Back to Journals » OncoTargets and Therapy » Volume 11
PET-CT evaluation of the curative effect of crizotinib on malignant myofibroblastoma with rare mutation of ALK R401: a case report and literature review
Authors Yang L , Wu Y , Tang H, Zhao J, Zhao D, Yang S , Wang Q
Received 24 October 2017
Accepted for publication 16 February 2018
Published 5 April 2018 Volume 2018:11 Pages 1921—1927
DOI https://doi.org/10.2147/OTT.S155033
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Samir Farghaly
Li Yang,1,* Yufeng Wu,1,* Hong Tang,1 Jiuzhou Zhao,2 Dongdong Zhao,1 Sen Yang,1 Qiming Wang1
1Department of Internal Medicine, Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital, Zhengzhou, China; 2Department of Molecular Pathology, Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital, Zhengzhou, China
*These authors contributed equally to this work
Objective: The purpose of this article is to explore the targeted treatment of malignant myofibroblastoma and evaluate the role of neoplasm metabolite markers in the evaluation of efficacy after targeted therapy.
Method: This report described a case of myofibroblastic sarcoma with rare mutation of ALK R401 in a 58-year-old man prescribed with crizotinib, to evaluate its curative effect by positron emission tomography coupled with computed tomography (PET-CT). After the progressive disease in the brain, bevacizumab combined with crizotinib was administered. The Response Evaluation Criteria in Solid Tumors (RECIST), metabolic tumor volume (MTV), and total lesion glycolysis (TLG) were used to assess the efficacy. The efficacy was assessed by comparing changes in MTV and TLG.
Result: After the treatment of crizotinib, the tumor volume was decreased. However, bevacizumab combined with crizotinib had not improved the prognosis. The change of MTV and TLG was consistent with the efficacy. The increase of MTV and TLG is an early indicator of the poor prognosis of patients.
Conclusion: The treatment of the crizotinib patient with the mutation of ALK R401 was effective. The values of MTV and TLG reflected the prognosis earlier than RECIST.
Keywords: malignant myofibroblastoma, PET-CT, ALK R401, volume-dependent parameter
Introduction
Malignant myofibroblastoma is a rare tumor in adults and pediatric patients,1–4 the classification of which belongs to myofibroblastic tumors. The classification of the myofibroblastic tumors is myfibroblastic sarcoma (MS), inflammatory myofibroblastic, myofibromatosis, myofibroblastoma, myopericytoma, and angiomyofibrolastoma. This report is based on low-grade fibrous myofibroblasts, which are infrequently accompanied by metastasis, and surgical treatment is common. It has been known to arise mainly in the head and neck regions and the soft tissues of the extremities,5,6 although it could be rarely found at the bone and breast; only a small number of cases have been reported in the literature worldwide.7 Inflammatory myofibroblastic tumor (IMT) and MS belong to soft tissue tumors, but the malignant level was different. The expression of anaplastic lymphoma kinase (ALK) protein occurs in 50%–70% of IMTs,8,9 while the ALK of MS are mostly immunonegative.10,11 ALK-R401 is a nonsense mutation, and its function is unknown.12 In this case, the immunohistochemistry (IHC) of our patient did not reveal ALK gene mutation, but next-generation sequencing (NGS) supported ALK R401 mutation in exon five. Treatment with an ALK inhibitor was found to be effective in reducing tumor size and tumor metabolism.
Tumor-response assessments are still using Response Evaluation Criteria in Solid Tumors (RECIST), which evaluate the effectiveness using the maximum and rear diameters.13–15 Although tumor size changes often take time (up to several months), it is necessary in the clinical treatment process to be able to predict early clinical response and to adjust treatment plans accordingly. Furthermore, metabolic changes in the tumor may reflect changes in the condition earlier. Positron emission tomography coupled with computed tomography (PET-CT) has long been a reference imaging tool for the diagnosis and staging of tumors,16 particularly in cases where the metastatic tumors of the primary lesion were unknown.17 There are some studies confirming that metabolic tumor volume (MTV) and total lesion glycolysis (TLG) as a tumor metabolic index can be used to assess the prognosis of patients.18 Herein, we reviewed the use of PET-CT to assess treatment response and found that the MTV can reflect the metabolic and volume changes, which are more conducive to predict the prognosis of patients.
Case report
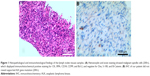
A 58-year-old male presented to the hospital with paroxysmal pain in the chest and discomfort in the head on August 10, 2015. The Karnofsky performance status (KPS) score was 60. PET-CT revealed a right upper chest mass was 51×41 mm, with a maximum standardized uptake value (SUV) of 5.6, accompanied with brain, bone, liver, and right subscapularis muscle metastases with high SUV. We recorded the tumor size of the primary tumor and metastases. The specimens showed malignant spindle cells, which displayed immunohistochemical positive staining for CK, SMA, CD34, CD99, and Bcl-2, and negative for Des, S-100, and B-Catenin. ALK was not supported (Ventana IHC) (Figure 1). The patient was then diagnosed with malignant myofibroblastoma with brain, bone, and liver metastases.
Considering the patient’s poor KPS score (60 at diagnosis) and metastases all over the body, chemotherapy, radio-chemotherapy, or surgery was excluded. Methylprednisolone was given at 800 mg/d, day (d)1-5 started from August 20, 2015. The patient then developed an aggravating headache. Fifteen days later, PET-CT imaging showed tumor volume enlargement. The MTV and TLG values are also increased. Subsequently, genetic tests revealed the fifth exon R401 mutation of the ALK gene. Considering the tumor histological and biological features, crizotinib was given at 250 mg daily from September 10, 2015. After 1 month of crizotinib at 250 mg daily, the headache was eased, and PET-CT (October 9, 2015) showed a dramatic reduction in tumor size and metabolism, resulting in stable disease (SD), based on criteria in RECIST 1.1 with KPS score of 70. Conversely, the MTV and TLG values were also significantly reduced.
Two months later, the patient had headaches again, so we reviewed his PET-CT on December 7, 2015. The brain imaging showed that the primary tumor was enlarged with edema, and new metastases could be found in the lung (Figure 2). The tumor size of the right upper chest wall, liver, and right subscapularis muscle metastases were stable, while the MTV and TLG values of them were almost doubled, representing progressive disease. The patient’s family rejected the second-generation ALK inhibitors. Thus, bevacizumab (500 mg/d, d1, every 21 days), combined with crizotinib at 250 mg daily, was administered. Twenty days later, the headache was eased, and the KPS score was 80. Two months after treatment with bevacizumab (500 mg/d, d1, every 21 days) combined with crizotinib 250 mg daily, the headache was aggravated, and the patient developed a poor diet, nausea, and vomiting. The KPS score was 50, PET-CT imaging showed right pleural effusion, all body metastases were enlarged, and the MTV and TLG values were significantly increased, indicating progressive disease (Figure 3). The patient was administered nutritional supportive treatment. Unfortunately, the patient died 1 month later in March 2016 (Figure 4).
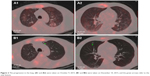  | Figure 2 The progression in the lung. (A1 and A2) were taken on October 9, 2015. (B1 and B2) were taken on December 10, 2015, and the green arrows refer to the new lesions. |
Written informed consent was obtained from the patient’s wife (the patient’s next of kin) for publication of this case report and accompanying images.
Discussion
Crizotinib is an ATP competitor that inhibits the multitarget protein kinase inhibitor of Met/ALK/ROS. Crizotinib has been used in ALK positive non-small cell lung cancer (NSCLC) to lengthen the period without progression. ALK inhibitors have been reported as effective treatments for ALK-rearranged IMT.19,20 It was reported that a patient who was diagnosed ALK-1-rearranged IMT with brain metastases was initially treated with crizotinib, and the disease progressed after 3 months.21 After that, alectinib, the second-generation ALK inhibitor, was taken as the second-line therapy with 8-month progression-free survival. Our patient had malignant myofibroblastoma with the mutation of ALK-R401. There is no report about the function and treatment effect of the ALK-R401, but it was considered implicated in tumorigenesis. In this case, after the treatment of crizotinib, the progression-free survival was 3 months. It has been known that crizotinib has poor central nervous system (CNS) penetration, as evidenced by low concentrations detected in CNS samples during the treatment course,22 which was likely the cause of treatment failure in our patient. Also, alectinib, which is a selective ALK inhibitor with high CNS penetration, is active against several secondary mutations that confer acquired resistance to crizotinib.23 It may be effective to administer the second generation ALK inhibitor after drug resistance.
Bevacizumab, a recombinant, humanized monoclonal antibody against vascular endothelial growth factor (VEGF), is a vascular-targeted therapy that may inhibit neovascularization,24 such as slower tumor development, reduced metastasis development, and improved drug delivery through vascular normalization.25 Bevacizumab is more effective when combined with other conventional therapies, such as chemotherapy or radiotherapy, and may be added to treatment regimens to increase their efficacy or to reduce developing therapeutic resistance.26,27 Several studies have demonstrated that bevacizumab could reduce brain edema and intracranial pressure, reducing the symptoms of headache or dizziness. Currently, the clinical trials of the EGFR-TKI (Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitor), combined with bevacizumab, have shown good curative effects in the treatment of lung adenocarcinoma.28,29 So, in our treatment, when the disease progressed after oral crizotinib, bevacizumab combined with crizotinib was given. Then the headache was eased transient, but the treatment effect was unsatisfactory. Whether bevacizumab combined with an ALK inhibitor is efficacious is worth exploring.
PET-CT is not only a reference imaging tool for the diagnosis and staging of tumors, but can also reflect the metabolic status of the tumor, to be used for evaluation of efficacy and prognosis.30 Currently, the prognostic information was deduced from the SUV, MTV, and TLG, which were significantly associated with an early metabolic response.31 Additionally, once PET-CT was used to evaluate the effects of a patient with multiple metastases, clinicians can comprehensively assess patient’s condition changes. In targeted therapy, PET-CT can reflect the metabolic abilities of the cells to predict the curative effect early and adjust the treatment plan in time. At present, MTV is considered a prognostic indicator of tumor survival, being a volumetric and metabolic biomarker of the tumor.32 Thus, unlike SUVmax, MTV can quantify the overall tumor burden. In some studies, higher MTV and TLG were significantly associated with shorter progression-free survival (PFS). In this case, the multiple growth of MTV may be related to the poor prognosis of the tumor.
Conclusion
Targeted therapy can be considered in the treatment of the poor KPS scores patient of advanced tumor with multiple metastases. Patients with mutated ALK-R401 are effective in the treatment of crizotinib. However, crizotinib has low penetration into the central nervous system, patient with head metastases is responded poorly to it. Bevacizumab, combined with crizotinib, had not improved the prognosis. Bevacizumab combined with crizotinib had not achieved excellent outcome in this advanced tumor patient with head metastases. PET-CT can be used to comprehensively assess the condition of patients with advanced tumor. MTV and TLG can reflect the metabolic changes of the tumor, and the sudden increase in its value may be related to the poor prognosis of the patient.
Acknowledgments
This study was supported, in part, by the National Natural Science Foundation of China (No 81272600), a project co-sponsored by the Henan Province and Ministry of Health of Medical Science and Technology Program (No 201601026), “51282 project”, leading talent of Henan Provincial Health Science and Technology Innovation Talents (No [2016]32), Natural Science Foundation of Henan Province (No 162300410300), Wu Jieping Medical Foundation for Clinical Research (No 320.6799.15018), Science and Technology Research Program of Henan Province (No 162102310327), Henan Province medical science and technology research project (No 201702249). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Hadjigeorgiou GF, Samaras V, Varsos V. Low-grade myofibroblastic sarcoma of the thoracic spine: report of an extreme rare case. Br J Neurosurg. 2017;31(6):731–733. | ||
Schurch W, Seemayer TA, Gabbiani G. The myofibroblast: a quarter century after its discovery. Am J Surg Pathol. 1998;22(2):141–147. | ||
Jo VY, Fletcher CD. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology. 2014;46(2):95–104. | ||
Singh SK, Raheja AA, Jagdevan AK, Singh PK, Purkait S, Suri VA. Malignant temporal muscle myofibroblastoma with an early recurrence in a child. Neurol India. 2017;65(2):408–410. | ||
Humphries WE 3rd, Satyan KB, Relyea K, et al. Low-grade myofibroblastic sarcoma of the sacrum. J Neurosurg Pediatr. 2010;6(3):286–290. | ||
Majno G. The story of the myofibroblasts. Am J Surg Pathol. 1979;3(6):535–542. | ||
Stark M, Hoffmann A, Xiong Z. Mammary myofibrosarcoma: case report and literature review. Breast J. 2011;17(3):300–304. | ||
Montgomery EA, Shuster DD, Burkart AL, et al. Inflammatory myofibroblastic tumors of the urinary tract: a clinicopathologic study of 46 cases, including a malignant example inflammatory fibrosarcoma and a subset associated with high-grade urothelial carcinoma. Am J Surg Pathol. 2006;30(12):1502–1512. | ||
Sukov WR, Cheville JC, Carlson AW, et al. Utility of ALK-1 protein expression and ALK rearrangements in distinguishing inflammatory myofibroblastic tumor from malignant spindle cell lesions of the urinary bladder. Mod Pathol. 2007;20(5):592–603. | ||
Lu ZJ, Zhou SH, Yan SX, Yao HT. Anaplastic lymphoma kinase expression and prognosis in inflammatory myofibroblastic tumours of the maxillary sinus. J Int Med Res. 2009;37(6):2000–2008. | ||
Qiu X, Montgomery E, Sun B. Inflammatory myofibroblastic tumor and low-grade myofibroblastic sarcoma: a comparative study of clinicopathologic features and further observations on the immunohistochemical profile of myofibroblasts. Hum Pathol. 2008;39(6):846–856. | ||
Yau NK, Fong AY, Leung HF, et al. A pan-cancer review of ALK mutations: implications for carcinogenesis and therapy. Curr Cancer Drug Targets. 2015;15(4):327–336. | ||
Choi H. Role of imaging in response assessment and individualised treatment for sarcomas. Clin Oncol (R Coll Radiol). 2017;29(8):481–488. | ||
Riedl CC, Pinker K, Ulaner GA, et al. Comparison of FDG-PET/CT and contrast-enhanced CT for monitoring therapy response in patients with metastatic breast cancer. Eur J Nucl Med Mol Imaging. 2017;44(9):1428–1437. | ||
Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. | ||
Bang JI, Lim Y, Paeng JC, et al. Comparison of quantitative methods on FDG PET/CT for treatment response evaluation of metastatic colorectal cancer. Nucl Med Mol Imaging. 2017;51(2):147–153. | ||
Schafer JF, Gatidis S, Schmidt H, et al. Simultaneous whole-body PET/MR imaging in comparison to PET/CT in pediatric oncology: initial results. Radiology. 2014;273(1):220–231. | ||
Ben Bouallegue F, Tabaa YA, Kafrouni M, Cartron G, Vauchot F, Mariano-Goulart D. Association between textural and morphological tumor indices on baseline PET-CT and early metabolic response on interim PET-CT in bulky malignant lymphomas. Med Phys. 2017;44(9):4608–4619. | ||
Butrynski JE, D’Adamo DR, Hornick JL, et al. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N Engl J Med. 2010;363(18):1727–1733. | ||
Liu Q, Kan Y, Zhao Y, He H, Kong L. Epithelioid inflammatory myofibroblastic sarcoma treated with ALK inhibitor: a case report and review of literature. Int J Clin Exp Pathol. 2015;8(11):15328–15332. | ||
Yuan C, Ma MJ, Parker JV, Mekhail TM. Metastatic anaplastic lymphoma kinase-1 (ALK-1)-rearranged inflammatory myofibroblastic sarcoma to the brain with leptomeningeal involvement: favorable response to serial ALK inhibitors: a case report. Am J Case Rep. 2017;18:799–804. | ||
Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. 2011;29(15):e443–e445. | ||
Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017;390(10089):29–39. | ||
Stefanou D, Stamatopoulou S, Sakellaropoulou A, et al. Bevacizumab, pemetrexed and carboplatin in first-line treatment of non-small cell lung cancer patients: focus on patients with brain metastases. Oncol Lett. 2016;12(6):4635–4642. | ||
Aravantinos G, Pectasides D. Bevacizumab in combination with chemotherapy for the treatment of advanced ovarian cancer: a systematic review. J Ovarian Res. 2014;7:57. | ||
Horn L, Sandler A. Chemotherapy and antiangiogenic agents in non-small-cell lung cancer. Clin Lung Cancer. 2007;8(Suppl 2):S68–S73. | ||
Teng LS, Jin KT, He KF, Wang HH, Cao J, Yu DC. Advances in combination of antiangiogenic agents targeting VEGF-binding and conventional chemotherapy and radiation for cancer treatment. J Chin Med Assoc. 2010;73(6):281–288. | ||
Jiang T, Li A, Su C, et al. Addition of bevacizumab for malignant pleural effusion as the manifestation of acquired EGFR-TKI resistance in NSCLC patients. Oncotarget. 2017;8(37):62648–62657. | ||
Otsuka K, Hata A, Takeshita J, et al. EGFR-TKI rechallenge with bevacizumab in EGFR-mutant non-small cell lung cancer. Cancer Chemother Pharmacol. 2015;76(4):835–841. | ||
Kim KR, Shim HJ, Hwang JE, et al. The role of interim FDG PET-CT after induction chemotherapy as a predictor of concurrent chemoradiotherapy efficacy and prognosis for head and neck cancer. Eur J Nucl Med Mol Imaging. 2018;45(2):170–178. | ||
Matsumoto Y, Baba S, Endo M, et al. Metabolic tumor volume by 18F-FDG PET/CT can predict the clinical outcome of primary malignant spine/spinal tumors. Biomed Res Int. 2017;2017:8132676. | ||
Choi ES, Ha SG, Kim HS, Ha JH, Paeng JC, Han I. Total lesion glycolysis by 18F-FDG PET/CT is a reliable predictor of prognosis in soft-tissue sarcoma. Eur J Nucl Med Mol Imaging. 2013;40(12):1836–1842. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.