 40% of the drug was released and 65% at pH 5.0 after 60 hours incubation. The amount of drugs released was less than that of DOX-GCS7.4-CMβ-CD NPs due to the different drug-loading capacities, which led to a large concentration gap between the polymeric microspheres and the release medium and caused a higher diffusion rate.35 That is to say, the drug-release rate was affected by the amount of drugs loaded. In this study, with increasing CMβ-CD content (NGCS/NCMβ-CD ratio from 13.0 to 7.4), the percentage of drugs released increased ~25% within 60 hours.
SPR online detection of drug release
The pH-responsive ability of NPs was confirmed by in vitro study with fluorescence spectroscopy analysis. To learn more about the process of drug release in different pH conditions, SPR was used to record drug release in real time. The SPR signals of DOX-GCS7.4-CMβ-CD NPs and GCS7.4-CMβ-CD NPs immobilized substrate after injection of pH 5.0 PBS and pH 7.4 PBS are shown in Figure 4. When PBS (pH =5.0) was introduced into the flow cell, an obvious decrease in SPR signal could be observed as displayed in Figure 4A, indicating a large decrease in refractive index on the surface of the substrate. As noted earlier, the pKa of the amino group of GCS is 6.3–6.7, so with the pH change from 6.0 to 5.0, the positive charge of NPs should increase. The electrostatic interaction between PGM and NPs would also increase, which may make the film become thinner and result in decreased SPR signal. After the pH 5.0 solution flowed out the cell, for GCS7.4-CMβ-CD NPs, the SPR signal almost returned to the original baseline (curve a′), meaning that the film returned to its original state. However, for DOX-GCS7.4-CMβ-CD NPs, the signal did not return to the baseline, showing a net SPR signal decrease approximately -0.00137 (curve a) after the PBS flowed out of the flow cell. The signal decrease is due to the release of DOX from the DOX-GCS7.4-CMβ-CD NPs. As mentioned earlier, in the pH 6.0 flow phase, the carboxymethyl groups (pKa ≈4.7) dissociate into carboxylic acid groups. There is a strong electrostatic interaction between the negatively charged carboxylic acid anions and positively charged DOX (pKa ≈8.6).26,36 Both the electrostatic and the hydrophobic interactions between CMβ-CD and DOX result in relatively stable formation of NPs. pH 5.0 PBS is close to the pKa of the carboxymethyl group, so the degree of dissociation of carboxymethyl groups would decrease, and the electrostatic interaction between the carboxymethyl groups and DOX would become weaker. Thus, DOX could be released from NPs into the carrier solution, changing the refractive index and decreasing the SPR signal.
Figure 4B shows the SPR signal change in release properties of DOX-GCS7.4-CMβ-CD NPs (curve b) and GCS7.4-CMβ-CD NPs (curve b′) when injected into pH 7.4 PBS. After pH 7.4 PBS was injected, there was an abrupt increase and then a slow decrease of the SPR signal, but the signal did not go below the original baseline for the trailing phenomenon (curve b and curve b′). The SPR signal increase is in contrast to the situation after pH 5.0 medium was introduced to the flow cell. With pH increasing from 6.0 to 7.4, the positive charge of the NPs decreases, decreasing the electrostatic interaction between PGM and NPs, which may make the film become thicker and result in increased SPR signal. However, the SPR signals were still higher than baseline after pH 7.4 PBS solution flowed out of the flow cell, which indicated that DOX is not released from the drug carrier. In pH 7.4 solution, more carboxymethyl group dissociation into carboxylic acid anions around the CMβ-CD cavity occurred, which strengthens the electrostatic interaction with DOX. In this case, drugs are not released in the short time frame of SPR detection, so the drug-release signal was not observed (curve b).
The drug-release behavior of DOX-GCS13.0-CMβ-CD NPs was also studied by SPR (Figure 5), and the result was similar to that with DOX-GCS7.4-CMβ-CD NPs. After injecting pH 5.0 PBS, the SPR signal of DOX-GCS13.0-CMβ-CD NPs did not return to the original baseline, in contrast to GCS13.0-CMβ-CD NPs, indicating that DOX was released from the drug carrier. However, in pH 7.4 PBS, both GCS7.4-CMβ-CD NPs and DOX-GCS13.0-CMβ-CD NPs showed signals higher than baseline, which indicated that the drug was not released from the NPs. This phenomenon further proves that the carriers are pH sensitive and are suitable for the transport and release of anticancer drugs. Both exhibit a slow return of the film to its initial state, so the SPR signal trails when pH 7.4 solution is used.
Based on the earlier results, NPs have the potential to release drugs in the partially acidic environment of cancer cells but do not release drugs in normal blood conditions, which could protect normal cells from damage by the anticancer drug. In addition, the drug-release behavior by SPR demonstrated that GCS-CMβ-CD NPs were immobilized on the PGM-modified Au substrate. PGM is a mucin, and mucins are aberrantly overexpressed in various malignant tumors and play unique roles in the pathogenesis of cancer.37 Therefore, the adhesion of GCS-CMβ-CD NPs on PGM means that GCS-CMβ-CD NPs could deliver more DOX to the tumor by recognizing overexpressed mucins.
The obtained GCS-CMβ-CD NP carrier combines the mucoadhesive property of GSC and the hydrophobic cavities of β-CDs. In addition, the free carboxymethyl groups around β-CD cavity make the carrier pH sensitive. All these phenomena enable the GCS-CMβ-CD NPs carrier to perform targeted delivery of hydrophobic anticancer agents.
Cell viability assay
To further verify the superior properties of the GCS-CMβ-CD NPs carrier, the cytotoxicity of blank and drug-loaded GCS7.4-CMβ-CD NPs and GCS13.0-CMβ-CD NPs against SW480 and MCF-7 cells was evaluated. Free DOX was also used as a control. Figure 6 shows the SW480 cell viability after incubation with the four types of NPs and free DOX for 24 hours (A), 48 hours (B), and 72 hours (C).
Obviously, the two blank NPs had no noticeable cytotoxicity against SW480 cells within the measured concentrations (the pink and blue lines in Figure 6). The cell viability remained >90% even after 72 hours at high NPs concentration. The high cell-survival rate indicated that GCS-CMβ-CD NPs have good biocompatibility and are expected to be a suitable material for a drug-delivery system.
For drug-loaded NPs and free DOX, cell viabilities decreased rapidly with increased DOX concentration. There was no significant difference in the IC50 at three time points among DOX-GCS7.4-CMβ-CD NPs (red line), DOX-GCS13.0-CMβ-CD NPs (green line), and free DOX (black line), which showed effective inhibition of the growth of cancer cells, indicating that drug-loaded NPs have effective drug-release properties. That is to say, compared with free DOX, NPs containing the same concentration of drug had equal ability to inhibit cancer cells. Considering the mucoadhesive property of NPs, and the aberrant overexpression of mucins in various malignant tumors, it can be speculated that smaller concentrations of drug-loaded NPs could achieve the same therapeutic effect as free DOX.
In addition, at relatively high sample concentration (20–100 μg/mL), drug-loaded NPs showed slightly lower inhibition of cell growth than free DOX after 24 hours (Figure 6A). For DOX-GCS7.4-CMβ-CD NPs (red line), the cell viability was ~25.3% after 24 hours incubation with 100 μg/mL NPs, while the cell viability was almost 0 with free DOX (black line). After 48 hours, the inhibition of the growth of the cells treated with DOX-GCS7.4-CMβ-CD NPs decreased to 14.2% (Figure 6B). After incubation for 72 hours, the growth of the cells was completely inhibited (Figure 6C, red line). DOX-GCS13.0-CMβ-CD NPs showed the same pattern. The cell viability was 17.3%, 9.1%, and 3.2%, respectively, after incubation with DOX-GCS13.0-CMβ-CD NPs for 24, 48, and 72 hours. These results indicated that NPs have the sustained release properties for DOX,21 likely due to the electrostatic and hydrophobic interactions of CMβ-CD to DOX, which limited the burst drug release. Gradual drug release is good for minimizing drug loss in the blood38 as well as decreasing the toxicity to normal tissues.
To further verify the properties of NPs, another cancer cell line, MCF-7, was chosen to evaluate their cytotoxicity. The live MCF-7 cells at three time points (24, 48, and 72 hours) are shown in Figure 7. According to the curve for blank NPs, there was a minimal effect on the cell viability even at high sample concentration for a long incubation, which indicated that these carriers are not toxic to MCF-7 cells either. Drug-loaded NPs exhibited excellent antitumor activity against MCF-7. After incubation for 24 hours (Figure 7A), 48 hours (Figure 7B), and 72 hours (Figure 7C), the cell viability after exposure to DOX-GCS7.4-CMβ-CD NPs and DOX-GCS13.0-CMβ-CD NPs was similar to that after exposure to DOX, showing that drug-loaded NPs and DOX have the same efficacy. The sustained release properties are shown in Figure 7. The MCF-7 cell viability was 21.1%, 9.2%, and 4.6% after incubation with DOX-GCS7.4-CMβ-CD NPs for 24, 48, and 72 hours, respectively. For the DOX-GCS13.0-CMβ-CD NPs, it was 7.46%, 0.49%, and 0.22%. These data indicate that both DOX-GCS7.4-CMβ-CD NPs and DOX-GCS13.0-CMβ-CD NPs have sustained release character in the presence of MCF-7 cells, although it was not obvious difference compared with SW480 cells. In addition, we also observed that the sustained release ability is not very obvious because most of the drug (~72%) was released within 24 hours based on the in vitro drug-release experiment (Figure 3), while cell viability is best assessed 24 hours after the NPs were added. The newly obtained nanocarrier may achieve better sustained release ability through further modifications.
Fluorescence imaging of DOX-GCS13.0-CMβ-CD NP in MCF-7 cells at different time points was recorded in order to know more about the intracellular behavior of NPs. As shown in Figure S9, DOX-GCS-CMβ-CD NP distributed around extracellular matrix and the outside of cell membrane evenly when the NP added (Figure S9A). However, DOX-GCS-CMβ-CD NP mainly attached to cell membrane after 2 hours (Figure S9B); this phenomenon also indicated the mucoadhesive effect of CS. After 4 hours of incubation, the detection of weak fluorescence signal in cytoplasm (Figure S9C) demonstrated that a small part of NPs have entered into cells. After 6 hours, more red fluorescence dots were detected in cytoplasm (Figure S9D), which indicated that more DOX-GCS-CMβ-CD NP had entered the cells. It may be due to the capacity of CS to open the tight junctions between epithelial cells.10 After 12 hours of incubation, most of the red fluorescence was diffusely distributed in cytoplasm and the majority of cells showed death state, which indicated that DOX could be released from DOX-GCS-CMβ-CD NP and kill the cancer cells.
Combining the results of experiment on MCF-7 and SW480 cells, it is clear that the carriers have favorable biocompatibility and mucoadhesive. Meanwhile, drug-loaded NPs also showed good bioactivity and drug-release ability to some extent.
Conclusion
Anticancer drug carriers that combine the notable properties of GCS and desirable features of CMβ-CD were synthesized. The CD cavity allowed easy association with DOX, and the introduction of multiple carboxymethyl groups around the CD cavity enabled differential response to varying pH, which could release the drug faster in the low pH environment near cancer tissues than in the normal bloodstream environment. The CTG assay further illustrated the good biocompatibility of the carrier. The drug-loaded NPs also showed good inhibitory effect on the growth of the cancer cells and sustained drug-release properties. Combined with passive targeting and preferential drug release within the target cells, the GCS-CMβ-CD NPs are a promising candidate to deliver poorly water-soluble anticancer drugs. They are expected to increase drug effectiveness and reduce adverse effects.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (21271184), the Ministry of Science and Technology of China (973 programs 2014CB931900 and 2012CB932504), and “Strategic Priority Research Program” of the Chinese Academy of Science, (grant XDA09030301).
Disclosure
The authors report no conflicts of interest in this work.
References
Jaracz S, Chen J, Kuznetsova LV, Ojima I. Recent advances in tumor-targeting anticancer drug conjugates. Bioorg Med Chem. 2005;13:5043–5054. | ||
Gao WW, Chan JM, Farokhzad OC. pH-Responsive nanoparticles for drug delivery. Mol Pharm. 2010;7:1913–1920. | ||
Li YY, Zhang XZ, Kim GC, Cheng H, Cheng SX, Zhuo RX. Thermosensitive Y-shaped micelles of poly(oleic acid-Y-N-isopropylacrylamide) for drug delivery. Small. 2006;2:917–923. | ||
Meng L, Huang W, Wang D, Huang X, Zhu X, Yan D. Chitosan-based nanocarriers with pH and light dual response for anticancer drug delivery. Biomacromolecules. 2013;14:2601–2610. | ||
Chen W, Cheng Y, Wang B. Dual-responsive boronate crosslinked micelles for targeted drug delivery. Angew Chem Int Ed Engl. 2012;51:5293–5295. | ||
Li Y, Xiao W, Xiao K, et al. Well-defined, reversible boronate crosslinked nanocarriers for targeted drug delivery in response to acidic pH values and cis-diols. Angew Chem Int Ed Engl. 2012;51:2864–2869. | ||
She W, Luo K, Zhang C, et al. The potential of self-assembled, pH-responsive nanoparticles of mPEGylated peptide dendron-doxorubicin conjugates for cancer therapy. Biomaterials. 2013;34:1613–1623. | ||
Thomas MB, Radhakrishnan K, Gnanadhas DP, Chakravortty D, Raichur AM. Intracellular delivery of doxorubicin encapsulated in novel pH-responsive chitosan/heparin nanocapsules. Int J Nanomedicine. 2013;8:267–273. | ||
Sampathkumar K, Arulkumar S, Ramalingam M. Advances in stimuli responsive nanobiomaterials for cancer therapy. J Biomed Nanotechnol. 2014;10:367–382. | ||
Amidi M, Mastrobattista E, Jiskoot W, Hennink WE. Chitosan-based delivery systems for protein therapeutics and antigens. Adv Drug Deliv Rev. 2010;62:59–82. | ||
Hyung Park J, Kwon S, Lee M, et al. Self-assembled nanoparticles based on glycol chitosan bearing hydrophobic moieties as carriers for doxorubicin: in vivo biodistribution and anti-tumor activity. Biomaterials. 2006;27:119–126. | ||
Kim JH, Kim YS, Park K, et al. Self-assembled glycol chitosan nanoparticles for the sustained and prolonged delivery of antiangiogenic small peptide drugs in cancer therapy. Biomaterials. 2008;29:1920–1930. | ||
Nogueira DR, Tavano L, Mitjans M, Pérez L, Infante MR, Vinardell MP. In vitro antitumor activity of methotrexate via pH-sensitive chitosan nanoparticles. Biomaterials. 2013;34:2758–2772. | ||
Alamdarnejad G, Sharif A, Taranejoo S, et al. Synthesis and characterization of thiolated carboxymethyl chitosan-graft-cyclodextrin nanoparticles as a drug delivery vehicle for albendazole. J Mater Sci Mater Med. 2013;24:1939–1949. | ||
Min KH, Park K, Kim YS, et al. Hydrophobically modified glycol chitosan nanoparticles-encapsulated camptothecin enhance the drug stability and tumor targeting in cancer therapy. J Control Release. 2008;127:208–218. | ||
Janes KA, Fresneau MP, Marazuela A. Chitosan nanoparticles as delivery systems for doxorubicin. J Control Release. 2001;73:255–267. | ||
Ji JG, Hao SL, Liu WQ, et al. Preparation and evaluation of O-carboxymethyl chitosan/cyclodextrin nanoparticles as hydrophobic drug delivery carriers. Polym Bull. 2011;67:1201–1213. | ||
Park JM, Lee SY, Lee GH, et al. Design and characterisation of doxorubicin-releasing chitosan microspheres for anti-cancer chemoembolisation. J Microencapsul. 2012;29:695–705. | ||
Davis ME, Brewster ME. Cyclodextrin-based pharmaceutics: past, present and future. Nat Rev Drug Discov. 2004;3:1023–1035. | ||
Zhang XG, Wu ZM, Gao XJ, et al. Chitosan bearing pendant cyclodextrin as a carrier for controlled protein release. Carbohydr Polym. 2009;77:394–401. | ||
Wang X, Chen CJ, Huo D, et al. Synthesis of β-cyclodextrin modified chitosan–poly(acrylic acid) nanoparticles and use as drug carriers. Carbohydr Polym. 2012;90:361–369. | ||
Trapani A, Lopedota A, Franco M, et al. A comparative study of chitosan and chitosan/cyclodextrin nanoparticles as potential carriers for the oral delivery of small peptides. Eur J Pharm Biopharm. 2010;75:26–32. | ||
Yuan Z, Ye Y, Gao F, et al. Chitosan-graft-beta-cyclodextrin nanoparticles as a carrier for controlled drug release. Int J Pharm. 2013;446:191–198. | ||
Prabaharan M, Mano JF. Hydroxypropyl chitosan bearing beta-cyclodextrin cavities: synthesis and slow release of its inclusion complex with a model hydrophobic drug. Macromol Biosci. 2005;5:965–973. | ||
Reuben J, Rao CT, Pitha J. Distribution of substituents in carboxymethyl ethers of cyclomaltoheptaose. Carbohydr Res. 1994;258:281–285. | ||
Tan HN, Xue Y, Luan QF, Yao X. Evaluation of glycol chitosan-graft-carboxymethyl β-cyclodextrin as potential pH-sensitive anticancer drug carrier by surface plasmon resonance. Anal Methods. 2012;4:2784–2790. | ||
Krauland A, Alonso M. Chitosan/cyclodextrin nanoparticles as macromolecular drug delivery system. Int J Pharm. 2007;340:134–142. | ||
Mahmoud AA, El-Feky GS, Kamel R, Awad GE. Chitosan/sulfobutylether-beta-cyclodextrin nanoparticles as a potential approach for ocular drug delivery. Int J Pharm. 2011;413:229–236. | ||
Sims JT, Plattner R. MTT assays cannot be utilized to study the effects of STI571/Gleevec on the viability of solid tumor cell lines. Cancer Chemother Pharmacol. 2009;64:629–633. | ||
Tanaka F, Koga T, Kojima H, Francoise MW. Hydration and phase separation of temperature-sensitive water-soluble polymers. Chin J Polym Sci. 2010;29:13–21. | ||
Oyarzun-Ampuero FA, Brea J, Loza MI, Torres D, Alonso MJ. Chitosan–hyaluronic acid nanoparticles loaded with heparin for the treatment of asthma. Int J Pharm. 2009;381:122–129. | ||
Lu C, Xing MM, Zhong W. Shell cross-linked and hepatocyte-targeting nanoparticles containing doxorubicin via acid-cleavable linkage. Nanomedicine. 2011;7:80–87. | ||
Yaroslavov AA, Kuchenkova OY, Okuneva IB, et al. Effect of polylysine on transformations and permeability of negative vesicular membranes. Biochim Biophys Acta. 2003;1611:44–54. | ||
Badruddoza AZ, Tay AS, Tan PY, Hidajat K, Uddin MS. Carboxymethyl-β-cyclodextrin conjugated magnetic nanoparticles as nano-adsorbents for removal of copper ions: synthesis and adsorption studies. J Hazard Mater. 2011;185:1177–1186. | ||
Xu YM, Du YM. Effect of molecular structure of chitosan on protein delivery properties of chitosan nanoparticles. Int J Pharm. 2003;250:215–226. | ||
Bodek KH, Kufelnicki A. Protolytic and complexing properties of microcrystalline chitosan with Co(ll), Zn(ll), and Cu(ll) Ions. J Appl Polym Sci. 1995;57:645–651. | ||
Bafna S, Kaur S, Batra SK. Membrane-bound mucins: the mechanistic basis for alterations in the growth and survival of cancer cells. Oncogene. 2010;29:2893–2904. | ||
Lee SJ, Min KH, Lee HJ, et al. Ketal cross-linked poly(ethylene glycol)-poly(amino acid)s copolymer micelles for efficient intracellular delivery of doxorubicin. Biomacromolecules. 2011;12:1224–1233. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.