Back to Journals » Journal of Asthma and Allergy » Volume 15
Perspectives for the Use of Bacterial Lysates for the Treatment of Allergic Rhinitis: A Systematic Review
Authors Janeczek K , Kaczyńska A , Emeryk A, Cingi C
Received 2 February 2022
Accepted for publication 13 June 2022
Published 23 June 2022 Volume 2022:15 Pages 839—850
DOI https://doi.org/10.2147/JAA.S360828
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Luis Garcia-Marcos
Kamil Janeczek,1 Agnieszka Kaczyńska,1 Andrzej Emeryk,1 Cemal Cingi2
1Department of Pulmonary Diseases and Children Rheumatology, Medical University of Lublin, Lublin, Poland; 2Department of Otorhinolaryngology, Faculty of Medicine, Eskişehir Osmangazi University, Eskişehir, Turkey
Correspondence: Kamil Janeczek, Department of Pulmonary Diseases and Children Rheumatology, Medical University of Lublin, Prof. A. Gębali 6, Lublin, 20-093, Poland, Tel +48817185477, Email [email protected]
Abstract: Bacterial lysates (BLs) are mixtures of bacterial antigens that have been used for many decades to minimize the risk of recurrent respiratory tract infections in both pediatric and adult populations. Research on the use of BLs is also conducted in allergology. Biomedical databases were searched for articles on the use of BLs in the treatment of allergic rhinitis (AR). After rejecting ineligible articles, six remaining reports were reviewed. Based on this review, it can be concluded that adding BL to standard therapy for seasonal or perennial AR reduces the severity of nasal symptoms and the need for antiallergic medications in both children and adults. Concurrently, these formulations have a high safety profile. An analysis of studies shows that the first effects of BLs therapy appear at the earliest 2– 6 weeks after the start of treatment and persist at least 3 months after treatment.
Keywords: bacterial lysate, allergic rhinitis, immunostimulation, treatment
Introduction
Allergic rhinitis (AR) is a widely prevalent condition of the upper respiratory tract. It affects about 10% to 40% of the population worldwide (10–30% in adults and up to 40% in children).1–3 The prevalence of the disease in some countries is continuously increasing, possibly due to lifestyle changes and increased urbanization. The described tendency is associated not only with AR but also with other atopic diseases, such as asthma and atopic dermatitis.4
AR is characterized by rhinorrhoea, blocked nose, sneezing and itchy nose. In most cases also ocular symptoms are observed.2,5 Described manifestations affect a patient’s quality of life by sleep disturbance, which could be a risk factor of sleep-disordered breathing, learning disabilities and changes in a child’s behaviour.6 AR can also lead to sinus infections, otitis, development or exacerbations of asthma.7
Multiple complications of the disease and a substantial impact on the patient’s quality of life make it essential to conduct effective treatment devoid of side effects. According to the current guidelines, intranasal corticosteroids, intranasal and oral H1-antihistamines, anticholinergic, antileukotriene, alpha-mimetic agents and cromones are used.8 However, the effectiveness of these medications may be limited, and most of them are burdened with side effects, which create a need to find new treatment methods.
Based on the available literature, it appears that bacterial lysates (BLs) may be one of the therapeutic options for patients with allergic diseases. BLs are antigen mixtures obtained from inactivated pathogenic respiratory bacteria. They can be divided into two groups, based on the methods that are used to prepare the mixture: polyvalent mechanical bacterial lysates (PMBLs) and polyvalent chemical bacterial lysates (PCBLs).9 BLs have been used since the 1950s to minimize the risk of recurrent respiratory tract infections in both pediatric and adult populations. Moreover, they have been proven to shorten the duration of respiratory infections and reduce the frequency of antibiotic therapy.10–12
In the past decades, different studies investigating BLs have shown also their beneficial effect in prevention and therapy of atopic dermatitis in children,13,14 seasonal and perennial AR therapy in children and adults15–20 and preschool wheezing and childhood asthma therapy.21,22
In this review, our purpose is to analyse the trial results and describe new ideas for bacterial lysate treatment in patients with AR.
Methods
Data Sources and Search
In August 2021, the authors searched biomedical databases (PubMed, Web of Science, Scopus, Cochrane Library) for articles about the usage of BLs in the treatment of AR. In all the databases, we used the following keywords: “allergic rhinitis”, “bacterial lysate” and “bacterial extract”. The search was performed by two independent persons (KJ, AE). All the relevant studies were identified by title and abstract reading. Duplicated articles were initially excluded. A careful analysis of full texts was done. Any disagreements were resolved by discussion. The search strategies are shown in Figure 1.
 |
Figure 1 The search strategies. |
Study Selection
Studies to be included in this descriptive review had to meet all of the following inclusion criteria: clinical trial (double-blind RCT or open-label RCT or sequential trial); study on humans (children or/and adults) with AR (seasonal or/and perennial – diagnosis made according to international guidelines); BL (PMBL or PCBL) as an intervention (alone or combined with conventional antiallergic medications); control group receiving only standard AR treatment or placebo or both; and the studies had to be written in English. Study size and publication type were not included as criteria. Studies were excluded if the study design was ineligible (observation studies), if they included tests performed on animals, if they included outcomes other than AR (diagnosis of other allergic diseases without AR) or evaluated the use of BLs for AR prevention.
Data Extraction
Two authors (KJ, AK) independently extracted data from the included articles. The following data were extracted from eligible studies: study design, sample size, patient characteristics, interventions, comparators, follow-up period, concomitant medications, clinical outcomes, laboratory outcomes and adverse events.
Quality Assessment
Two reviewers (KJ, AK) independently assessed the quality of enrolled studies. The Cochrane Risk of Bias Tool for Randomized Trials23 and the Risk of Bias in Non-Randomized Studies of Interventions24 were used. Bias was judged for individual elements in several domains suitable for the study design. Any discrepancies between the reviewers were resolved by the third reviewer (AE), or by consensus of all three.
Results
A total of 125 articles were screened. After the eligibility assessment, 6 studies were included in the qualitative synthesis.15–20 Table 1 presents the characteristics of the studies that met the criteria for inclusion in this systematic review (Table 1).
 |
Table 1 Characteristics of Included Studies |
Risk of Bias in Included Studies
One study20 fall to the low risk of bias, two studies15,17 to the unclear and three studies16,18,19 to the high risk of bias. The risk of bias summaries and graph are presented in Figures 2 and 3, respectively.
 |
Figure 2 Summaries of the risk of bias in the RCT (A) and non-RCT (B) studies. |
 |
Figure 3 Graph of the risk of bias in the RCT studies.15,17–20 |
Polyvalent Mechanical Bacterial Lysates
PMBLs are antigen mixtures obtained by means of mechanical disruption of bacterial cells most frequently responsible for respiratory tract infections. The most common product used in recent studies is Ismigen. It comes in the form of sublingual tablets containing 7 mg of lysate of the following bacteria: Neisseria catarrhalis, Streptococcus pneumoniae, Streptococcus pyogenes, Streptococcus viridans, Staphylococcus aureus, Haemophilus influenzae, Klebsiella pneumoniae and Klebsiella ozaenae. It is obtained by sonification, whereby ultrasound is used to inactivate the bacterial cell wall. The use of mechanical cellular lysis results in less damage to bacterial antigens and less chemical contamination compared to chemical lysis. Consequently, PMBLs may exhibit greater immunogenicity and thus clinical efficacy than PCBLs.10 In recent years, three clinical trials concerning the use of PMBL in AR were conducted.15,18,20
The first study evaluating the effect of PMBLs on the clinical course of AR was published in 2007.15 Banche et al selected 41 patients with seasonal and perennial AR (SAR, PAR) and randomly assigned them to the PMBL (26 patients) or placebo group (15 patients). The participants of the study took PMBL sublingual tablets for 10 days each month for 3 consecutive months or placebo following the same regimen as the PMBL group. The use of allergy medication was prohibited. Blood was drawn at the beginning of and after treatment to assess the effect of the intervention on changes in IgE, IL-4, and IFN-γ concentrations. AR symptom severity was assessed using a 4-point scale, where 0 meant no symptoms and 3 meant severe symptoms. In the group taking PMBL, a significant improvement in AR symptom severity was observed in 61.5% of the patients; the rest exhibited no change. Fourteen patients (53.8%) experienced a decrease in nasal congestion and rhinorrhoea, 13 (50%) decrease in ocular symptoms and 10 (38.5%) the improvement in asthma control. The described improvement was noticeable 2–3 weeks after the start of treatment at the earliest and persisted for a 3-month follow-up period. In contrast, in the placebo group only 1 patient (6.6%) experienced improvement, with the remaining patients experiencing exacerbated allergic symptoms (53.4%) or no change (40%). Laboratory testing showed a statistically significant reduction in IL-4 levels and no change in IgE or IFN-γ concentrations.
Based on these results, we can conclude that sublingual administration of PMBL provides a significant improvement of the clinical course of AR, which may be associated with the attenuation of the allergic Th2 lymphocyte response (decline of IL-4 concentration). Concurrently, this preparation has a high safety profile (no side effects have been reported).
Study limitations:
- small sample size,
- no subgroup analysis by AR type (seasonal and perennial),
- no statistical test results and their significance for assessing the effect of PMBL on changes in AR symptom severity,
- no information about the time of year when PMBL was administered and AR symptom severity was assessed (key information for SAR – symptom severity is modulated by changes in pollen grain concentrations in ambient air).
Twelve years later, Janeczek et al published the results of a randomized, open-label study concerning the use of PMBL in childhood SAR.18 The study included 38 children aged 5–17 diagnosed with SAR caused by grass pollen. Twenty patients were randomly assigned to the group receiving PMBL and 18 to the control group receiving standard AR treatment only. Throughout the study, all patients were allowed to take desloratadine and/or mometasone furoate on request. Parents gave their children PMBL sublingual tablets as described above during the grass pollen season. The severity of nasal and ocular symptoms was assessed using four-point standardized scoring scales – total nasal symptom score (TNSS) and total ocular symptom score (TOSS). Additionally, the degree of nasal duct obstruction was checked at each visit by measuring peak nasal inspiratory flow (PNIF). During the second half of the grass pollen season, there was a statistically significant decrease in TNSS (p = 0.001) and an increase in PNIF (p = 0.009) in the PMBL treatment group, while the control group had a statistically significant increase in TNSS (p = 0.009) and a statistically insignificant increase in PNIF (p = 0.724). PMBL therapy has not been shown to have any effect on allergic conjunctivitis symptoms.
Based on these results, we can conclude that adding sublingual PMBL to standard allergy therapy during the grass pollen season improves the clinical course of SAR in children. Concurrently, this preparation has a high safety profile (no side effects have been reported).
Study limitations:
- open-label study,
- no placebo comparison group,
- small sample size,
- short follow-up period after treatment.
In the study discussed above, a clinically significant reduction in TNSS was achieved in the PMBL treatment group (a reduction of 1.54, with a minimal clinically important difference of 0.55), so Janeczek et al decided to conduct a randomized, double-blind, placebo-controlled trial in the next grass pollen season to confirm the effectiveness of this therapy.20 Seventy children aged 5–17 years with SAR to grass pollen allergens were enrolled in the study and randomly assigned to the PMBL-treated (35 patients) and placebo-treated group (35 patients). The first group took PMBL tablets sublingually, according to the dosing regimen as described above, while the second group took placebo tablets indistinguishable from PMBL tablets. If applicable, patients are allowed to use desloratadine and/or mometasone furoate. The study included 3 visits, which dates were based on retrospective measurements of the concentration of grass pollen grains in the ambient air, so that they were performed before, at the peak and 3 weeks before the end of the grass pollen season. On each visit, the severity of allergic symptoms was assessed using visual analogue scale (VAS); PNIF was also measured. Moreover, patients were told to record their daily symptoms in a diary using TNSS and TOSS. To evaluate the effect of PMBL therapy on Th1/Th2 balance, nasal swabs for eosinophils and nasal lavage fluids for asIgE against timothy grass pollen were collected from children. During the grass pollen season, a statistically and clinically significant decrease in TNSS (p = 0.001) and VAS for nasal symptoms (p < 0.001) and a statistically and clinically significant increase in PNIF (p = 0.04) in the PMBL-treated group were observed. Children taking PMBL presented less severe nasal SAR symptoms compared to the control group. Described effects were noticeable 4–6 weeks after the start of the treatment. The study group also reported a decrease in TOSS (p = 0.04) and VAS for ocular symptoms (p < 0.001), but the groups compared were not found to differ in the intensity of ocular symptoms. PMBL therapy was linked with 36% less allergy medication use when compared to the placebo group. Changes in the number of eosinophils in nasal swabs dependent on the concentration of grass pollen grains in ambient air were observed in both groups. However, it was in the PMBL group that the number of eosinophils was significantly lower (p = 0.01). Moreover, in the study group, there were no changes in asIgE concentrations during the grass pollen season, while in the placebo group, these concentrations increased significantly (p = 0.03).
Based on these results, we can conclude that sublingual administration of PMBL during the grass pollen season improves the clinical course of SAR in children sensitized to grass pollen allergens which may be due to attenuation of Th2 lymphocyte response (inhibition of eosinophil count increase and asIgE levels in nasal mucosa). This therapy reduces the need for antiallergic medications and importantly, has a high safety profile (only one case of mild abdominal pain).
Study limitations:
- insufficient power of statistical test for TOSS, which may be due to small sample size,
- authors provide only indirect evidence for the effect of PMBL on the attenuation of the allergic response of Th2 cells in the nasal mucosa. No evaluation of Th2-type cytokines in nasal lavage.
Polyvalent Chemical Bacterial Lysates
PCBLs are oral, intranasal or injectable immunostimulating nonspecific vaccines obtained from bacteria that are the major aetiologic agents of respiratory tract infections. PCBLs differ significantly in the number and selection of bacteria that determine their clinical efficacy.10 One of the better known immunostimulating drugs obtained by chemical proteolysis is OM-85. It is an orally administered drug consisting of antigens from the following bacteria: Neisseria catarrhalis, Streptococcus viridans, Streptococcus pyogenes, Streptococcus pneumoniae, Staphylococcus aureus, Haemophilus influenzae, Klebsiella pneumoniae, Klebsiella ozaenae.11 Another PCBL with a different bacterial composition and route of administration is Polyvaccinum mite. This vaccine is applied intranasally and, compared to OM-85, does not contain Klebsiella ozaenae and Streptococcus viridans antigens. The conducted literature search resulted in three studies concerning the use of PCBLs in AR.16,17,19
Between 2008 and 2010, Koatz et al conducted an open-label, sequential study evaluating the effect of PCBL therapy on respiratory infection rates, primary disease exacerbation rates, and symptom severity in patients with PAR, asthma, and chronic obstructive pulmonary disease.16 Twenty-nine patients with PAR and three or more respiratory infections in the year prior to the study were included. In 2009, patients used only standard optimized care, and in 2010, patients additionally used oral PCBL for 10 days per month for 3 consecutive months and were followed up for 6 months. Patients rated the severity of allergic symptoms based on a four-point scale, as described previously. PAR exacerbations were confirmed by a physician. Additionally, blood and saliva were collected from patients for measurement of IgA level. The researchers showed that PCBL therapy reduced the number of respiratory infections and the number of PAR exacerbations by 33% and 46% (p < 0.05), respectively, and the severity of allergic symptoms by 48% (for nasal congestion) to 65% (for nasal secretion) compared to the previous year when patients received only standard optimized care. Bacterial lysate immunostimulation resulted in statistically significant increases in serum (200 mg% vs 296 mg%, p < 0.001) and salivary secretory IgA (25 mg% vs 125 mg%, p < 0.001).
Based on these results, we can conclude that PCBL reduces the number of respiratory infections and thus improves the clinical course of PAR in patients with recurrent respiratory tract infections.
Study limitations:
- open-label study,
- small sample size – a smaller number of patients than assumed were included in the study, consistent with the sample size calculation,
- lack of demographic data on PAR patients,
- no information on additional medications allowed during the study,
- different methodology compared to the other studies discussed.
In mid-2019, Meng et al published the results of a study in which, in addition to clinically evaluating the efficacy of oral PCBL in AR therapy, they also examined its effects on selected immunological parameters.17 The study included 60 adult patients with moderate-to-severe PAR, 30 of whom were randomly assigned to the PCBL treatment group and another 30 to the placebo group. Loratadine was an approved medication to relieve allergic symptoms. At the beginning of the study, immediately after the third cycle of PCBL, and 4 and 8 weeks after the end of treatment, each of the four AR symptoms was assessed individually on a scale of 0 to 3 (individual nasal symptom score), and the individual scores were summed to obtain the TNSS. At the same measurement points, the levels of IL-4, IL-13 and IFN-γ in nasal lavage fluid and the number of eosinophils in nasal smears were assessed. Researchers showed that PCBL therapy reduced TNSS by 28.33%, itching score by 23.72%, nasal rhinorrhoea score by 18.59% and sneezing score by 23.08% compared to the placebo group (all p < 0.05). There were no statistically significant changes in nasal congestion score. Furthermore, the described improvement was maintained for the next 8 weeks after the end of treatment. Laboratory tests showed a statistically significant decrease in IL-4 and IL-13 (by 31.69% and 20.45%, respectively, for both p < 0.05) and increase in IFN-γ (by 72.02%, p < 0.01) in the PCBL group versus placebo group. Eosinophil counts in nasal swabs collected from patients taking PCBL were reduced, with an average decrease of 23% (p < 0.05). The inclusion of PCBL was associated with a 37.18% reduction in loratadine requirement compared to the placebo group (p < 0.05).
Based on these results, we can conclude that orally administered PCBL may be an alternative therapeutic strategy for adults with PAR. This therapy reduces the severity of nasal symptoms, the need for antiallergic medications and importantly, has a high safety profile (three cases of mild abdominal pain). The observed improvement is probably due to the restoration and maintenance of the Th1/Th2 cytokine balance.
Study limitations:
- small sample size,
- no assessment of AR symptom severity during the time patients were taking PCBL, and consequently inability to determine when the first effects of therapy were noticeable.
The databases search provided only one study that evaluated the effect of intranasally applied PCBL on the clinical course of SAR in children.19 For first 6 weeks of the grass pollen season, 18 patients took intranasal PCBL in addition to standard AR therapy, while the remaining 22 took placebo. Nasal and ocular symptom severity was assessed at the beginning of the study and at the end of therapy using standard scoring scales (TNSS, TOSS, VAS) and objective methods (PNIF measurement). The researchers showed a statistically significant increase in PNIF (p = 0.01) and decrease in VAS for nasal symptoms (p = 0.03) in the PCBL group compared to the placebo group. Changes in TNSS, TOSS, and VAS for ocular symptoms did not reach the level of statistical significance.
Based on these results, we can conclude that intranasal PCBL added to standard antiallergy treatment during the grass pollen season does not improve the process of SAR in children, but improves PNIF and VAS.
Study limitations:
- preliminary study,
- small sample size,
- difference in group size,
- no follow-up period after completion of PCBL therapy.
Discussion
To begin the discussion, we would like to mention the meta-analysis of clinical trials evaluating the efficacy of BLs in the treatment of allergic diseases, including AR, which was published online in July 2021.25 Based on an analysis of three studies,15,26,27 the authors demonstrated that improvement of AR symptoms was 3 times higher in the BL group compared with controls (RR 2.64, 95% CI: 1.88–3.72). However, two studies26,27 included children with chronic rhinosinusitis who did not have an allergy diagnosis and lacked information about previously detected allergies. Consequently, these studies should not be included in the meta-analysis, because they do not meet the inclusion criteria adopted by the authors (criterion 2: “children and adults receiving a diagnosis of any type of allergic disease (asthma, dermatitis, allergic rhinitis)”).
Although in recent years there has been an increase in the frequency of clinical trials using BLs, the data regarding the treatment of AR remains limited. Nevertheless, this review enable us to draw some conclusions.
BLs offer significant efficacy in alleviating symptoms of SAR and PAR, both in children and adults.15–20 The addition of BL to standard AR therapy results in a reduction in the severity of nasal symptoms and the need for antiallergic medications.17,18,20 Four of the studies discussed above demonstrated a high safety profile for BLs.15,17,18,20 The most common adverse event was abdominal pain, which occurred with similar frequency to the placebo group.17,20 Although one study19 demonstrated a small effect of BLs on improving the clinical course of AR, given the high safety of these agents, their addition to standard therapy should be considered.
The authors of the discussed studies agree that the first effects of BL treatment are noticeable with some delay, most often 2–6 weeks after the start of therapy.15,18,20 Based on this observation, a practical recommendation can be made for patients allergic to seasonal allergens. Patients with SAR should begin BL therapy at least 2–6 weeks before expected exposure to the allergen. This can provide better disease control from the beginning of the pollen season.
The summarized studies differ in the length of the observation period (0–12 weeks). The longest one concerns the study by Banche et al15 – 3 months (the study by Koatz et al was excluded from consideration16 due to differences in methodology). Italian researchers have shown that the beneficial effect of PMBL persists for at least 3 months after treatment. Based on the available scientific data, it is not clear whether repeated immunostimulation with BL is necessary for patients with PAR to maintain good disease control throughout the year.
As mentioned earlier, BLs are divided into mechanical and chemical by the method of preparation. The former has been shown to be more effective in patients with recurrent respiratory infections.10 This is also confirmed by a study conducted by La Mantia et al on a group of 120 children with otitis media or nasopharyngitis, in which the use of PMBL was associated with greater protective efficacy compared to PCBL.28 This could be because PMBLs contain bacterial antigens with less damage and fewer chemical contaminants compared to PCBLs, which results in their greater immunogenicity. Studies included in this systematic review have demonstrated high efficacy of both PMBLs and PCBLs in the treatment of AR.15–18,20 The route of administration may be another factor affecting the effectiveness of BLs in allergic diseases. There are oral, sublingual, intranasal, and subcutaneous/intramuscular preparations. Dendritic cells appear to be an important link in the mechanism of action of immunostimulants in AR. The density of distribution of these cells is highest in the upper respiratory tract and oral cavity.29,30 Therefore, it is speculated that BLs administered sublingually may have a greater effect on reducing allergic symptoms than oral preparations.15,20 However, at present, it is not possible to clearly determine which type of BLs is more effective in the treatment of AR, as there are no studies comparing BLs with different modes of receipt and routes of administration in this disease.
The function of dendritic cells is to introduce allergens to Th0 lymphocytes in lymphoid tissue. Through this process, Th0 lymphocytes differentiate into Th2 cells, which initiate the inflammatory process and release numerous cytokines, including IL-3, IL-4, IL-5, IL-9, IL-13, GM-CSF. IL-4 and IL-13 are responsible for stimulating B lymphocytes to produce asIgE, while IL-3 and IL-5 are responsible for the influx of eosinophils into nasal tissues.31 In patients with AR, we find an imbalance of the immune system that involves a dominance of Th2 cell responses over Th1 cell responses. The studies discussed above demonstrate that BLs therapy increases IgA, Th1-type cytokines (IFN-γ) and reduces Th2-type cytokines (IL-4, IL-13).15–17,20 The researchers note that these changes occur mainly locally, in the nasal mucosa.17,20 The immune effects of BLs described above are likely due to their ability to interact with immune cells via Toll-like receptors.32 Upon analysing the studies included in this systematic review, it can be concluded that BLs affect mucosal immunity, weakening the response of Th2 cells and thus restoring Th1/Th2 balance.15,17,20 The mechanism of action of BLs in the treatment of allergic diseases has been further discussed by the authors of this review in another article.20 Figure 4 shows the possible pathways of PMBL in AR (Figure 4).
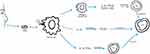 |
Figure 4 Possible pathways of PMBL action in AR.15–17,20,31,32 Abbreviations: CTLA-4, cytotoxic T lymphocyte-associated antigen-4; ICOS-L, inducible costimulator ligand; IDO, indoleamine 2,3-dioxygenase; IFN-γ, interferon gamma; IL, interleukin; PMBL, polyvalent mechanical bacterial lysate; TGF-β, transforming growth factor beta; Th1, T-helper type-1; Th2, T-helper type-2; TLR, toll-like receptors; Treg, regulatory T cell. |
There are also attempts to use BLs in the prevention of allergic diseases, including AR. One was undertaken by Roßberg et al who conducted a randomized, placebo-controlled study to evaluate the effect of BLs administered in infancy on the risk of developing atopic dermatitis, AR, and asthma.33 In total, 606 infants with a family history of allergic disease were enrolled in the study, half of them receiving oral BL three times daily from 5 weeks to 7 months of age. The patients were then observed until reaching the school age of 6–11 years. The use of an immunostimulant did not reduce the risk of developing an allergic disease, for instance AR was diagnosed in 35% of the patients in the observation group and in 38% of the patients in the placebo group.
Articles from different years and available in different databases have been reviewed. A limitation of our review is that we have only included articles in English.
Finally, there is an urgent need for high-quality and large sample size studies on the clinical efficacy and mechanisms of action of BLs with different bacterial antigen composition, method of preparation and route of administration. We hope that the benefits of adding BLs to standard AR therapy as well as the high safety thereof will be reflected in the recommendations of international scientific societies.
Conclusion
This systematic review showed that adding BL to standard therapy for seasonal or perennial AR reduces the severity of nasal symptoms and the need for oral H1-antihistamines and intranasal corticosteroids in both children and adults, as well as demonstrated the high safety profile of these formulations. A practical guideline for clinicians is that BLs for AR therapy should be used according to their dosing regimen for recurrent respiratory infections and that, for patients with seasonal AR, therapy should be initiated 2–6 weeks before the expected pollen season.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas. All authors took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors have no competing interests to declare that are relevant to the content of this article. No funding was received to assist with the preparation of this manuscript.
References
1. Meirlaen L, Levy EI, Vandenplas Y. Prevention and management with pro-, pre and synbiotics in children with asthma and allergic rhinitis: a narrative review. Nutrients. 2021;13(3):934. doi:10.3390/nu13030934
2. Asher MI, Montefort S, Björkstén B, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368(9537):733–743. doi:10.1016/S0140-6736(06)69283-0
3. Cingi C, Bayar Muluk N, Scadding GK. Will every child have allergic rhinitis soon? Int J Pediatr Otorhinolaryngol. 2019;118:53–58. doi:10.1016/j.ijporl.2018.12.019
4. Hallit S, Raherison C, Malaeb D, Hallit R, Kheir N, Salameh P. The AAA risk factors scale: a new model to screen for the risk of asthma, allergic rhinitis and atopic dermatitis in children. Med Princ Pract. 2018;27(5):472–480. doi:10.1159/000490704
5. Small P, Keith PK, Kim H. Allergic rhinitis. Allergy Asthma Clin Immunol. 2018;14(Suppl 2):51. doi:10.1186/s13223-018-0280-7
6. Mir E, Panjabi C, Shah A. Impact of allergic rhinitis in school going children. Asia Pac Allergy. 2012;2(2):93–100. doi:10.5415/apallergy.2012.2.2.93
7. Sih T, Mion O. Allergic rhinitis in the child and associated comorbidities. Pediatr Allergy Immunol. 2010;21(1 Pt 2):e107–e113. doi:10.1111/j.1399-3038.2009.00933.x
8. Bousquet J, Schünemann HJ, Togias A, et al. Next-generation Allergic Rhinitis and Its Impact on Asthma (ARIA) guidelines for allergic rhinitis based on Grading of Recommendations Assessment, Development and Evaluation (GRADE) and real-world evidence. J Allergy Clin Immunol. 2020;145(1):70–80. doi:10.1016/j.jaci.2019.06.049
9. De Benedetto F, Sevieri G. Prevention of respiratory tract infections with bacterial lysate OM-85 bronchomunal in children and adults: a state of the art. Multidiscip Respir Med. 2013;8(1):33. doi:10.1186/2049-6958-8-33
10. Cazzola M, Anapurapu S, Page CP. Polyvalent mechanical bacterial lysate for the prevention of recurrent respiratory infections: a meta-analysis. Pulm Pharmacol Ther. 2012;25(1):62–68. doi:10.1016/j.pupt.2011.11.002
11. Yin J, Xu B, Zeng X, Shen K. Broncho-Vaxom in pediatric recurrent respiratory tract infections: a systematic review and meta-analysis. Int Immunopharmacol. 2018;54:198–209. doi:10.1016/j.intimp.2017.10.032
12. Cao C, Wang J, Li Y, et al. Efficacy and safety of OM-85 in paediatric recurrent respiratory tract infections which could have a possible protective effect on COVID-19 pandemic: a meta-analysis. Int J Clin Pract. 2021;75(5):e13981. doi:10.1111/ijcp.13981
13. Lau S, Gerhold K, Zimmermann K, et al. Oral application of bacterial lysate in infancy decreases the risk of atopic dermatitis in children with 1 atopic parent in a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2012;129(4):1040–1047. doi:10.1016/j.jaci.2012.02.005
14. Bodemer C, Guillet G, Cambazard F, et al. Adjuvant treatment with the bacterial lysate (OM-85) improves management of atopic dermatitis: a randomized study. PLoS One. 2017;12(3):e0161555. doi:10.1371/journal.pone.0161555
15. Banche G, Allizond V, Mandras N, et al. Improvement of clinical response in allergic rhinitis patients treated with an oral immunostimulating bacterial lysate: in vivo immunological effects. Int J Immunopathol Pharmacol. 2007;20(1):129–138. doi:10.1177/039463200702000115
16. Koatz AM, Coe NA, Cicerán A, Alter AJ. Clinical and immunological benefits of OM-85 bacterial lysate in patients with allergic rhinitis, asthma, and COPD and recurrent respiratory infections. Lung. 2016;194(4):687–697. doi:10.1007/s00408-016-9880-5
17. Meng Q, Li P, Li Y, et al. Broncho-vaxom alleviates persistent allergic rhinitis in patients by improving Th1/Th2 cytokine balance of nasal mucosa. Rhinology. 2019;57(6):451–459. doi:10.4193/Rhin19.161
18. Janeczek KP, Emeryk A, Rapiejko P. Effect of polyvalent bacterial lysate on the clinical course of pollen allergic rhinitis in children. Postepy Dermatol Alergol. 2019;36(4):504–505. doi:10.5114/ada.2019.87457
19. Kowalska M, Emeryk A, Janeczek K, Czrwińska-Pawluk I. Effect of nasal polivalent bacterial lysate on the clinical course of seasonal allergic rhinitis in children - preliminary study. Eur Respir J. 2020;56(Suppl 64):1208.
20. Janeczek K, Emeryk A, Rachel M, Duma D, Zimmer Ł, Poleszak E. Polyvalent mechanical bacterial lysate administration improves the clinical course of grass pollen-induced allergic rhinitis in children: a randomized controlled trial. J Allergy Clin Immunol Pract. 2021;9(1):453–462. doi:10.1016/j.jaip.2020.08.025
21. Emeryk A, Bartkowiak-Emeryk M, Raus Z, Braido F, Ferlazzo G, Melioli G. Mechanical bacterial lysate administration prevents exacerbation in allergic asthmatic children-The EOLIA study. Pediatr Allergy Immunol. 2018;29(4):394–401. doi:10.1111/pai.12894
22. de Boer GM, Żółkiewicz J, Strzelec KP, et al. Bacterial lysate therapy for the prevention of wheezing episodes and asthma exacerbations: a systematic review and meta-analysis. Eur Respir Rev. 2020;29(158):190175. doi:10.1183/16000617.0175-2019
23. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi:10.1136/bmj.l4898
24. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi:10.1136/bmj.i4919
25. Li C, Zhou H, Zhang W, Che D, Peters R. Bacterial lysate treatment in allergic disease: a systematic review and meta-analysis. Pediatr Allergy Immunol. 2021;32(8):1813–1823. doi:10.1111/pai.13572
26. Chen J, Zhou Y, Nie J, et al. Bacterial lysate for the prevention of chronic rhinosinusitis recurrence in children. J Laryngol Otol. 2017;131(6):523–528. doi:10.1017/S0022215117000524
27. Zagar S, Löfler-Badzek D. Broncho-Vaxom in children with rhinosinusitis: a double-blind clinical trial. ORL J Otorhinolaryngol Relat Spec. 1988;50(6):397–404. doi:10.1159/000276020
28. La Mantia I, Nicolosi F, Maiolino L, Serra A. Immunoprophylaxis of recurring bacterial infections of respiratory tracts in pediatric age: clinical experience through a new immune stimulating vaccine. GIMMOC. 2007;9:1–8.
29. Holt PG. Regulation of antigen-presenting cell function(s) in lung and airway tissues. Eur Respir J. 1993;6(1):120–129.
30. Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106(3):255–258. doi:10.1016/S0092-8674(01)00449-4
31. Rosenwasser LJ. Current understanding of the pathophysiology of allergic rhinitis. Immunol Allergy Clin North Am. 2011;31(3):433–439. doi:10.1016/j.iac.2011.05.009
32. Kearney SC, Dziekiewicz M, Feleszko W. Immunoregulatory and immunostimulatory responses of bacterial lysates in respiratory infections and asthma. Ann Allergy Asthma Immunol. 2015;114(5):364–369. doi:10.1016/j.anai.2015.02.008
33. Roßberg S, Keller T, Icke K, et al. Orally applied bacterial lysate in infants at risk for atopy does not prevent atopic dermatitis, allergic rhinitis, asthma or allergic sensitization at school age: follow-up of a randomized trial. Allergy. 2020;75(8):2020–2025. doi:10.1111/all.14247
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
