Back to Journals » OncoTargets and Therapy » Volume 13
PFND1 Predicts Poor Prognosis of Gastric Cancer and Promotes Cell Metastasis by Activating the Wnt/β-Catenin Pathway
Authors Zhou C, Guo Z, Xu L, Jiang H, Sun P, Zhu X, Mu X
Received 1 November 2019
Accepted for publication 23 March 2020
Published 16 April 2020 Volume 2020:13 Pages 3177—3186
DOI https://doi.org/10.2147/OTT.S236929
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sanjay Singh
Cheng Zhou,1,2,* Zhiyuan Guo,1,2,* Liqun Xu,2 Haohai Jiang,2 Pengfei Sun,2 Xinguo Zhu,1 Xiangming Mu2
1Department of General Surgery, The First Affiliated Hospital of Soochow University, Suzhou, People’s Republic of China; 2Department of General Surgery, Yancheng City No.1 People’s Hospital, Yancheng, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xinguo Zhu
Department of General Surgery, The First Affiliated Hospital of Soochow University, 899 Pinghai Road, Suzhou 215006, People’s republic of china
Email [email protected]
Xiangming Mu
Department of General Surgery, Yancheng City No.1 People’s Hospital, 166 Yulongxi Road, Yancheng 224001 Email [email protected]
Background: Prefoldin (PFDN) subunits have recently been found to function importantly in various tumor types, while the role of PFDN subunit 1 (PFDN1) in gastric cancer (GC) remains largely unknown. Herein, we aimed to investigate the clinical significance, the biological role and the underlying mechanism of PFDN1 in GC development.
Materials and Methods: PFDN1 expression levels were measured in human GC specimens by quantitative real-time PCR (qRT-PCR), Western blot and immunohistochemistry. Furthermore, the effects of aberrant PFDN1 expression on GC cells behavior were assessed by wound-healing assay and transwell assay in vitro, and metastasis assay in nude mice, as well as Wnt/β-catenin signaling-induced epithelial–mesenchymal transition (EMT)-related markers by qRT-PCR and Western blot.
Results: PFDN1 levels were significantly upregulated in GC tissues compared with those in matched adjacent normal tissues. PFDN1 upregulation correlated strongly with clinical metastasis and unfavorable prognosis for GC patients. In vitro and in vivo studies revealed that PFDN1 facilitated GC cell migration, invasion and metastasis. Mechanically, PFDN1 modulated GC cell behavior by activating Wnt/β-catenin signaling-mediated EMT.
Conclusion: These results suggested a central role of PFDN1 in GC metastatic development via the Wnt/β-catenin pathway, thus providing a potential therapeutic target for patients with GC.
Keywords: PFDN1, gastric cancer, epithelial–mesenchymal transition
Introduction
Gastric cancer (GC) is one of the most prevalent cancers, ranking as the third leading cause of cancer-related mortality worldwide.1 With the development of early detection and surgical skills in combination with various therapeutic strategies, such as chemotherapy and radiotherapy, however, the 5-year postoperative survival rate of GC remains poor, partly due to tumor metastasis.2 Therefore, it is of great importance to identify novel metastasis genes and the molecular mechanisms underlying GC progression, probably providing potential therapeutic targets to suppress GC metastasis.
Metastasis, cell detachment from primary tumors, can be initiated by the transdifferentiation of epithelial cells into motile mesenchymal cells, a process commonly known as epithelial-mesenchymal transition (EMT) to symbolize its transient nature.3,4 Recently, extensive evidence has suggested EMT as a well-documented molecular event that facilitates cancer cell invasion and metastasis.5,6 EMT describes the process whereby tumor epithelial cells undergo molecular and genetic changes, leading to the disappearance of polarity, the loss of cell-cell adhesion and the acquisition of migratory and invasive properties.7 And genetically, EMT is accompanied by a decrease in the expression of epithelial cell adhesive factor E-cadherin, and an increase in the expression of mesenchymal cell marker vimentin. Besides, the switch in EMT is regulated by a network of interconnected signalling pathways such as Wnt/β-catenin, PI3K/AKT, Notch and TGF-β/Smad signalling cascade.7 Since the incidence of metastasis and its impact on the prognosis of cancer patients, novel therapeutic approaches are required to prevent cancer cells dissemination or eradicate existing metastatic cancer cells. Interestingly, recent data have suggested that the pharmacological targeting of EMT in certain cancer types might represent such a strategy.8
Chaperone proteins have been demonstrated to be involved in cancer development and progression. Prefoldin (PFDN) is a jellyfish-shaped chaperone that captures newly synthesized proteins (especially actin and tubulin) and delivers them to chaperonin-containing t-complex polypeptide 1, which correlates with poor prognosis in various types of cancer.9 Interestingly, recent studies reveal that prefoldin subunit 1 (PFDN1), a subunit of the PFDN complex, has an important role in cancer development and progression. For example, research reveals that PFDN1 contributes to colorectal cancer (CRC) metastasis via activation of cytoskeletal proteins, especially F-actin and a-tubulin, and thus serves as a poor predictor for CRC prognosis.9 In lung cancer, gain- and loss-of-function studies demonstrate that PFDN1 promotes EMT and lung cancer progression by suppressing cyclin A expression, describing PFDN1 as a tumor promoter and a candidate therapeutic target.10 Despite these findings, to our knowledge, the PFDN1 expression and its role in GC remains largely unknown.
In this study, we examined the expression level of PFDN1 and its function in GC development and progression, and uncovered a novel mechanism whereby PFDN1 induced EMT and GC metastasis.
Materials and Methods
Tissue Samples
A total of 86 matched cancerous and normal tissues were collected from patients with gastric adenocarcinoma who underwent radical gastrectomy at the Department of General Surgery of the First Affiliated Hospital of Soochow University. None of these patients had received preoperative chemotherapy or radiotherapy. All samples were snap frozen in liquid nitrogen immediately after surgical removal, followed by storage at −80°C until the subsequent assays were performed. Samples were evaluated for PFDN1 mRNA and protein expressions by quantitative real-time PCR (qRT-PCR), Western blot and immunohistochemical staining, respectively. The study protocol was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University, and written informed consents were obtained from all participants in compliance with the Declaration of Helsinki.
Cell Lines
Cell lines used in this study included six human GC cell lines (MKN45, SGC7901, AGS, HGC27, MGC803 and MKN7) and one normal gastric epithelial cell line GES-1. All cells were obtained from American Type Culture Collection (ATCC, USA), and were cultured in RPMI-1640 medium (Thermo Fisher Scientific, USA) containing 10% FBS (Thermo Fisher Scientific, USA) at 37°C in a humidified 5% CO2 incubator.
qRT-PCR
Total RNA was extracted from tissue samples and cell lines using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and employed to synthesize cDNA sequence using PrimeScript RT Reagent Kit (TaKaRa, Japan) following the manufacturer’s recommendation. qRT-PCR amplification was carried out on a 7500 RT-PCR System (Applied Biosystems, USA) using the SYBR Green assay kit (Takara, Japan). The relative mRNA level of transcript was quantified using the 2−ΔΔCt method. Primers are listed in Supplementary 1.
Western Blot
Briefly, protein was extracted from tissue or cell lysate, separated by SDS-PAGE, and transferred onto polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA, USA). Then, membranes were blocked with 5% nonfat milk and incubated with indicated primary antibodies at 4°C overnight. The next day, membranes were incubated with an appropriate HRP-conjugated secondary antibody. Protein bands were visualized by an enhanced chemiluminescence detection kit (Thermo Fisher Scientific) following the manufacturer’s protocol. Primary antibodies against PFDN1, β-catenin, c-myc, cyclin D1, and survivin used in this study were purchased from Abcam (UK). Primary antibodies against E-cadherin and vimentin were bought from CST (Massachusetts, USA). GAPDH antibody was obtained from Bioworld Technology (St Louis Park, MN, USA).
Immunohistochemistry
Briefly, the paraffin-embedded sections were deparaffinized, rehydrated and heat-treated to repair antigenicity, followed by incubation with 3% H2O2 to inactivate endogenous peroxidase activity. Then, the sections were treated with goat serum to reduce nonspecific antibody binding, and incubated with a primary antibody at 4°C overnight. The next day, the sections were incubated with a secondary antibody. Afterwards, the sections were incubated with an SABC solution, and counterstained with hematoxylin. Finally, the sections were observed and evaluated under a light microscopy by two independent pathologists.
The IRS (immunoreactive score) was determined by the sum of the intensity scores (0, negative; 1, weak; 2, moderate; 3, strong) and the area scores (0, 0–5%; 1, 6%–25%; 2, 26%–50%; 3, >50%). The IRS <3 points were classified as negative, otherwise were defined as positive.
Cell Transfection
Commercially available PFDN1-specific short hairpin RNA (shRNA) construct was obtained from Hanbio (Shanghai, China),9 and overexpression construct was purchased from Addgene (USA).10 Cell transfected with control-shRNA or vector was employed as control. Transfections were carried out using Lipofectamine 2000 (Invitrogen, USA) following the manufacturer’s protocols. The transfection efficiency was confirmed by Western blot.
Wound-Healing Assay
Cells were seeded in a 24-well plate to reach an approximately 90% confluence. An artificial scratch wound was generated using a sterile pipette tip, followed by a removal of floating cells. A series of images of the scratches were taken at the appropriate time points (0 h and 48 h). Wound closure rate = (0 h wound width − 48 h wound width)/0 h wound width × 100%.
Cell Migration and Invasion Assays
For transwell migration and invasion assays, cells in serum-free medium were placed in the upper compartment of a 24-well 8-µm pore size transwell plate (Corning Incorporated, Corning, NY, USA), which was pre-coated with Matrigel (BD Biosciences, San Jose, CA, USA) for transwell invasion assay. Medium containing 20% FBS was placed in the bottom chamber as a chemoattractant. After incubation for 24 h, the invaded cells on the lower surface of the chamber were fixed with paraformaldehyde, stained with crystal violet, and calculated under an inverted light microscope in five random fields.
In vivo Metastasis Assay
To evaluate the effect of aberrant PFDN1 expression on GC cells metastasis in vivo, The BALB/c nude mice aged 6 weeks were injected intravenously with MKN45 cells (shNC/shPFDN1) or AGS cells (Vector/PFDN1) via the tail vein. At 30 days after injection, the mice were sacrificed. Meanwhile, the lungs were surgically collected for the count of the metastatic foci to assess the development of pulmonary metastases, and then processed for Western blot analysis of the expression levels of PFDN1, E-cadherin and vimentin. All animal procedures were approved by the Ethics Committee of the First Affiliated Hospital of Soochow University and performed according to the guide for the Care and Use of Laboratory Animals issued by the National Institute of Health (NIH).
Statistical Analysis
Chi-square test was used to analyze the relationship between PFDN1 expression and the clinicopathologic features. The Kaplan–Meier survival curve was assessed by the Log rank test. The data were presented using mean ± SD, and analyzed by Student’s t-test or One-Way ANOVA. Statistical analyses were performed using the SPSS 21.0 software (IBM Corporation, Armonk, NY, USA). P<0.05 was considered to be statistically significant.
Results
Clinical Significance of PFDN1 in GC Tissues
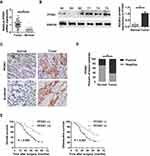
To evaluate the clinical significance of PFDN1 in GC progression, we detected the expression levels of PFDN1 mRNA and protein in GC tissues. As shown in Figure 1A, PFDN1 mRNA was found to be significantly higher in tumor tissues compared with that in adjacent normal samples in 43 pairs of cases. Consistently, elevated PFDN1 protein expression was confirmed by Western blot analysis in 43 pairs of cases (Figure 1B). Furthermore, IHC analysis in 86 pairs of cases revealed that the expression levels of PFDN1 and β-catenin were differentially upregulated in tumor tissues compared with them in normal tissues (Figure 1C and D). As shown in Table 1, PFDN1 expression was significantly associated with T stage, pTNM stage and lymph node metastasis (P<0.05). Meanwhile, Table 2 manifested that PFDN1 expression was positively related to β-catenin expression (P<0.001, contingency coefficient = 0.529) in GC samples. Kaplan–Meier survival analysis further revealed that high PFDN1 expression observably correlated with poor overall survival and/or disease-free survival in patients with GC (P<0.05) (Figure 1E). Taken together, these results suggest that PFDN1 expression is upregulated in tumour tissues and correlated with unfavorable prognosis in patients with GC, and more remarkably, PFDN1 might function importantly in metastasis.
 |
Table 1 Relationship Between PFDN1 Expression Level and Clinicopathologic Variables in Gastric Cancer |
 |
Table 2 Correlation Analysis Between PFDN1 Expression and β-Catenin Expression in Gastric Cancer Tissues by Chi-Square Test |
PFDN1 Expression in GC Cell Lines
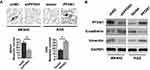
To select the appropriate GC cells for a further examination of the role of PFDN1 in GC cells, we firstly evaluated PFDN1 expression in a panel of 6 GC cell lines and a normal gastric epithelial cell line (GES-1). Obviously, the expression levels of PFDN1 mRNA and protein were markedly upregulated in GC cell lines than those in GES-1 cell. Among them, MKN45 and SGC7901 cells showed the highest levels of PFDN1 mRNA and protein expression. Conversely, AGS and HGC27 cells exhibited the opposite outcomes (Figure 2A and B). Secondly, MKN45 and SGC7901 cells were selected for stable transfection with shRNA-PFDN1 (Figure 2C). Meanwhile, AGS and HGC27 cells were selected for stable transfection with PFDN1 overexpression (Figure 2D).
PFDN1 Enhances GC Cell Migration Invasion in vitro
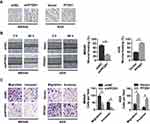
To assess the potential effects of PFDN1 on GC cells, we selected MKN45-shPFDN1 cells and AGS-PFDN1 cells for follow-up experiments. As shown in Figure 3A, PFDN1 knockdown in MKN45 cells resulted in a cellular morphological change form a long spindle-shaped mesenchymal profile to a short spindle-shaped epithelial phenotype. Conversely, PFDN1 overexpression in AGS cells led to an opposite result. The wound-healing assay revealed that PFDN1 silencing in MKN45 cells suppressed cell scratch repair ability; however, PFDN1 upregulation in AGS cells promoted cell scratch repair (Figure 3B). Consistently, the transwell assays indicated that MKN45-shPFDN1 cells showed weaker migration and invasiveness capacities compared with control group. Inversely, this result was reversed by treatment with PFDN1 overexpression in AGS cells (Figure 3C). Hence, we concluded that PFDN1 promoted GC cell migration and invasion in vitro.
PFDN1 Facilitates GC Cells Metastasis in vivo
Interestingly, a similar result was observed in a lung metastases model. As shown in Figure 4A and B, PFDN1 downregulation in MKN45 cells suppressed lung metastasis and EMT, as evidenced by a significantly less number of lung metastatic nodules. In contrast, PFDN1 upregulation in AGS cells facilitated lung metastasis and EMT (Figure 4A and B). Taken together, these findings indicated that PFDN1 promoted GC cells metastasis in vivo.
Wnt/β-Catenin Signaling-Mediated EMT Involved in PFDN1-Induced GC Cell Migration and Invasion
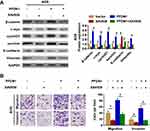
It’s widely acceptable that Wnt/β-catenin signaling pathway is involved in cancer migration and invasion.11 Herein, we found that PFDN1 expression was positively correlated with β-catenin expression in GC tissues. Hence, we reasoned that Wnt/β-catenin signaling may be involved in PFDN1-induced GC cell migration and invasion. To confirm this hypothesis, we firstly sought to determine the expression levels of Wnt/β-catenin signaling-related genes with PFDN1 alteration in GC cells. The results revealed that PFDN1 silencing in MKN45 cells significantly inhibited the mRNA expression levels of β-catenin, the transcriptional targets of Wnt/β-catenin signaling (including c-myc, cyclin D1 and survivin), and vimentin; whereas remarkably elevated E-cadherin mRNA expression. On the contrary, PFDN1 overexpression in AGS cells manifested an opposite outcome (Figure 5A). Additionally, the Western blot analysis further confirmed that PFDN1 was positively correlated with β-catenin, the transcriptional targets of Wnt/β-catenin signaling and vimentin; but negatively correlated with E-cadherin (Figure 5B).
Secondly, to further validate Wnt/β-catenin signaling-induced EMT involved in PFDN1-induced GC cell migration and invasion, XAV939 (the specific Wnt/β-catenin signaling inhibitor) was employed in GC cells. The results demonstrated that XAV939 pretreatment decreased the expression levels of c-myc, cyclin D1 and survivin, and promoted a noticeable reversal of PFDN1-induced EMT (Figure 6A). Moreover, XAV939 pretreatment further suppressed PFDN1-facilitated cell migration and invasion by transwell assays (Figure 6B). Collectively, these findings suggested that PFDN1 could promote GC cell migration and invasion via EMT alteration, probably mediated by activating Wnt/β-catenin signaling.
Discussion
As with other cancers, GC remains a highly heterogeneous disease with poor prognosis. Over the past decades, although the comprehensive treatment of GC has developed rapidly; most afflicted individuals do not survive partly owing to tumor metastasis.12 Therefore, increased efforts are urgently needed to identify cancer-specific cellular targets and gain more in-depth insight into the underlying mechanisms involved in cancer metastasis. Interestingly, recent reports have revealed that PFDN1 was highly expressed in human malignancies and was involved in tumor invasion and metastasis.9,10
Previous studies have shown that chaperone proteins correlated with poor clinical outcomes in various types of cancer and contributed to cancer development and progression.13,14 Notably, PFDN is a jellyfish-shaped chaperone comprised of six subunits, binding partially newly synthesized proteins such as actin and tubulin.9,15 PFDN1, an important subunit of the PFDN complex, binds specifically to cytosolic chaperonin. Meanwhile, as in other studies, research suggested that PFDN1 had an instrumental role in the remodeling of cytoskeletal system.16 In consideration of these findings and the close relationship between cytoskeletal rearrangement and tumor metastasis, we could reasonably assume that PFDN1 is an essential factor involved in tumor progression and metastasis, as well as its accompanying biological events.
Interestingly, recent studies have demonstrated that PFDN1 functions as a metastatic gene in CRC and lung cancer.9,10 In the current study, we found that PFDN1 upregulation was significantly associated with clinical metastasis and a lower progression-free survival in patients with GC. In addition, we confirmed that a decrease in PFDN1 levels suppressed GC cells invasion and metastasis, whereas an increase in PFDN1 levels resulted in the opposite outcome. Mechanistically, we revealed that PFDN1 facilitated GC cell invasion and metastasis via Wnt/β-catenin signaling-mediated EMT.
As mentioned in the introduction, metastasis is the spread of cancer cells from the primary lesion to distant organs through a process involving several defined stages. EMT is adopted early in the initial step of the metastatic cascade to permit migration and invasion of metastatic cells to distant organs, upon which mesenchymal–epithelial transition (MET) is an effective approach for inhibiting EMT pathway to suppress tumor dissemination and eradicate existing metastatic cancer cells.3,17 Hence, a very substantial effort is urgently needed to identify novel proposed EMT targets to prevent cancer metastasis. Herein, our findings suggested PFDN1 as an important regulator of EMT, as evidenced by PFDN1 knockdown-induced a decrease in vimentin level and an increase in E-cadherin level, as well as PFDN1 overexpression-induced an opposite outcome.
Besides, an important finding is that we suggested Wnt/β-catenin signaling as a key participant involved in PFDN1-mediated EMT. Firstly, previous studies have confirmed that the blockade of the Wnt/β-catenin signaling suppressed EMT, migration and metastasis of cancer cells.11,18 Β-catenin, a central molecule of the Wnt/β-catenin signaling, could activate the downstream targets (including c-myc, cyclin D1, and survivin) and subsequently cause tumor metastasis.19 Notably, we found not only that PFDN1 expression significantly correlated with β-catenin expression in GC specimens, but also that PFDN1 silencing decreased the expressions of the Wnt/β-catenin signaling targets, whereas PFDN1 overexpression achieved the opposite outcomes. Secondly, XAV939, the specific Wnt/β-catenin signaling inhibitor,18 was employed to further prove Wnt/β-catenin signaling involved in PFDN1-induced EMT, migration and invasion of GC cells. The results revealed that the blockade of Wnt/β-catenin signaling weakened PFDN1-facilitated EMT, migration and invasion. Based on these observations, we therefore concluded that PFDN1 promoted GC cell invasion and metastasis via Wnt/β-catenin-regulated EMT.
Conclusion
In summary, these findings indicated that PFDN1 was closely associated with clinical metastasis and was an unfavorable predictor of overall survival in patients with GC. Besides, PFDN1 facilitated GC cell invasion and metastasis by activating the Wnt/β-catenin signaling-mediated EMT, thus possibly providing a potential target for therapeutic intervention in GC development.
Acknowledgments
This work was supported in part by funding from the Natural Science Foundation of Jiangsu Province (No. BK20161225).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi:10.3322/caac.21442
2. Lee SY, Oh SC. Changing strategies for target therapy in gastric cancer. World J Gastroenterol. 2016;22(3):1179–1189. doi:10.3748/wjg.v22.i3.1179
3. Singh M, Yelle N, Venugopal C, Singh SK. EMT: mechanisms and therapeutic implications. Pharmacol Ther. 2018;182:80–94. doi:10.1016/j.pharmthera.2017.08.009
4. Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–196. doi:10.1038/nrm3758
5. Shi M, Duan G, Nie S, Shen S, Zou X. Elevated FAM3C promotes cell epithelial- mesenchymal transition and cell migration in gastric cancer. Onco Targets Ther. 2018;11:8491–8505. doi:10.2147/OTT.S178455
6. Wang X, Lin M, Zhao J, Zhu S, Xu M, Zhou X. TSPAN7 promotes the migration and proliferation of lung cancer cells via epithelial-to-mesenchymal transition. Onco Targets Ther. 2018;11:8815–8822. doi:10.2147/OTT.S167902
7. Tang J, Li Y, Wang J, Wen Z, Lai M, Zhang H. Molecular mechanisms of microRNAs in regulating epithelial-mesenchymal transitions in human cancers. Cancer Lett. 2016;371(2):301–313. doi:10.1016/j.canlet.2015.11.043
8. Davis FM, Stewart TA, Thompson EW, Monteith GR. Targeting EMT in cancer: opportunities for pharmacological intervention. Trends Pharmacol Sci. 2014;35(9):479–488. doi:10.1016/j.tips.2014.06.006
9. Wang P, Zhao J, Yang X, et al. PFDN1, an indicator for colorectal cancer prognosis, enhances tumor cell proliferation and motility through cytoskeletal reorganization. Med Oncol. 2015;32(12):264. doi:10.1007/s12032-015-0710-z
10. Wang D, Shi W, Tang Y, et al. Prefoldin 1 promotes EMT and lung cancer progression by suppressing cyclin A expression. Oncogene. 2017;36(7):885–898. doi:10.1038/onc.2016.257
11. Sun J, Zhang T, Cheng M, et al. TRIM29 facilitates the epithelial-to-mesenchymal transition and the progression of colorectal cancer via the activation of the Wnt/β-catenin signaling pathway. J Exp Clin Cancer Res. 2019;38(1):104. doi:10.1186/s13046-019-1098-y
12. Catalano V, Labianca R, Beretta GD, Gatta G, de Braud F, Van Cutsem E. Gastric cancer. Crit Rev Oncol Hematol. 2009;71(2):127–164. doi:10.1016/j.critrevonc.2009.01.004
13. Zhu J, Xiong G, Fu H, Evers BM, Zhou BP, Xu R. Chaperone Hsp47 drives malignant growth and invasion by modulating an ECM gene network. Cancer Res. 2015;75(8):1580–1591. doi:10.1158/0008-5472.CAN-14-1027
14. Luo B, Lee AS. The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies. Oncogene. 2013;32(7):805–818. doi:10.1038/onc.2012.130
15. Siegert R, Leroux MR, Scheufler C, Hartl FU, Moarefi I. Structure of the molecular chaperone prefoldin: unique interaction of multiple coiled coil tentacles with unfolded proteins. Cell. 2000;103(4):621–632. doi:10.1016/S0092-8674(00)00165-3
16. Cao S, Carlesso G, Osipovich AB. Subunit 1 of the prefoldin chaperone complex is required for lymphocyte development and function. J Immunol. 2008;181(1):476–484. doi:10.4049/jimmunol.181.1.476
17. Singh M, Manoranjan B, Mahendram S, McFarlane N, Venugopal C, Singh SK. Brain metastasis-initiating cells: survival of the fittest. Int J Mol Sci. 2014;15(5):9117–9133. doi:10.3390/ijms15059117
18. Liang TS, Zheng YJ, Wang J, Zhao JY, Yang DK, Liu ZS. MicroRNA-506 inhibits tumor growth and metastasis in nasopharyngeal carcinoma through the inactivation of the Wnt/β-catenin signaling pathway by down-regulating LHX2. J Exp Clin Cancer Res. 2019;38(1):97. doi:10.1186/s13046-019-1023-4
19. Tian X, Liu Z, Niu B, et al. E-cadherin/β-catenin complex and the epithelial barrier. J Biomed Biotechnol. 2011;2011:567305. doi:10.1155/2011/567305
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.