Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Perspectives on the Underlying Etiology of HCC and Its Effects on Treatment Outcomes
Received 17 January 2023
Accepted for publication 25 February 2023
Published 10 March 2023 Volume 2023:10 Pages 413—428
DOI https://doi.org/10.2147/JHC.S347959
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr David Gerber
Takanori Ito,1 Mindie H Nguyen2,3
1Department of Gastroenterology and Hepatology, Nagoya University Graduate School of Medicine, Nagoya, Japan; 2Division of Gastroenterology and Hepatology, Department of Medicine, Stanford University Medical Center, Palo Alto, CA, USA; 3Department of Epidemiology and Population Health, Stanford University Medical Center, Palo Alto, CA, USA
Correspondence: Mindie H Nguyen, Division of Gastroenterology and Hepatology, Department of Medicine, and Department of Epidemiology and Population Health, Stanford University Medical Center, 780 Welch Road, CJ250K, Palo Alto, CA, 94304, USA, Tel +1 650-498-6084, Fax +1 650-498-5692, Email [email protected]
Abstract: Hepatocellular carcinoma (HCC) continues to be a serious medical problem with poor prognosis worldwide. The distribution of the major etiologies of HCC is changing due to the progress of anti-viral treatments, including hepatitis B virus (HBV) suppression by nucleoside/nucleotide analogues (NAs) and increased sustained virologic response (SVR) rates by direct-acting antivirals (DAAs) for hepatitis C virus (HCV), as well as the rising trend of nonviral liver disease. Although viral hepatitis remains the most common cause of HCC, non-alcoholic liver disease (NAFLD) with metabolic syndrome and alcohol-associated liver disease (ALD) are increasing. Effective and well-tolerated NAs treatment can slow the disease progression of chronic HBV infection to cirrhosis, end-stage liver disease, and reduce HCC risk. Treatment with NAs is also associated with significant improvement in the long-term survival of patients with HBV infection who already have HCC. DAAs have achieved viral elimination in almost all patients with HCV without significant adverse events, even in patients with decompensated liver cirrhosis and HCC. Similarly, DAA therapy can reduce disease progression, liver and non-liver complications, and improve the long-term survival of patients with chronic HCV infection with or without HCC. Meanwhile, NAFLD is a rapidly increasing cause of HCC along with the epidemics of obesity and type 2 diabetes globally. NAFLD-related HCC can occur in patients without cirrhosis and is known to have a lower survival rate than viral hepatitis-related HCC. Since there is currently no specific pharmacotherapy effective for NAFLD, lifestyle modification and prevention of complications are important to improve prognosis. Additionally, ALD is the second fastest-growing cause of HCC-related deaths, especially with an accelerated trend since the COVID-19 pandemic. This review provides an overview of the epidemiologic trends in the etiologies of HCC, and the progress of treatments for each etiology and the impact on outcome in the patients with HCC.
Keywords: hepatocellular carcinoma, etiology, hepatitis B, hepatitis C, non-alcoholic fatty liver disease, alcohol-associated liver disease
Introduction
Liver cancer is the sixth most common malignant tumor (n = 905,677; 4.7%) and is the third leading cause of cancer-related death (n = 830,180; 8.3%) in 2020 based on GLOBOCAN database.1 Among liver cancers, hepatocellular carcinoma (HCC) accounts for around 90% of primary malignant liver tumors.2,3 Thus, HCC is one of the most common cancers worldwide and represents a major global health challenge.4–6 Additionally, the incidence rate of HCC has been increasing for the last 3 decades in the United States and is projected to continue to rise through 2030.7,8
Meanwhile, the distribution of the major etiologies of HCC which include hepatitis B virus (HBV), hepatitis C virus (HCV), alcohol, and metabolic disease (Figure 1) in contemporary clinical practice are changing along with increasing numbers of patients with sustained virological response (SVR) after HCV eradication by direct-acting antivirals (DAAs), of patients with suppressed HBV during nucleoside/nucleotide analogues (NAs) treatment, and of patients with non-alcoholic fatty liver disease (NAFLD) while alcohol-associated liver disease (ALD) remains a major contributor of HCC.9–11 Prior studies have also reported differential disease presentation and natural history of nonviral HCC as compared to those of viral HCC.12 Most recently, the COVID-19 pandemic has brought further changes with great impact on the changing epidemiology of chronic liver disease, particularly that of ALD as a result of increasing social isolation, increasing alcohol dependence, and poor access to mental health care.13
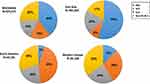 |
Figure 1 The etiology of Liver cancer diagnosed as new cases based on GLOBOCAN 2020.Reproduced from GLOBOCAN 2020. Available from: https://gco.iarc.fr/.1 East Asia includes China, Japan, Republic of Korea, Democratic People’s Republic of Korea, and Mongolia. North America includes USA and Canada. Western Europe includes Austria, Belgium, France, Germany, Luxembourg, Switzerland, and Netherlands. Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus; ALD, alcohol-related liver disease. |
In this review, we discuss the epidemiologic trends in the etiologies of HCC, and the progress and impact of the treatment on the incidence and outcome of patients with HCC, with a focus on viral hepatitis, NAFLD, and ALD.
Hepatitis B
HBV infection is a major global public health problem and is responsible for about 40% of the global HCC burden as of 2020 (Figure 1). The World Health Organization (WHO) estimates that worldwide, over 296 million people are positive for hepatitis B surface antigen (HBsAg) in 2019.14 Chronic HBV infection progresses from an asymptomatic, persistently infected state to chronic hepatitis, cirrhosis, or HCC. Although there are no curative treatments for HBV currently, there are effective and well-tolerated suppressive anti-viral medications, which can slow the disease progression of chronic HBV infection to cirrhosis, end-stage liver disease (ESLD) and HCC.15
The two main classes of antiviral treatments for HBV are pegylated interferon-α (Peg-IFNα) and nucleos(t)ide analogs (NAs).16,17 However, the relatively high variability of response and unfavorable safety profile limit the Peg-IFNα use.16 Due to its excellent safety and tolerability profile coupled with excellent effectiveness in suppressing viral replication, NAs may be the preferred option to Peg-IFNα for the vast majority of patients and has been the most commonly used antiviral agents for chronic HBV infection worldwide. Since 2005 and 2008, entecavir (ETV) and tenofovir disoproxil fumarate (TDF), respectively, have been the mainstay of treatment for chronic HBV infection because of their high genetic barrier against HBV resistance.17–19 More recently, tenofovir alafenamide (TAF), a prodrug of tenofovir modified to be more quickly and thoroughly absorbed into the cells and thus reducing potential risks of renal impairment and bone loss, is another first-line treatment for chronic HBV infection.20
Though the use of NAs does not eliminate the risk of HCC in treated patients, it has been shown to significantly reduce the risk of HCC while also reducing the risk of progression to cirrhosis and ESLD or even reversing ESLD.15 Several studies have reported that suppression of viruses using NAs by keeping a low or undetectable HBV DNA leads to a reduction of hepatocarcinogenesis.17,19,21 An early study demonstrated reduced HCC incidence with long-term treatment with ETV in HBV patients with cirrhosis but not in non-cirrhosis.22 Since then, additional studies have confirmed the benefit of HCC prevention with other antiviral agents for a wider range of patients with chronic HBV infection to include those without cirrhosis.23 There have also been conflicting reports on the effectiveness of ETV and TDF in HCC prevention with some finding TDF to be superior while some found no significant differences between the two.24–26 However, it appears that the source of heterogeneity and inconsistencies lies in the differences of not only the baseline characteristics but also the length of follow-up observation between the ETV vs TDF study populations among these various studies. Though TDF was approved only 3 years after ETV, reimbursement for TDF came many years after ETV, the higher HCC occurrence rates observed among ETV-treated patients may have been due to the fact that they were just observed for a longer time. Additionally, due to the “warehousing” effect for ETV as the first “good” antiviral agent, ETV-treated patients tended to have more advanced liver disease and thus higher risk for HCC and background risks between ETV-treated and TDF-treated patients may not have been adequately balanced by some studies, especially those relying on administrative claims database which usually lack detailed clinical and/or laboratory data. Indeed, in a recent meta-analysis involving 24,269 patients (tenofovir, n = 10,534 and entecavir n = 13,735) from 14 studies, no significant difference in the risk of HCC between patients who received ETV and patients who received TDF was found.27 In the same meta-analysis, between study heterogeneity was resolved once studies based on administrative database and studies with significant differences in the follow-up time between the study groups were excluded. Currently, there are no long-term data regarding HCC risk for the newer first-line HBV medication TAF, though some studies have demonstrated that switching from ETV or TDF to TAF can lead to further increase in virologic response, while preserving renal function.28–35
Currently, professional society guidelines recommend antiviral treatment for patients without cirrhosis with elevated HBV DNA (≥2000-20,000 IU/mL) and elevated alanine aminotransferase (ALT) levels or fibrosis stage 2 or higher (Table 1) 16,17,36–38. For patients with cirrhosis especially those with decompensated cirrhosis, antiviral therapy is generally recommended for patients with any level of detectable HBV DNA and irrespective of ALT levels.39–41 However, viral suppression could reduce liver-related mortality, the effect of NAs on hepatocarcinogenesis in decompensated HBV cirrhosis may be limited as HCC incidence and non-liver mortality were not found to improve with NA therapy in a prior study.42 On the other hand, among patients with existing HCC, antiviral therapy has been shown to associate with significant improvement in overall survival even in patients with decompensated cirrhosis, patients with advanced HCC stage, and in patients with supportive care only43,44.
 |
Table 1 Summary of Latest Society Guidelines for Treatment of Chronic Hepatitis B |
Unfortunately, it is estimated that only about 15% of patients with HBV infection in the US have been diagnosed, only about 70% of diagnosed patients who were linked to care undergo adequate evaluation for treatment, and only about 70% of those evaluated and met treatment criteria would receive treatment.44–47 Most disturbingly, less than half of patients with chronic HBV infection and advanced disease such as cirrhosis or HCC received antiviral therapy.
Mother-to-child transmission (MTCT) is a major route for transmitting HBV. In order to prevent MTCT, all pregnant women should first be screened for HBsAg. Additionally, the combination of HBV vaccination and hepatitis B immune globulin (HBIG) is the most effective way to reduce the risk of MTCT.17 On the other hand, infants born to women with high HBV viral load (>2×105 IU/mL) and with HBeAg positive are at high risk of MTCT, even if HBIG and HBV vaccine administration is completed immediately after birth. For these pregnant women with high HBV loads, NA therapy can reduce the incidence of MTCT of HBV without increasing adverse events related to pregnancy outcomes of the fetus.16,17,38,48 As for the type of NA used, WHO recommends that pregnant women with high HBV viral load take TDF from the 28th week of pregnancy until at least delivery.49 Thus, HBV-related HCC is a vaccine-preventable cancer.
In summary, HBV contributes to almost half of the global HCC burden. While HBV-related HCC can be prevented by effective and well-tolerated NAs and the overall mortality in patients with HBV-related HCC can be reduced by NA treatment, HBV diagnosis and treatment rates are suboptimal, even for patients with severe complications such as HCC. Further education and public health efforts are needed to improve the currently poor linkage to care for patients with HBV infection.44
Hepatitis C
HCV affects approximately 58 million people and contributes to 18% of the HCC burden worldwide (Figure 1).50 The chronic infection of HCV is one of the leading causes of liver cirrhosis and HCC, but the progression to cirrhosis is often clinically silent as well as HBV infection, and some patients are unaware of the HCV infection.51–53 Among patients with cirrhosis and HCV infection, HCC develops at an average rate of 3% to 7% per year based on the data before interferon (IFN) free direct‐acting antivirals era (DAAs).54,55 The achievement of a sustained virologic response (SVR) to antiviral treatment for HCV has been shown to prevent liver-related complications in patients with HCV-related cirrhosis.56–58
Although IFN had been used for anti-HCV therapy for decades, peg-IFN-based therapy was contraindicated in some patients due to its adverse event (AE) and serious adverse event (SAE) profiles.59 Since 2013, highly efficacious and well-tolerated IFN-free-DAA regimens have revolutionized HCV treatment with cure rate of over 90% including most historically difficult-to-treat patient groups such as those with decompensated cirrhosis and HCC60–69 (Figure 2).
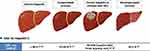 |
Figure 2 Sustained virologic response (SVR) rate with interferon-free direct-acting antivirals (DAAs) in hepatitis C patients with or without cirrhosis / HCC. Abbreviations: SVR, Sustained virologic response; DAA, direct-acting antivirals; HCC, hepatocellular carcinoma; SVR, sustained viral response.62–69 |
For patients with chronic HCV infection who have not yet developed HCC, the first reports regarding the preventive effect of DAA on HCC development in patients with chronic hepatitis C (CHC) indicated conflicting results. Early small case series reported a higher de novo HCC incidence and higher rates of HCC recurrence following HCV cure by IFN-free DAA therapy,70,71 while some other studies showed that an SVR was associated with reduced risk of HCC development.72–74 A landmark meta-analysis of 41 studies on HCC occurrence and recurrence in both IFN- and DAA-treated HCV patients found that DAA-treated patients were much older biasing towards higher HCC rate in such patients, and that most HCC occurrences in DAA-treated patients occurred within 6–12 months of DAA therapy, suggesting those HCC cases were probably prevalent HCC rather than new HCC development.75 In fact, following meta-regression analysis adjusting for relevant confounders, there was no significant difference in HCC risk among patients treated with IFN versus those treated with DAA in this meta-analysis. The findings of this meta-analysis of early studies have been confirmed by large cohort studies of patients for both HCC recurrence and HCC occurrence and in both Western and Eastern patients.61,76 As a result, professional society guidelines have recommended IFN free-DAA treatment to reduce the risk of HCC.60,77 Taken together, it has been shown that DAAs treatment reduces HCC risk and does not increase the risk of HCC incidence/recurrence. In the case of patients with HCV infection and decompensated, cirrhosis, DAA therapy has also shown a high rate of viral clearance (SVR rate >90%),60 improved hepatic function,78 reduced liver transplantation due to hepatic decompensation,79 as well as reduced new HCC development (HCC developed 6 months after DAA treatment).80
As in the case with HBV, antiviral treatment reduces HCC risk but does not eliminate the risk though in the case of HCV, the risk mainly persists in patients who already developed advanced fibrosis or cirrhosis prior to HCV treatment and cure.81 Reported risk factors of de-novo HCC after SVR include older age, male sex, the presence of diabetes, alcohol consumption, and viral co-infections.82 Although no established therapy exists for preventing hepatocarcinogenesis after the achievement of SVR, there are several reports on medications showing efficacy in preventing the development of HCC in CHC patients with diabetes and dyslipidemia. Very recently, Tsai et al showed that the use of metformin reduced the HCC risk after SVR by IFN-based antiviral therapy in diabetic patients with CHC. A simple risk stratification composed of cirrhosis and DM non-metformin use could predict the long-term outcomes of CHC patients who achieved SVR.83 Statin use has been reported to be associated with the reduction of HCC and cirrhosis risk in a dose-dependent manner in CHC patients or those without antiviral therapy.84,85 Nevertheless, since HCC risk persists after HCV cure, higher risk patients should continue to undergo regular HCC surveillance with tumor markers (eg, α-fetoprotein) and imaging every 6 months.86–88
For patients with HCV infection who have already developed HCC, early reports also suggested concerns for lower SVR with DAA though DAAs were shown to be safe and well tolerated.81,89,90 However, the number of patients included in these early studies was relatively small and there was heterogeneity of clinical characteristics. A large systematic review and meta-analysis showed significantly lower SVR rate in active HCC (HCC that have not been treated or treated adequately) but not in inactive/ablated HCC (HCC that have been treated and without evidence of residual viable HCC on imaging) (Figure 2, Table 2).68 Another study with individual data of 6081 patients (HCC, n = 465; non-HCC, n = 5616) treated by interferon-free DAAs from a multi-center international cohort indicated a similar result with inactive HCC having a similar SVR rate as non-HCC patients and active HCC having lower SVR compared with inactive HCC or non-HCC patients, though the cure rate was still relatively high (85%) in active HCC patients (Table 2).62,68,91 Therefore, to achieve optimal cure rate with DAA, DAA therapy should be delayed in patients with HCV-related HCC until HCC treatment has been completed, except in cases where early DAA treatment may help improve liver function to allow for HCC treatment that would otherwise not be an option due to impaired hepatic function.
 |
Table 2 Sustained Virologic Response (SVR) with Interferon-Free Direct-Acting Antivirals (DAAs) and Outcome in Patients with HCV-Related HCC Who Have Achieved SVR |
Another issue in patients with HCV infection who already developed HCC is whether treatment with DAA can improve overall survival. A large multicenter US study showed significant improvement in survival of DAA-treated patients with HCC, as well as a large multinational study inclusive of patients from the US and Asia Pacific region.61,92 In the latter study, HCV-related HCC patients who achieved SVR had a 63% lower risk of 5-year all-cause and 66% lower risk of 5-year liver-related mortality compared with patients untreated for HCV after adjusting for relevant confounders.92 Taken together, DAA therapy should be considered in all HCV-related HCC patients who are not yet moribund.61,92
In summary, DAA therapy is safe, efficacious with cure rate of >90%, and highly effective in the prevention of HCC in HCV-infected patients who have not developed HCC including those with decompensated cirrhosis and should be considered in all such patients. For patients with HCV infection who have developed HCC, DAA therapy is also safe, efficacious (>90% in well-treated HCC and 85% in active HCC), and can improve overall survival by over 60%. Therefore, DAA therapy should be considered in patients with HCV-related HCC whose HCC is not pre-terminal, though treatment should be delayed until after adequate HCC treatment in most cases.
NAFLD
Another major and emerging cause of HCC globally is NAFLD (Figure 1). In both Asia and Western countries, NAFLD is increasing along with the epidemics of obesity and type 2 diabetes.93,94 NAFLD is now a leading cause of cirrhosis, and is becoming the most common underlying chronic liver disease in patients with HCC awaiting orthotropic liver transplantation (OLT).95 The prevalence of NAFLD is estimated to be 30% of the overall global population and this number is predicted to increase up to 55.7% by 2040.96 Even in areas with historically lower NAFLD prevalence such as Japan, a recent systematic review and meta-analysis with forecasting found an overall NAFLD prevalence for Japan to be 25.5%, with an annual increase of 0.64% to reach 39.3% in 2030 and 44.8% in 204093. NAFLD associated with metabolic syndrome has been considered a typical Western condition, but its prevalence has been increasing in Asia in recent years.94 This is related to lifestyle changes associated with rapid economic growth in Asia (eg, westernized diets and physical activity).97
The percentage of cases with NAFLD/nonalcoholic steatohepatitis (NASH)-related HCC has also increased.10 Compared with other etiologies, including viral hepatitis, NAFLD-related HCC was reported to be associated with lower surveillance receipt for HCC, and a lower rate of early-stage detection.98 Due to a lack of awareness of NAFLD-related HCC in primary care and often delayed diagnosis, some patients with NAFLD-related HCC are diagnosed as advanced-stage with poor overall survival.99,100 In addition, patients with NAFLD-related HCC are often older and with more comorbidities such as metabolic syndrome, cardiovascular diseases, and chronic kidney disease as compared to HCC patients due to the other etiologies.101,102 For these reasons, curative treatment such as surgery may not be possible in many cases, and the prognosis for NAFLD-related HCC is poor.
Previous studies have shown that liver fibrosis is a well-known key driver of mortality in patients with NAFLD, and is strongly associated with the incidence of liver-related events, including hepatic carcinogenesis.103,104 Meanwhile, it is also known that HCC can develop in non-cirrhotic patients with NAFLD. In a systematic review and meta-analysis based on the 19 studies with 168,571 participants, the prevalence of HCC in non-cirrhotic NASH is significantly higher than that in non-cirrhotic patients with chronic liver diseases of different etiologies, with a prevalence of 38% and 14%, respectively (P < 0.001).105 Currently, there is no established regular HCC surveillance system for patients with non-cirrhotic NAFLD.106
The mechanism of hepatocarcinogenesis in patients with NAFLD/NASH is complex and not fully understood. As with chronic liver disease of other etiologies, well-known common risk factors for HCC in patients with NAFLD also include older age, male sex, fibrosis stage, cigarette smoking, and alcohol consumption (Figure 3). Diabetes and obesity are also risk factors of HCC in NAFLD patients.107,108 The presence of type 2 diabetes and metabolic syndrome along with diabetes increases the risk of HCC 2-fold and 5-fold, respectively, while obesity (body mass index [BMI] >30 kg/m2) and severe obesity (BMI >35 kg/m2) can increase the risk of HCC 2-fold and 4-fold, respectively.100 Inflamed visceral adipose tissue induced by obesity secretes pro-inflammatory adipocytokines such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), thereby promoting systemic inflammation and insulin resistance, further exacerbating NAFLD.109,110 Besides, the development of HCC in NAFLD is influenced by genetic variation. A single nucleotide polymorphism (SNP) of patatin-like phospholipase domain-containing 3 (PNPLA3) (rs738409) is among the most well-known risks associated with hepatic steatosis, steatohepatitis, cirrhosis, and risk of HCC and mortality in NAFLD patients.111–118 Changes of gut microbiota are also thought to be a cause of HCC in NAFLD patients.119 In patients with NAFLD, increased intestinal permeability associated with disruption of tight junctions deteriorates gastrointestinal leakage, allowing bacterial translocation or invasion of bacterial-derived products, such as lipopolysaccharide (LPS). Alterations in the gut microbiome that affect the gut–liver axis are common in individuals with obesity and are also associated with the progression of chronic liver disease and advanced fibrosis, such as NAFLD and cirrhosis.120–123 Taken together, the causes of NAFLD progression and HCC are not simple and are intricately related to each other, which makes it more difficult for NAFLD-related HCC prevention.
NAFLD is generally associated with metabolic syndrome accompanied with obesity and insulin resistance, but it can develop in lean individuals, namely “lean NAFLD”. Of note, recent studies have shown that lean NAFLD, despite the absence of obesity, has a similar or poorer mortality risk as that of individuals with obesity and NAFLD.93,124,125 However, whether the incidence of HCC in lean/non-obese NAFLD differs from those with obese/overweight NAFLD remains undetermined. It is possible that a subpopulation at high risk for HCC, such as “burned-out NASH” and sarcopenic obesity with metabolic syndrome, may be included in lean NAFLD.
The presence of hepatic steatosis also affects the risk of developing HCC in patients with viral hepatitis. Hepatic steatosis is among the factors associated with increased risk of HCC in HCV patients after DAA treatment.76 The impact of hepatic steatosis on the natural history of chronic HBV infection is more controversial with studies showing conflicting results.126–128 Recently, a panel of international experts from 22 countries proposed a new diagnostic entity called metabolic dysfunction-associated fatty liver disease (MAFLD), which can coexist with other liver diseases.129 MAFLD would include patients with other causes of liver disease such as viral hepatitis, as opposed to NAFLD which requires exclusion of other liver disease.
For patients with NAFLD/NASH, the changes in lifestyle and management of metabolic risk factors may help prevent disease progression and consequently HCC (Figure 3). The primary treatment for overweight/obese patients with NAFLD is weight loss, which can improve liver steatosis and inflammation and suppress the progression of liver fibrosis.130 However, its effect on decreasing HCC incidence has not yet been established. On the other hand, a recent study of 467,336 cases from a European cohort showed that physical activity is associated with a reduced risk of developing liver cancers over the next decade. For those reporting >2 hours/week compared to those with no vigorous activity, the hazard ratio (HR) for HCC was 0.50 (95% CI 0.33–0.76).131 Turati et al reported that closer adherence to the Mediterranean diet can decrease HCC risk based on case-control studies undertaken in Italy and Greece that included 518 cases of HCC and 772 controls.132 The Mediterranean diet is recommended by the European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD) and European Association for the Study of Obesity (EASO).133
There have also been several studies regarding medications with the effect of decreasing the risk of HCC in patients with fatty liver (Figure 3). Prior studies have demonstrated reduced HCC risk with long-term metformin use in diabetic patients with NASH and advanced fibrosis,134 with incremental decrease of HCC risk with each year of metformin use in diabetic patients.135 Currently, there is insufficient evidence to support the regular use of metformin for preventing HCC in NAFLD patients without diabetes, but it should be considered in patients with NAFLD complicated by type 2 diabetes. Interestingly, in a large cohort study of 85,963 NAFLD patients with diabetes, while the use of metformin reduced 20% lower risk of HCC compared with no medication, the use of combination therapy with insulin and other oral medications was associated with increased risk, though adequate glycemic control overall was associated with a 31% lower risk of HCC.136
As seen with patients with HCV infection, statins have also been found to have a protective effect in reducing HCC risk in NAFLD patients. Statin use was significantly associated with 53% lower hazards of developing HCC in NAFLD in a nationwide cohort of 272,431 patients with NAFLD in the U.S., with consistent finding after adjusting for liver fibrosis.137 A dose-dependent effect was also observed in this study.
Vitamin E use (800 IU/day) is another commonly recommended chemotherapy for patients with NAFLD and it has been shown to associate with significant reductions in overall mortality and hepatic decompensation failure in patients with bridging fibrosis and cirrhosis due to NAFLD, irrespective of the presence of diabetes, though it did not reduce the risks of HCC, vascular events, or extra-hepatic malignancies.138
Also, as observed with chronic viral hepatitis,139 daily aspirin use was associated with less advanced histologic features of NAFLD and NASH, and lower risk for progression to advanced fibrosis with time.140 Therefore, low-dose aspirin use has been advocated for patients with NAFLD to prevent HCC and progression of liver histology, as long as the risk of bleeding is acceptable.
In summary, NAFLD is a fast-emerging cause of HCC worldwide. NAFLD-related HCC often present in late-stage, can occur in patients without cirrhosis, and has poorer survival rates than viral-related HCC. Improved diagnosis and more accurate risk stratification for patients with NAFLD are needed, so appropriate patients can be identified to enter HCC surveillance as well as other therapeutic intervention.
Alcohol-Associated Liver Disease
ALD refers to a wide spectrum of direct liver injuries ranging from steatosis, alcohol-associated hepatitis, cirrhosis, and HCC that are caused by excess alcohol consumption. As the International Agency for Research on Cancer (IARC) classifies alcohol as a group 1 human carcinogen,141 alcohol consumption leads to approximately 5% of the global burden of cancer.142 As mentioned above, alcohol is the second fastest-growing cause of liver cancer-related deaths from 2010 to 2019 after NAFLD,10 and alcohol use disorder (AUD) has become an urgent global issue.
Liver cancer accounted for 830,180 deaths, and 150,000 cases of those were associated with alcohol drinking in 2020.1 Although varied by region/country, an estimated 19% of deaths from liver cancer and 25% of cirrhosis deaths were associated with alcohol in 2019 (Figure 1).10,11,143 The global estimated age-standardized death rate (ASDR) for alcohol-associated cirrhosis and liver cancer was 4.5 cases (95% confidence interval (CI) 3.8–5.3) and 1.1 deaths (95% CI 0.9–1.3) per 100,000 population in 2019, respectively.10,11,143,144 The average level of alcohol consumption worldwide increased steadily from 5.5 liters in 2005 to 5.8 liters of pure alcohol per capita in 2019 and is predicted to increase to 7.6 liters in 2030.145 In parallel with the increasing global per-capita alcohol consumption, ASDR for alcohol-associated HCC is increasing.144
The annual incidence of HCC among patients with alcohol-associated cirrhosis was reported at 0.9% to 5.6%,146,147 and the cumulative incidence of HCC in alcohol-associated cirrhosis at 1, 5, and 10 years were reported 1%, 3%, and 9%, respectively.148 Whereas alcohol consumption was found to have a strong association with increased risks of mortality and hepatic decompensation in a dose-dependent manner among patients with alcohol-associated cirrhosis, an association between alcohol use and the development of HCC was controversial149. Since women are more susceptible to alcohol-induced liver injury than men, women are at higher risk for developing cirrhosis than men with the same amount of alcohol consumption.150,151 However, as alcohol consumption is substantially higher in men, ASDRs of alcohol-associated liver cancer are higher in men compared with women.152
The COVID-19 pandemic has inflicted a large impact on both the physical and mental well-being of people leading to increased alcohol consumption and dependence. There are diverse findings in trends of alcohol consumption during the COVID-19 pandemic.153–155 A nationwide USA survey demonstrated a 14% increase in alcohol consumption in 2020 compared with 2019156,157 and predicted the increase of ALD over the next few years as a result.158 Indeed, age-standardized mortality rates (ASMR) for ALD and NAFLD substantially increased during the COVID-19 pandemic with the largest disparities among the young, non-Hispanic White, and Alaska Indian/Native American populations.159 The one-year increase in alcohol consumption during the COVID-19 pandemic was estimated to result in 8000 additional ALD-related deaths (95% uncertainty interval [UI], 7500–8600), 18,700 cases of decompensated cirrhosis (95% UI: 17,600–19,900), and 1000 cases of HCC (95% UI: 1000–1100) between 2020 and 2040.158
Since the proportion of HCCs diagnosed during the cirrhosis follow-up was lower in the non-abstinent cirrhosis patients compared to the abstinent patients (11.9% vs 28.4%; P < 0.0001) while overall survival was also demonstrated to increase from 7.6 months in non-abstinent alcohol-related HCC patients to 11.7 months in abstinent alcohol-related HCC,160 the importance of alcohol abstinence cannot be over-emphasized. However, it should also be noted that the association between alcohol abstinence and reduced HCC risk was only observed in patients with compensated alcohol-associated cirrhosis and not in decompensated patients161. Furthermore, while 27% of ALD patients can achieve histological normalization with abstinence, 18% would still progress to cirrhosis.162,163 These findings indicated the need for an early diagnosis of ALD and early intervention for AUD including abstinence, treatment of withdrawal syndrome, relapse prevention medications and nutritional support.164,165
As with NAFLD-related HCC, ALD-related HCC also tend to present at later stage with poorer prognosis, and HCC surveillance in patients with alcohol-associated cirrhosis is also poorer than those of patients with viral-related cirrhosis.161
In summary, ALD is the second-fastest cause of HCC with an accelerated trend since the COVID-19 pandemic. Alcohol is a modifiable risk factor for HCC, but AUD is a disease and should be approached as such and without stigmatization. Disease control and prevention for AUD is urgently needed, and closer monitoring of patients with ALD is needed for intervention for AUD as well as HCC surveillance for those with cirrhosis.
Conclusion
Viral hepatitis B and C remain the major cause of HCC globally but advances in antiviral treatment have helped to reduce HCC burden related to viral hepatitis and have helped improve the survival of viral-related HCC patients. However, viral hepatitis B and C remained underdiagnosed and undertreated, especially for patients with HCC, so further patient, provider education and public health intervention are needed to improve the linkage of care for patients with viral hepatitis. Alcohol and non-alcohol associated fatty liver diseases are two other major causes of HCC and are the two fastest rising contributors. Currently, there is no effective specific pharmacotherapy for either of these conditions, so disease control and prevention rely on behavioral modification which is complex and requires concerted effort and commitment from all stakeholders.
Abbreviations
HCC, Hepatocellular carcinoma; HBV, hepatitis B virus; HCV, hepatitis C virus; NAs, nucleoside/nucleotide analogues; DAAs; direct-acting antivirals; SVR, sustained virologic response; NAFLD, non-alcoholic liver disease; ALD, alcohol-associated liver disease; WHO, World Health Organization; HBsAg, hepatitis B surface antigen; ESLD, end-stage liver disease; Peg-IFNα, pegylated interferon-α; ETV, entecavir; TDF, tenofovir disoproxil fumarate; TAF, tenofovir alafenamide; IFN, interferon; AE, adverse event; SAE, serious adverse event; MTCT, mother-to-child transmission; HBIG, hepatitis B immune globulin; CHC, chronic hepatitis C; OLT, orthotropic liver transplantation; NASH, nonalcoholic steatohepatitis; BMI, body mass index; IL-6, interleukin-6; TNF-α, tumor necrosis factor-alpha; SNP, single nucleotide polymorphism; PNPLA3, patatin-like phospholipase domain-containing 3; LPS, lipopolysaccharide; MAFLD, metabolic dysfunction-associated fatty liver disease; EASL, the European Association for the Study of the Liver; EASD, European Association for the Study of Diabetes; EASO, European Association for the Study of Obesity; IARC, the International Agency for Research on Cancer; AUD, alcohol use disorder; ASDR, age-standardized death rate; CI, confidence interval; ASMR, age-standardized mortality rates; UI, uncertainty interval.
Acknowledgments
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No external funding to disclose.
Disclosure
MHN received Research support from Pfizer, Enanta, CurveBio, Delfi Biotech, Innogen, Astra Zeneca, Gilead, Exact Sciences, Vir Biotech, Helio Health, National Cancer Institute, Glycotest, and B.K. Kee Foundation; also Consulting and/or Advisory Board for Intercept, Exact Science, Gilead, GSK, Eli Lilly, Laboratory of Advanced Medicine, and Janssen outside the submitted work. TI received speaker’s fees from Chugai Pharmaceutical Co., Ltd outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. GLOBOCAN 2020. Available from: https://gco.iarc.fr/.
2. Llovet JM, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018.
3. Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology. 2019;156(2):477–491 e471.
4. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33.
5. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249.
6. Hyun JY, Kim SK, Yoon SJ, et al. Microbiome-based metabolic therapeutic approaches in alcoholic liver disease. Int J Mol Sci. 2022;23(15):8749.
7. Kim HS, El-Serag HB. The epidemiology of hepatocellular carcinoma in the USA. Curr Gastroenterol Rep. 2019;21(4):17.
8. Petrick JL, Kelly SP, Altekruse SF, McGlynn KA, Rosenberg PS. Future of hepatocellular carcinoma incidence in the United States forecast through 2030. J Clin Oncol. 2016;34(15):1787–1794.
9. Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400(10360):1345–1362.
10. Huang DQ, Singal AG, Kono Y, Tan DJH, El-Serag HB, Loomba R. Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer. Cell Metab. 2022;34(7):969–977 e962. doi:10.1016/j.cmet.2022.05.003
11. Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18(4):223–238. doi:10.1038/s41575-020-00381-6
12. Degasperi E, Colombo M. Distinctive features of hepatocellular carcinoma in non-alcoholic fatty liver disease. Lancet Gastroenterol Hepatol. 2016;1(2):156–164. doi:10.1016/S2468-1253(16)30018-8
13. Da BL, Im GY, Schiano TD. Coronavirus disease 2019 hangover: a rising tide of alcohol use disorder and alcohol-associated liver disease. Hepatology. 2020;72(3):1102–1108. doi:10.1002/hep.31307
14. World Health Organization (WHO). Newsroom on hepatitis B. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b.
15. Nguyen MH, Wong G, Gane E, Kao JH, Dusheiko G. Hepatitis B virus: advances in prevention, diagnosis, and therapy. Clin Microbiol Rev. 2020;33(2). doi:10.1128/CMR.00046-19
16. Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560–1599. doi:10.1002/hep.29800
17. Lampertico P, Agarwal K, Berg T; European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–398. doi:10.1016/j.jhep.2017.03.021
18. Ahn J, Lee HM, Lim JK, et al. Entecavir safety and effectiveness in a national cohort of treatment-naive chronic hepatitis B patients in the US – The ENUMERATE study. Aliment Pharmacol Ther. 2016;43(1):134–144. doi:10.1111/apt.13440
19. Liaw YF, Sung JJ, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351(15):1521–1531. doi:10.1056/NEJMoa033364
20. Hsu YC, Wei MT, Nguyen MH. Tenofovir alafenamide as compared to tenofovir disoproxil fumarate in the management of chronic hepatitis B with recent trends in patient demographics. Expert Rev Gastroenterol Hepatol. 2017;11(11):999–1008.
21. Papatheodoridis GV, Chan HL, Hansen BE, Janssen HL, Lampertico P. Risk of hepatocellular carcinoma in chronic hepatitis B: assessment and modification with current antiviral therapy. J Hepatol. 2015;62(4):956–967.
22. Hosaka T, Suzuki F, Kobayashi M, et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58(1):98–107.
23. Lok AS, McMahon BJ, Brown RS, et al. Antiviral therapy for chronic hepatitis B viral infection in adults: a systematic review and meta-analysis. Hepatology. 2016;63(1):284–306.
24. Yip TC, Wong VW, Chan HL, Tse YK, Lui GC, Wong GL. Tenofovir Is associated with lower risk of hepatocellular carcinoma than entecavir in patients with chronic HBV Infection in China. Gastroenterology. 2020;158(1):215–225 e216.
25. Hsu YC, Wong GL, Chen CH, et al. Tenofovir versus entecavir for hepatocellular carcinoma prevention in an international consortium of chronic hepatitis B. Am J Gastroenterol. 2020;115(2):271–280.
26. Chon HY, Ahn SH, Kim YJ, et al. Efficacy of entecavir, tenofovir disoproxil fumarate, and tenofovir alafenamide in treatment-naive hepatitis B patients. Hepatol Int. 2021;15(6):1328–1336.
27. Tseng CH, Hsu YC, Chen TH, et al. Hepatocellular carcinoma incidence with tenofovir versus entecavir in chronic hepatitis B: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(12):1039–1052.
28. Nguyen MH, Atsukawa M, Ishikawa T, et al. Outcomes of sequential therapy with tenofovir alafenamide after long-term entecavir. Am J Gastroenterol. 2021;116(6):1264–1273.
29. Ogawa E, Nakamuta M, Koyanagi T, et al. Sequential HBV treatment with tenofovir alafenamide for patients with chronic hepatitis B: week 96 results from a real-world, multicenter cohort study. Hepatol Int. 2022;16(2):282–293.
30. Toyoda H, Leong J, Landis C, et al. Treatment and renal outcomes up to 96 weeks after tenofovir alafenamide switch from tenofovir disoproxil fumarate in routine practice. Hepatology. 2021;74(2):656–666.
31. Kumada T, Toyoda H, Yasuda S, Ito T, Tanaka J. Impact of switching to tenofovir alafenamide fumarate in patients with entecavir-treated chronic hepatitis B. Eur J Gastroenterol Hepatol. 2021;33(1SSuppl 1):e898–e904.
32. Kaneko S, Kurosaki M, Tamaki N, et al. Tenofovir alafenamide for hepatitis B virus infection including switching therapy from tenofovir disoproxil fumarate. J Gastroenterol Hepatol. 2019;34(11):2004–2010.
33. Farag MS, Fung S, Tam E, et al. Effectiveness and renal safety of tenofovir alafenamide fumarate among chronic hepatitis B patients: real-world study. J Viral Hepat. 2021;28(6):942–950.
34. Sakamoto Y, Shimada S, Kamiyama T, et al. Impact of comorbid renal dysfunction in patients with hepatocellular carcinoma on long-term outcomes after curative resection. World J Gastrointest Surg. 2022;14(7):670–684.
35. Yeh H, Chiang CC, Yen TH. Hepatocellular carcinoma in patients with renal dysfunction: pathophysiology, prognosis, and treatment challenges. World J Gastroenterol. 2021;27(26):4104–4142.
36. Terrault NA, Bzowej NH, Chang KM, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261–283.
37. Martin P, Nguyen MH, Dieterich DT, et al. Treatment algorithm for managing chronic hepatitis B virus infection in the United States: 2021 update. Clin Gastroenterol Hepatol. 2022;20(8):1766–1775.
38. Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10(1):1–98.
39. Jang JW, Choi JY, Kim YS, et al. Long-term effect of antiviral therapy on disease course after decompensation in patients with hepatitis B virus-related cirrhosis. Hepatology. 2015;61(6):1809–1820.
40. Peng CY, Chien RN, Liaw YF. Hepatitis B virus-related decompensated liver cirrhosis: benefits of antiviral therapy. J Hepatol. 2012;57(2):442–450.
41. Terrault N. Is low level viremia acceptable during antiviral therapy of patients with HBV infection and decompensated cirrhosis? Clin Gastroenterol Hepatol. 2018;16(12):1876–1878.
42. Kumada T, Toyoda H, Yasuda S, Miyake N, Ito T, Tanaka J. Long-term prognosis with or without nucleot(s)ide analogue therapy in hepatitis B virus-related decompensated cirrhosis. J Viral Hepat. 2021;28(3):508–516.
43. Chen VL, Yeh ML, Le AK, et al. Anti-viral therapy is associated with improved survival but is underutilised in patients with hepatitis B virus-related hepatocellular carcinoma: real-world east and west experience. Aliment Pharmacol Ther. 2018;48(1):44–54.
44. Yeo YH, Nguyen MH. Review article: current gaps and opportunities in HBV prevention, testing and linkage to care in the United States – a call for action. Aliment Pharmacol Ther. 2021;53(1):63–78.
45. Ye Q, Kam LY, Yeo YH, et al. Substantial gaps in evaluation and treatment of patients with hepatitis B in the US. J Hepatol. 2022;76(1):63–74.
46. Ogawa E, Yeo YH, Dang N, et al. Diagnosis rates of chronic hepatitis b in privately insured patients in the United States. JAMA Netw Open. 2020;3(4):e201844.
47. Le MH, Yeo YH, Cheung R, Henry L, Lok AS, Nguyen MH. Chronic hepatitis B prevalence among foreign-born and U.S.-born adults in the United States, 1999–2016. Hepatology. 2020;71(2):431–443.
48. Hou J, Cui F, Ding Y, et al. Management algorithm for interrupting mother-to-child transmission of hepatitis B virus. Clin Gastroenterol Hepatol. 2019;17(10):1929–1936 e1921.
49. World Health Organization. Prevention of Mother-to-Child Transmission of Hepatitis B Virus: Guidelines on Antiviral Prophylaxis in Pregnancy. Geneva: World Health Organization; 2020.
50. World Health Organization (WHO). Newsroom on hepatitis C. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c.
51. Zou B, Yeo YH, Le MH, et al. Prevalence of viremic hepatitis C virus infection by age, race/ethnicity, and birthplace and disease awareness among viremic persons in the United States, 1999–2016. J Infect Dis. 2020;221(3):408–418.
52. Hofmeister MG, Rosenthal EM, Barker LK, et al. Estimating prevalence of hepatitis C virus infection in the United States, 2013–2016. Hepatology. 2019;69(3):1020–1031.
53. Polaris Observatory HCVC. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2(3):161–176.
54. European Association For The Study Of The L, European Organisation For R, Treatment Of C. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–943.
55. Lok AS, Seeff LB, Morgan TR, et al. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. 2009;136(1):138–148.
56. van der Meer AJ, Feld JJ, Hofer H, et al. Risk of cirrhosis-related complications in patients with advanced fibrosis following hepatitis C virus eradication. J Hepatol. 2017;66(3):485–493.
57. Bruno S, Di Marco V, Iavarone M, et al. Survival of patients with HCV cirrhosis and sustained virologic response is similar to the general population. J Hepatol. 2016;64(6):1217–1223.
58. Alavi M, Law MG, Valerio H, et al. Declining hepatitis C virus-related liver disease burden in the direct-acting antiviral therapy era in New South Wales, Australia. J Hepatol. 2019;71(2):281–288.
59. Druyts E, Thorlund K, Wu P, et al. Efficacy and safety of pegylated interferon alfa-2a or alfa-2b plus ribavirin for the treatment of chronic hepatitis C in children and adolescents: a systematic review and meta-analysis. Clin Infect Dis. 2013;56(7):961–967.
60. Ghany MG, Morgan TR; Panel A-IHCG. Hepatitis C guidance 2019 update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America Recommendations for testing, managing, and treating hepatitis C virus infection. Hepatology. 2020;71(2):686–721.
61. Singal AG, Rich NE, Mehta N, et al. Direct-acting antiviral therapy for hepatitis C virus infection is associated with increased survival in patients with a history of hepatocellular carcinoma. Gastroenterology. 2019;157(5):1253–1263 e1252.
62. Ogawa E, Toyoda H, Iio E, et al. Hepatitis C virus cure rates are reduced in patients with active but not inactive hepatocellular carcinoma: a practice implication. Clin Infect Dis. 2020;71(11):2840–2848.
63. Feld JJ, Jacobson IM, Hezode C, et al. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med. 2015;373(27):2599–2607.
64. Mizokami M, Yokosuka O, Takehara T, et al. Ledipasvir and sofosbuvir fixed-dose combination with and without ribavirin for 12 weeks in treatment-naive and previously treated Japanese patients with genotype 1 hepatitis C: an open-label, randomised, Phase 3 trial. Lancet Infect Dis. 2015;15(6):645–653.
65. Zeuzem S, Foster GR, Wang S, et al. Glecaprevir-pibrentasvir for 8 or 12 weeks in HCV genotype 1 or 3 infection. N Engl J Med. 2018;378(4):354–369.
66. Forns X, Lee SS, Valdes J, et al. Glecaprevir plus pibrentasvir for chronic hepatitis C virus genotype 1, 2, 4, 5, or 6 infection in adults with compensated cirrhosis (EXPEDITION-1): a single-arm, open-label, multicentre phase 3 trial. Lancet Infect Dis. 2017;17(10):1062–1068.
67. Brown RS, Buti M, Rodrigues L, et al. Glecaprevir/pibrentasvir for 8 weeks in treatment-naive patients with chronic HCV genotypes 1–6 and compensated cirrhosis: the EXPEDITION-8 trial. J Hepatol. 2020;72(3):441–449.
68. Ji F, Yeo YH, Wei MT, et al. Sustained virologic response to direct-acting antiviral therapy in patients with chronic hepatitis C and hepatocellular carcinoma: a systematic review and meta-analysis. J Hepatol. 2019;71(3):473–485.
69. Curry MP, O’Leary JG, Bzowej N, et al. Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N Engl J Med. 2015;373(27):2618–2628.
70. Reig M, Marino Z, Perello C, et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol. 2016;65(4):719–726.
71. Conti F, Buonfiglioli F, Scuteri A, et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol. 2016;65(4):727–733.
72. Cheung MCM, Walker AJ, Hudson BE, et al. Outcomes after successful direct-acting antiviral therapy for patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;65(4):741–747.
73. Romano A, Angeli P, Piovesan S, et al. Newly diagnosed hepatocellular carcinoma in patients with advanced hepatitis C treated with DAAs: a prospective population study. J Hepatol. 2018;69(2):345–352.
74. ANRS collaborative study group on hepatocellular carcinoma, . Lack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: data from three ANRS cohorts. J Hepatol. 2016;65(4):734–740.
75. Waziry R, Hajarizadeh B, Grebely J, et al. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: a systematic review, meta-analyses, and meta-regression. J Hepatol. 2017;67(6):1204–1212.
76. Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153(4):996–1005 e1001.
77. European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69(2):461–511.
78. El-Sherif O, Jiang ZG, Tapper EB, et al. Baseline factors associated with improvements in decompensated cirrhosis after direct-acting antiviral therapy for hepatitis C virus infection. Gastroenterology. 2018;154(8):2111–2121 e2118.
79. Flemming JA, Kim WR, Brosgart CL, Terrault NA. Reduction in liver transplant wait-listing in the era of direct-acting antiviral therapy. Hepatology. 2017;65(3):804–812.
80. Lusivika-Nzinga C, Fontaine H, Dorival C, et al. The dynamic effect of direct-acting antiviral treatments on the risk of hepatocellular carcinoma in patients with cirrhosis and chronic hepatitis C. J Viral Hepat. 2019;26(12):1489–1492.
81. Beste LA, Green PK, Berry K, Kogut MJ, Allison SK, Ioannou GN. Effectiveness of hepatitis C antiviral treatment in a USA cohort of veteran patients with hepatocellular carcinoma. J Hepatol. 2017;67(1):32–39.
82. D’Ambrosio R, Degasperi E, Lampertico P. Predicting hepatocellular carcinoma risk in patients with chronic HCV infection and a sustained virological response to direct-acting antivirals. J Hepatocell Carcinoma. 2021;8:713–739.
83. Tsai PC, Kuo HT, Hung CH, et al. Metformin reduces hepatocellular carcinoma incidence after successful antiviral therapy in patients with diabetes and chronic hepatitis C in Taiwan. J Hepatol. 2022;78(2):281–292.
84. Simon TG, Bonilla H, Yan P, Chung RT, Butt AA. Atorvastatin and fluvastatin are associated with dose-dependent reductions in cirrhosis and hepatocellular carcinoma, among patients with hepatitis C virus: results from ERCHIVES. Hepatology. 2016;64(1):47–57.
85. Tsan YT, Lee CH, Ho WC, Lin MH, Wang JD, Chen PC. Statins and the risk of hepatocellular carcinoma in patients with hepatitis C virus infection. J Clin Oncol. 2013;31(12):1514–1521.
86. Semmler G, Meyer EL, Kozbial K, et al. HCC risk stratification after cure of hepatitis C in patients with compensated advanced chronic liver disease. J Hepatol. 2022;76(4):812–821.
87. Carrat F, Fontaine H, Dorival C, et al. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study. Lancet. 2019;393(10179):1453–1464.
88. Tanaka Y, Ogawa E, Huang CF, et al. HCC risk post-SVR with DAAs in East Asians: findings from the REAL-C cohort. Hepatol Int. 2020;14(6):1023–1033.
89. Prenner SB, VanWagner LB, Flamm SL, Salem R, Lewandowski RJ, Kulik L. Hepatocellular carcinoma decreases the chance of successful hepatitis C virus therapy with direct-acting antivirals. J Hepatol. 2017;66(6):1173–1181.
90. He S, Lockart I, Alavi M, Danta M, Hajarizadeh B, Dore GJ. Systematic review with meta-analysis: effectiveness of direct-acting antiviral treatment for hepatitis C in patients with hepatocellular carcinoma. Aliment Pharmacol Ther. 2020;51(1):34–52.
91. Chen CY, Huang CF, Cheng PN, et al. Factors associated with treatment failure of direct-acting antivirals for chronic hepatitis C: a real-world nationwide hepatitis C virus registry programme in Taiwan. Liver Int. 2021;41(6):1265–1277.
92. Dang H, Yeo YH, Yasuda S, et al. Cure with interferon-free direct-acting antiviral is associated with increased survival in patients with hepatitis C virus-related hepatocellular carcinoma from both east and west. Hepatology. 2020;71(6):1910–1922.
93. Ito T, Ishigami M, Zou B, et al. The epidemiology of NAFLD and lean NAFLD in Japan: a meta-analysis with individual and forecasting analysis, 1995–2040. Hepatol Int. 2021;15(2):366–379.
94. Estes C, Anstee QM, Arias-Loste MT, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J Hepatol. 2018;69(4):896–904.
95. Haldar D, Kern B, Hodson J, et al. Outcomes of liver transplantation for non-alcoholic steatohepatitis: a European Liver Transplant Registry study. J Hepatol. 2019;71(2):313–322.
96. Le MH, Yeo YH, Li X, et al. 2019 global NAFLD prevalence: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2022;20(12):2809–2817 e2828.
97. Kojima S, Watanabe N, Numata M, Ogawa T, Matsuzaki S. Increase in the prevalence of fatty liver in Japan over the past 12 years: analysis of clinical background. J Gastroenterol. 2003;38(10):954–961.
98. Karim MA, Singal AG, Kum HC, et al. Clinical characteristics and outcomes of nonalcoholic fatty liver disease-associated hepatocellular carcinoma in the United States. Clin Gastroenterol Hepatol. 2022. doi:10.1016/j.cgh.2022.03.010
99. Jun TW, Yeh ML, Yang JD, et al. More advanced disease and worse survival in cryptogenic compared to viral hepatocellular carcinoma. Liver Int. 2018;38(5):895–902.
100. Younossi ZM, Henry L. Epidemiology of non-alcoholic fatty liver disease and hepatocellular carcinoma. JHEP Rep. 2021;3(4):100305. doi:10.1016/j.jhepr.2021.100305
101. Mohamad B, Shah V, Onyshchenko M, et al. Characterization of hepatocellular carcinoma (HCC) in non-alcoholic fatty liver disease (NAFLD) patients without cirrhosis. Hepatol Int. 2016;10(4):632–639. doi:10.1007/s12072-015-9679-0
102. Kim NG, Nguyen PP, Dang H, et al. Temporal trends in disease presentation and survival of patients with hepatocellular carcinoma: a real-world experience from 1998 to 2015. Cancer. 2018;124(12):2588–2598. doi:10.1002/cncr.31373
103. Ito T, Ishigami M, Ishizu Y, et al. Utility and limitations of noninvasive fibrosis markers for predicting prognosis in biopsy-proven Japanese non-alcoholic fatty liver disease patients. J Gastroenterol Hepatol. 2019;34(1):207–214. doi:10.1111/jgh.14448
104. Fujii H, Iwaki M, Hayashi H, et al. Clinical outcomes in biopsy-proven nonalcoholic fatty liver disease patients: a multicenter registry-based cohort study. Clin Gastroenterol Hepatol. 2022;21(2):370–379.
105. Stine JG, Wentworth BJ, Zimmet A, et al. Systematic review with meta-analysis: risk of hepatocellular carcinoma in non-alcoholic steatohepatitis without cirrhosis compared to other liver diseases. Aliment Pharmacol Ther. 2018;48(7):696–703. doi:10.1111/apt.14937
106. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the study of liver diseases. Hepatology. 2018;67(1):328–357. doi:10.1002/hep.29367
107. Fujiwara N, Friedman SL, Goossens N, Hoshida Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J Hepatol. 2018;68(3):526–549. doi:10.1016/j.jhep.2017.09.016
108. Chavez-Tapia NC, Murua-Beltran Gall S, Ordonez-Vazquez AL, Nuno-Lambarri N, Vidal-Cevallos P, Uribe M. Understanding the role of metabolic syndrome as a risk factor for hepatocellular carcinoma. J Hepatocell Carcinoma. 2022;9:583–593. doi:10.2147/JHC.S283840
109. Azzu V, Vacca M, Virtue S, Allison M, Vidal-Puig A. Adipose tissue-liver cross talk in the control of whole-body metabolism: implications in nonalcoholic fatty liver disease. Gastroenterology. 2020;158(7):1899–1912. doi:10.1053/j.gastro.2019.12.054
110. Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24(7):908–922. doi:10.1038/s41591-018-0104-9
111. Liu YL, Patman GL, Leathart JB, et al. Carriage of the PNPLA3 rs738409 C >G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J Hepatol. 2014;61(1):75–81. doi:10.1016/j.jhep.2014.02.030
112. Carlsson B, Linden D, Brolen G, et al. Review article: the emerging role of genetics in precision medicine for patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2020;51(12):1305–1320. doi:10.1111/apt.15738
113. Kawaguchi T, Shima T, Mizuno M, et al. Risk estimation model for nonalcoholic fatty liver disease in the Japanese using multiple genetic markers. PLoS One. 2018;13(1):e0185490. doi:10.1371/journal.pone.0185490
114. Sookoian S, Pirola CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. 2011;53(6):1883–1894. doi:10.1002/hep.24283
115. Singal AG, Manjunath H, Yopp AC, et al. The effect of PNPLA3 on fibrosis progression and development of hepatocellular carcinoma: a meta-analysis. Am J Gastroenterol. 2014;109(3):325–334. doi:10.1038/ajg.2013.476
116. Shen J, Wong GL, Chan HL, et al. PNPLA3 gene polymorphism accounts for fatty liver in community subjects without metabolic syndrome. Aliment Pharmacol Ther. 2014;39(5):532–539. doi:10.1111/apt.12609
117. Valenti L, Al-Serri A, Daly AK, et al. Homozygosity for the patatin-like phospholipase-3/adiponutrin I148M polymorphism influences liver fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51(4):1209–1217. doi:10.1002/hep.23622
118. Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40(12):1461–1465. doi:10.1038/ng.257
119. Jayakumar S, Loomba R. Review article: emerging role of the gut microbiome in the progression of nonalcoholic fatty liver disease and potential therapeutic implications. Aliment Pharmacol Ther. 2019;50(2):144–158. doi:10.1111/apt.15314
120. Loomba R, Seguritan V, Li W, et al. Gut microbiome-based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metab. 2017;25(5):1054–1062 e1055. doi:10.1016/j.cmet.2017.04.001
121. Boursier J, Mueller O, Barret M, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63(3):764–775. doi:10.1002/hep.28356
122. Bajaj JS, Heuman DM, Hylemon PB, et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. 2014;60(5):940–947. doi:10.1016/j.jhep.2013.12.019
123. Qin N, Yang F, Li A, et al. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513(7516):59–64. doi:10.1038/nature13568
124. Ahmed OT, Gidener T, Mara KC, Larson JJ, Therneau TM, Allen AM. Natural history of nonalcoholic fatty liver disease with normal body mass index: a population-based study. Clin Gastroenterol Hepatol. 2022;20(6):1374–1381 e1376. doi:10.1016/j.cgh.2021.07.016
125. Zou B, Yeo YH, Nguyen VH, Cheung R, Ingelsson E, Nguyen MH. Prevalence, characteristics and mortality outcomes of obese, nonobese and lean NAFLD in the United States, 1999–2016. J Intern Med. 2020;288(1):139–151. doi:10.1111/joim.13069
126. Li J, Henry L, Nguyen MH. Chronic hepatitis B and fatty liver-weal or woe? Am J Gastroenterol. 2022;117(4):688. doi:10.14309/ajg.0000000000001644
127. Li J, Yang HI, Yeh ML, et al. Association between fatty liver and cirrhosis, hepatocellular carcinoma, and hepatitis B surface antigen seroclearance in chronic hepatitis B. J Infect Dis. 2021;224(2):294–302. doi:10.1093/infdis/jiaa739
128. Mao X, Cheung KS, Peng C, et al. Steatosis, HBV-related HCC, cirrhosis, and HBsAg seroclearance: a systematic review and meta-analysis. Hepatology. 2022. doi:10.1002/hep.32792
129. Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–209. doi:10.1016/j.jhep.2020.03.039
130. Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149(2):367–378e365; quiz e314–365. doi:10.1053/j.gastro.2015.04.005
131. Baumeister SE, Schlesinger S, Aleksandrova K, et al. Association between physical activity and risk of hepatobiliary cancers: a multinational cohort study. J Hepatol. 2019;70(5):885–892. doi:10.1016/j.jhep.2018.12.014
132. Turati F, Trichopoulos D, Polesel J, et al. Mediterranean diet and hepatocellular carcinoma. J Hepatol. 2014;60(3):606–611. doi:10.1016/j.jhep.2013.10.034
133. European Association for the Study of the L, European Association for the Study of D, European Association for the Study of O. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–1402. doi:10.1016/j.jhep.2015.11.004
134. Vilar-Gomez E, Vuppalanchi R, Desai AP, et al. Long-term metformin use may improve clinical outcomes in diabetic patients with non-alcoholic steatohepatitis and bridging fibrosis or compensated cirrhosis. Aliment Pharmacol Ther. 2019;50(3):317–328. doi:10.1111/apt.15331
135. Chen HP, Shieh JJ, Chang CC, et al. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut. 2013;62(4):606–615. doi:10.1136/gutjnl-2011-301708
136. Kramer JR, Natarajan Y, Dai J, et al. Effect of diabetes medications and glycemic control on risk of hepatocellular cancer in patients with nonalcoholic fatty liver disease. Hepatology. 2022;75(6):1420–1428. doi:10.1002/hep.32244
137. Zou B, Odden MC, Nguyen MH. Statin use and reduced hepatocellular carcinoma risk in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2022;21(2):435–444.
138. Vilar-Gomez E, Vuppalanchi R, Gawrieh S, et al. Vitamin E improves transplant-free survival and hepatic decompensation among patients with nonalcoholic steatohepatitis and advanced fibrosis. Hepatology. 2020;71(2):495–509. doi:10.1002/hep.30368
139. Simon TG, Ma Y, Ludvigsson JF, et al. Association between aspirin use and risk of hepatocellular carcinoma. JAMA Oncol. 2018;4(12):1683–1690. doi:10.1001/jamaoncol.2018.4154
140. Simon TG, Henson J, Osganian S, et al. Daily aspirin use associated with reduced risk for fibrosis progression in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2019;17(13):2776–2784 e2774. doi:10.1016/j.cgh.2019.04.061
141. International Agency for Research on Cancer (IARC). Available from: https://www.iarc.who.int.
142. Safiri S, Nejadghaderi SA, Karamzad N, et al. Global, regional, and national cancer deaths and disability-adjusted life-years (DALYs) attributable to alcohol consumption in 204 countries and territories, 1990–2019. Cancer. 2022;128(9):1840–1852. doi:10.1002/cncr.34111
143. Global health data exchange GBD 2019. Available from: https://vizhub.healthdata.org/gbd-results/.
144. Diseases GBD, Injuries C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222.
145. WHO global information system on alcohol and health. Available from: https://apps.who.int/gho/data/node.main.GISAH.
146. Hagstrom H, Thiele M, Sharma R, et al. Risk of cancer in biopsy-proven alcohol-related liver disease: a population-based cohort study of 3410 persons. Clin Gastroenterol Hepatol. 2022;20(4):918–929 e918. doi:10.1016/j.cgh.2021.01.005
147. Seitz HK, Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer. 2007;7(8):599–612. doi:10.1038/nrc2191
148. Huang DQ, Tan DJH, Ng CH, et al. Hepatocellular carcinoma incidence in alcohol-associated cirrhosis: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2022. doi:10.1016/j.cgh.2022.06.032
149. Pearson MM, Kim NJ, Berry K, et al. Associations between alcohol use and liver-related outcomes in a large national cohort of patients with cirrhosis. Hepatol Commun. 2021;5(12):2080–2095. doi:10.1002/hep4.1776
150. Rehm J, Taylor B, Mohapatra S, et al. Alcohol as a risk factor for liver cirrhosis: a systematic review and meta-analysis. Drug Alcohol Rev. 2010;29(4):437–445. doi:10.1111/j.1465-3362.2009.00153.x
151. Roerecke M, Vafaei A, Hasan OSM, et al. Alcohol consumption and risk of liver cirrhosis: a systematic review and meta-analysis. Am J Gastroenterol. 2019;114(10):1574–1586. doi:10.14309/ajg.0000000000000340
152. Tan DJH, Setiawan VW, Ng CH, et al. Global burden of liver cancer in males and females: changing etiological basis and the growing contribution of NASH. Hepatology. 2022. doi:10.1002/hep.32758
153. Acuff SF, Strickland JC, Tucker JA, Murphy JG. Changes in alcohol use during COVID-19 and associations with contextual and individual difference variables: a systematic review and meta-analysis. Psychol Addict Behav. 2022;36(4):386. doi:10.1037/adb0000852
154. Kilian C, O’Donnell A, Potapova N, et al. Changes in alcohol use during the COVID-19 pandemic in Europe: a meta-analysis of observational studies. Drug Alcohol Rev. 2022;41(4):918–931. doi:10.1111/dar.13446
155. Plata A, Motoki K, Spence C, Velasco C. Trends in alcohol consumption in relation to the COVID-19 pandemic: a cross-country analysis. Int J Gastron Food Sci. 2022;27:100397. doi:10.1016/j.ijgfs.2021.100397
156. Pollard MS, Tucker JS, Green HD. Changes in adult alcohol use and consequences during the COVID-19 pandemic in the US. JAMA Netw Open. 2020;3(9):e2022942. doi:10.1001/jamanetworkopen.2020.22942
157. Weerakoon SM, Jetelina KK, Knell G. Longer time spent at home during COVID-19 pandemic is associated with binge drinking among US adults. Am J Drug Alcohol Abuse. 2021;47(1):98–106. doi:10.1080/00952990.2020.1832508
158. Julien J, Ayer T, Tapper EB, Barbosa C, Dowd WN, Chhatwal J. Effect of increased alcohol consumption during COVID-19 pandemic on alcohol-associated liver disease: a modeling study. Hepatology. 2022;75(6):1480–1490. doi:10.1002/hep.32272
159. Gao X, Lv F, He X, et al. Impact of the COVID-19 pandemic on liver disease-related mortality rates in the United States. J Hepatol. 2022;78(1):16–27.
160. Costentin CE, Mourad A, Lahmek P, et al. Hepatocellular carcinoma is diagnosed at a later stage in alcoholic patients: results of a prospective, nationwide study. Cancer. 2018;124(9):1964–1972. doi:10.1002/cncr.31215
161. Rodriguez M, Gonzalez-Dieguez ML, Varela M, et al. Impact of alcohol abstinence on the risk of hepatocellular carcinoma in patients with alcohol-related liver cirrhosis. Am J Gastroenterol. 2021;116(12):2390–2398. doi:10.14309/ajg.0000000000001399
162. Lackner C, Spindelboeck W, Haybaeck J, et al. Histological parameters and alcohol abstinence determine long-term prognosis in patients with alcoholic liver disease. J Hepatol. 2017;66(3):610–618. doi:10.1016/j.jhep.2016.11.011
163. Hosseini N, Shor J, Szabo G. Alcoholic hepatitis: a review. Alcohol Alcohol. 2019;54(4):408–416. doi:10.1093/alcalc/agz036
164. Bajaj JS, Nagy LE. Natural history of alcohol-associated liver disease: understanding the changing landscape of pathophysiology and patient care. Gastroenterology. 2022;163(4):840–851. doi:10.1053/j.gastro.2022.05.031
165. Liu SY, Tsai IT, Hsu YC. Alcohol-related liver disease: basic mechanisms and clinical perspectives. Int J Mol Sci. 2021;22(10):5170.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.