Back to Journals » OncoTargets and Therapy » Volume 8
Personalized treatment strategies for non-small-cell lung cancer in Chinese patients: the role of crizotinib
Received 30 January 2015
Accepted for publication 26 February 2015
Published 2 May 2015 Volume 2015:8 Pages 999—1007
DOI https://doi.org/10.2147/OTT.S64664
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Daniele Santini
Fei-Yu Niu,1,2 Yi-Long Wu2
1Graduate School, Southern Medical University, Guangzhou, People’s Republic of China; 2Guangdong Lung Cancer Institute, Guangdong General Hospital, Guangdong Academy of Medical Sciences, Guangzhou, People’s Republic of China
Abstract: Anaplastic lymphoma kinase (ALK) rearrangement is an oncogene targeted with approved drugs second to epidermal growth factor receptor (EGFR) in lung cancer. Crizotinib was developed and introduced into clinical practice rapidly and successfully after the discovery of ALK rearrangement in non-small-cell lung cancer. Chinese and other Asian patients treated with crizotinib seem to have lower toxicity and higher efficacy compared with other ethnicities. Crizotinib showed potent antitumor activity and manageable toxicity in mesenchymal–epithelial transition factor (c-Met)/ROS1-positive non-small-cell lung cancer patients, but prospective clinical trials are still needed to confirm its efficacy and safety. Crizotinib appears to be effective against tumors originating from various organs that harbor ALK abnormalities. In the near future, we would classify the tumors by their genetic information beyond organs, such as ALKoma, EGFRoma, and RAFoma, and a single compound could be used for many different types of cancer in different organs. The major challenge of the widespread use of crizotinib in clinical practice is establishing convenient diagnostic techniques for the detection of ALK/c-Met/ROS1. In the present study, we reviewed the application of crizotinib in Chinese patients.
Keywords: NSCLC, crizotinib, ALK, c-Met, ROS1
Introduction
Since the discovery of the epidermal growth factor receptor (EGFR) mutation in 2004, personalized treatment based on genomic variations has significantly changed lung cancer clinical practice in the past 10 years. EGFR tyrosine kinase inhibitors (TKIs) such as gefitinib and erlotinib have opened the gate of precise medicine and become a standard therapy for patients with non-small-cell lung cancer (NSCLC) harboring the EGFR mutation. EGFR inhibitors came first and then target driver gene was discovered. Conversely, the anaplastic lymphoma kinase (ALK) rearrangement in NSCLC was discovered prior to the development of an effective inhibitor. Thus, the development of the ALK inhibitor crizotinib became a typical model in personalized lung cancer treatment. The first clinical trial of crizotinib, PROFILE 1001,1 was conducted for unselected patients with solid tumors in 2007. During the same year, ALK rearrangement was also first reported and a diagnostic fluorescence in situ hybridization (FISH) assay was developed for NSCLC. Fortunately, two NSCLC ALK-positive patients were enrolled in this Phase I trial and experienced a significant disease control. Subsequently, crizotinib was widely used in ALK-positive lung cancer patients. Only 4 years later in 2011, crizotinib was approved by the US Food and Drug Administration, a relatively short period from laboratory to market.
Crizotinib was approved by the Chinese Food and Drug Administration for ALK-positive patients in any line setting in 2013. It was only based on results of PROFILE 1001, 1005,2 and 1007,3 when the mature results of PROFILE 10144 and 10295 were not available. The crizotinib was not covered by the Chinese health insurance system, and most patients could not tolerate the financial burden. The charitable project of crizotinib in People’s Republic of China was started in April 2014. Chinese mainland citizens over 18 years of age with low income could apply for free crizotinib if they paid for the drug during the initial 4 months of treatment. The reduced economical burden greatly enhances patient adherence. To date, more than 3,000 Chinese ALK-positive patients have taken crizotinib in this charitable project (internal data provided by Pfizer). This review focused on the role of crizotinib in personalized treatment in People’s Republic of China.
Pharmacology, mode of action, and pharmacokinetics of crizotinib
Crizotinib (PF-02341066) is a potent and selective small-molecule inhibitor of MET kinase,6 ALK,7,8 and ROS.9 Crizotinib competes with ATP for binding to the catalytic pocket, which inhibits the receptor tyrosine kinase downstream signaling pathways that are critical for growth and survival.
The pharmacokinetics of crizotinib were assessed in 15 Chinese patients (seven males and eight females) with advanced ALK-positive NSCLC who were enrolled in PROFILE 1005.10 The median time for crizotinib to achieve peak concentration was 4–6 hours after absorption, which was similar to the first 80 patients of various ethnicities enrolled in PROFILE 1001.1 The mean Cmax of crizotinib was 117 ng/mL and AUCinf was 2,711 ng h/mL. Following attainment of Cmax, plasma concentrations of crizotinib declined in a multi-exponential manner with an average half-life terminal elimination of approximately 39 hours, which was slightly shorter than that in the 80 patients mentioned earlier, whose average terminal half-life was 43–51 hours. The plasma concentrations of crizotinib reached a steady state within 15 days and increased with a median accumulation ratio of 5.2 days. The geometric mean values for the apparent clearance were 57.7 L/h and 59.7 L/h, following 15 and 29 days of dosing, respectively, which were lower than those observed after a single dose (92.3 L/h), indicating that crizotinib exhibited nonlinear pharmacokinetics due to autoinhibition of CYP3A4.
Compared with non-Asian patients, Asian patients had higher crizotinib exposure. A comparison study evaluated crizotinib pharmacokinetics in 95 patients, including 32 Asian and 63 non-Asian patients, with advanced malignancies.11 The mean AUCtau-ss and Cmax-ss were, respectively, 56% and 70% higher in Asian than in non-Asian patients (body weight adjustment accounted for 30% of the difference) after repeated crizotinib, with median trough concentrations 41%–59% (median 50%) higher. An analysis of 167 patients from PROFILE 1001 included 42 Asian and 125 non-Asian patients and showed that mean values for crizotinib Cmax and AUC in Asian patients were 1.57-fold (90% confidence interval, 1.16–2.13) and 1.50-fold (90% confidence interval, 1.10–2.04) those seen in non-Asian patients, respectively.12 Another analysis included 8,973 pharmacokinetic samples from 1,214 patients treated with crizotinib in Phase I, II, and III trials; 43.1% were Asian patients.13 The AUCss in Asian patients were over 25% higher than typical AUCss values in non-Asian patients.
Diagnosis of ALK rearrangements in People’s Republic of China
The prevalence of ALK rearrangements is 3.3%–11.6% in Chinese patients14–16 similar to other Asian patients,17,18 slightly greater than that in non-Asian patients.18,19 and much lower than EGFR mutations – about 30% in Chinese patients.16 Currently, three major methods are used for the detection of ALK rearrangements in lung cancer; ie, break-apart FISH, Ventana immunohistochemistry (IHC), and polymerase chain reaction (PCR). All three methods were recommended by Chinese expert consensus opinion20 and approved as companion diagnostic tests by the Chinese Food and Drug Administration, which is quite different from other guidelines developed in the US and EU.
FISH is the standard method for the detection of ALK rearrangements in lung cancer globally. However, the application of FISH was restricted mainly by its cost-effectiveness and the lack of experienced pathologists in People’s Republic of China.
IHC is much more rapid and affordable. Ventana IHC was developed by Roche for detecting ALK rearrangements. The sensitivity and specificity of Ventana IHC are 100% and 99%, respectively,21–26 as shown in Table 1; thus, Chinese experts recommend the Ventana IHC as a diagnostic test for ALK rearrangements. This test has also been approved in Europe but still under consideration of National Comprehensive Cancer Network/College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology guidelines. A prospective observational trial of diagnosis of ALK rearrangements in unselected NSCLC patients using Ventana IHC in People’s Republic of China was launched in 2013.27 The primary objective is to obtain epidemiological data in unselected Chinese patients with ALK-positive NSCLC. The estimated enrollment is 10,000, and 3,000 patients have been enrolled to date.
The reverse transcription (RT)-PCR method is the least subjective methodology for the detection of ALK rearrangements.28 However, it requires high-quality RNA or is unable to detect the unknown fusion partners.15,23,29–31 RT-PCR is also recommended by the Japan Lung Cancer Society but not by the National Comprehensive Cancer Network or College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology guidelines. The three types of PCR recommended by Chinese expert consensus opinion include quantitative RT-PCR, rapid amplification of cDNA ends-coupled PCR sequencing, and specific primer-based RT-PCR coupling direct sequencing.20
The three ALK detection methods have advantages and disadvantages, as mentioned earlier. However, both Ventana IHC and RT-PCR have high sensitivity and specificity compared with FISH (Table 1). The methods used by clinicians in People’s Republic of China to detect ALK arrangements differ according to the equipment available. The ALK test procedures used in People’s Republic of China are shown in Figure 1.
Efficacy of crizotinib in ALK-positive patients
The efficacy and safety of crizotinib were confirmed through a series of clinical trials named PROFILE (Table 2). PROFILE 1001 is a Phase I dose-escalation trial that was the first to find a target population with ALK-positive and embedded biomarker in patient’s selection in the early stage of drug development. PROFILE 1005 is a Phase II trial that selected patients with ALK rearrangements to evaluate the efficacy and safety of crizotinib. Based on the results of these two trials, ALK rearrangement was defined as a definitive target biomarker. Subsequent Phase III trials, either second line (PROFILE 1007) or first line (PROFILE 1014 and PROFILE 1029), were based on biomarker selection. The results are shown in Table 3. All PROFILE trials showed good consistency; higher objective response rate (ORR), longer progression-free survival (PFS), overall survival, and better safety compared to chemotherapy; these are the characteristics of precise cancer treatment.
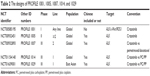  | Table 2 The designs of PROFILE 1001, 1005, 1007, 1014, and 1029 |
  | Table 3 The efficacy of crizotinib in Asian and non-Asian populations |
Asian patients accounted for 28%–46% of the overall population in the PROFILE series (Table 3).2–4,32 Asian patients had higher response rates than non-Asian patients, possibly related to the higher crizotinib exposure. Twenty-three Chinese patients were enrolled in PROFILE 1005 and 1007. The ORR was 73.9%,33 similar to that in Asian patients but higher than in non-Asian patients.
Although ORR was higher in Asian patients, the PFS was similar among ethnicities, as shown in Table 3. The median PFS of the 23 Chinese patients enrolled in PROFILE 1005 and 1007 was 7.0 months.32 Several retrospective analyses of Chinese patients reported median PFS of 6.0–7.6 months.34
Safety and tolerability of crizotinib
The normal ALK function in adult humans is unknown, but it is involved in gut development35 and retinal axon targeting36 in Drosophila. Therefore, the predominant adverse events (AEs) of crizotinib were visual effects and gastrointestinal events, which may represent on-target anti-ALK effects. The AEs of 1,255 patients taking crizotinib in three trials (PROFILE 1001, 1005, and 1007) were analyzed.37 The most common gastrointestinal events were nausea (49%), diarrhea (44%), vomiting (41%), and constipation (29%). The incidence of visual impairment was 42%. Most AEs mentioned earlier were grade 1–2 in severity, which was similar to other reports and our clinical experience in Chinese patients.34,38–40 The most common treatment-related grade 3/4 AEs were neutropenia (7%) and elevated alanine aminotransferase (ALT) (6%) and aspartate aminotransferase (AST) (<3%) levels. Among the AE grades, neutropenia and elevated ALT and AST levels occurred in 11%, 21%, and 15% patients, respectively.37,38,41
The incidence of AEs based on ethnicity differs slightly. The incidence of AEs was higher in non-Asian patients compared with Asian patients in the subgroup analysis of 901 patients enrolled in PROFILE 1005.42 An analysis of 95 patients (32 non-Asian and 63 Asian patients) with advanced malignancies taking crizotinib showed that Asian patients had a higher incidence of low-grade AEs (eg, gastrointestinal, visual impairment) but a lower incidence of high-grade AEs (eg, increased ALT).11 The treatment-related grade 3 and/or 4 AEs occurred in 2.4% (9/379) and 6.7% (35/522) and grade 5 serious AEs in 1.1% [4/379] and 1.3% [7/522] of Asian and non-Asian patients, respectively. Due to AEs, 12% of Asian patients and 18% of non-Asian patients discontinued their treatment. In another pooled analysis of 1,053 patients from PROFILE 1005 and 1007, Asian patients were less susceptible to sinus bradycardia compared with non-Asian patients (P=0.039),43 indicating that crizotinib was slightly safer in Asian patients.
Overcoming resistance to crizotinib
Nearly all patients develop resistance to crizotinib. The mechanism of resistance includes secondary mutations in the tyrosine kinase domain of ALK, ALK copy number gain, the aberrant activation of other driver genes, and several unknown mechanisms.44,45
The second-generation ALK TKIs, such as ceritinib (LDK378),46 alectinib (RO5424802),47 and AP26113,48 showed strong capability in patients resistant to crizotinib. Ceritinib was approved by the US Food and Drug Administration in early 2014 and alectinib has been approved by the Japanese government.
In addition to novel drugs, National Comprehensive Cancer Network guidelines recommended new strategies for patients resistant to crizotinib according to response evaluation criteria in solid tumors criteria. Patients with asymptomatic progression can continue taking crizotinib. The patients with symptomatic progression in the brain or an isolated extracranial lesion can receive local treatment and continue taking crizotinib. Patients with symptomatic progression in multiple lesions can change to chemotherapy. An analysis of 194 patients from PROFILE 1005 and 1007 was conducted; 120 (62%) patients continued taking crizotinib beyond disease progression because the investigators believed that ongoing clinical benefits could be obtained. The patients who continued taking crizotinib had significantly longer overall survival from the time of progressive disease (median 16.4 months vs 3.9 months; hazard ratio, 0.27; P<0.0001).49
Basket trial: crizotinib in different tumors
An increasing number of driver genes are being discovered in solid tumors, including NSCLCs. Based on different driver genes, NSCLC was subdivided in different rare diseases. Innovative clinical trials have been performed, including basket trials. The basket trial is defined as testing of one drug against the same genetic abnormality in different organs. The ALK abnormality has been discovered in many solid tumors, including anaplastic large-cell lymphoma,50 inflammatory myofibroblastic tumor,51 diffuse large B-cell lymphoma,52 esophageal squamous cell carcinoma,53 and several other tumors.54,55 Tumors originating from various organs carrying abnormal ALK as an essential growth driver were defined as “ALKoma”.56 Crizotinib appears to be effective against tumors originating from various organs that harbor ALK abnormalities.57,58 There is an ongoing basket clinical trial (A8081013, ClinicalTrials.gov identifier NCT01121588) evaluating the safety and clinical activity of crizotinib in patients with ALK-positive malignancies other than NSCLC. There is no basket trial of crizotinib in People’s Republic of China so far. In future, tumors could be classified based on genetic information beyond organs, such as EGFRoma and RAFoma and a single compound could be used for many cancer types in different organs. Similar to ALKoma cancers, EGFRoma and RAFoma cancers could be treated using EGFR TKIs and RAF TKIs.
Umbrella trail: crizotinib in lung cancers with different genetic abnormalities
The umbrella trial is another innovative clinical trial in which several drugs are tested against multiple genetic abnormalities within one type of tumor, such as lung cancer. Crizotinib showed antitumor activities in lung cancer patients with c-Met or ROS1 abnormalities and is particularly suitable for an umbrella trial.
Crizotinib in patients with ROS1 rearrangements
ROS1 rearrangement was discovered in NSCLC in 2007.59 The researchers detected the activation of oncogenic kinases in 41 NSCLC cell lines and over 150 Chinese NSCLC patients using a phosphoproteomic approach. ROS was identified to have high tyrosine kinase phosphorylation in the HCC78 cell line and one patient tumor sample.
The frequency of ROS1 was approximately 1.0%–2.0% in Chinese patients,60,61 similar to other ethnicities.62,63 However, the frequency of ROS1 rearrangements in the triple-negative (without EGFR/KRAS mutations or ALK rearrangements) population was 8.2%, higher than that in the nonselected population.64
Shaw et al65 reported the results of 50 patients with advanced NSCLC who tested positive for ROS1 rearrangement in an expansion cohort of the PROFILE 1001. The AEs of crizotinib were similar to those in NSCLC patients with ALK rearrangements. The efficacy of crizotinib was better in patients with ROS1 rearrangements than with ALK rearrangements. The ORR was 72%, including three complete responses. The median PFS was 19.2 months. One ROS1 trial in Asian patients is ongoing (ClinicalTrials.gov identifier: NCT01945021).
Crizotinib in patients with de novo c-Met abnormalities
Preliminary results on the safety and efficacy of crizotinib in NSCLC patients with c-Met amplification were reported in the 2014 American Society of Clinical Oncology, which was part of the ongoing Phase I trial (A8081001, ClinicalTrials.gov identifier: NCT00585195).40 The c-Met amplification status was divided into the following four categories: negative (MET/CEP7 ratio ≤1.8), low (MET/CEP7 ratio ≥1.8 to ≤2.2), intermediate (>2.2 to <5) and high (≥5). CEP7 refers to the chromosome 7 centromere. At data cutoff, 13 patients with amplified c-Met were treated with crizotinib. The median duration of response was 35 weeks (95% confidence interval, 16–112). The AEs were similar to crizotinib in ALK-positive patients; most were grade 1 in severity. The antitumor activity and general tolerability of crizotinib were first indicated in a prospective clinical trial.
De novo c-Met expression was detected using IHC in NSCLC patients in Guangdong Lung Cancer Institute in People’s Republic of China.66,67 c-Met overexpression was defined as more than 50% tumor cells with moderate- to high-intensity staining. Thirteen patients with c-Met overexpression treated with crizotinib were evaluable for response; six experienced partial response (PR), two stable disease, and five progressive disease. One patient died of interstitial lung disease attributed to crizotinib, suggesting that c-Met overexpression could be a predictive factor of crizotinib.
De novo c-Met overexpressions/amplifications coexisting with EGFR mutations, KRAS mutations, and ALK rearrangements were analyzed in Guangdong Lung Cancer Institute in People’s Republic of China. The definition of overexpression was as mentioned earlier and amplification was detected using FISH.68 Seven patients with concomitant de novo c-Met overexpression and EGFR mutations received first-line EGFR TKIs. The ORR was 71.4% (5/7). One patient with concomitant de novo c-Met overexpression and EGFR mutations developed intrinsic resistance to first-line crizotinib. Five patients with concomitant de novo c-Met overexpressions and ALK rearrangements received crizotinib, and the response rate was 80%. The patient with concomitant de novo c-Met overexpression and KRAS mutation was resistant to crizotinib. Based on these results, NSCLC patients with concomitant de novo c-Met overexpression and other driver genes can have diverse responses to TKIs, which may depend on the predominant pathway in the tumor.69,70
Crizotinib in patients with acquired c-Met abnormalities
c-Met amplification was found in 13%–33% of patients with acquired resistance to EGFR TKIs71,72 and is the second most common EGFR TKIs resistance mechanism, following the T790M mutation. The sensitivity to EGFR TKIs was restored by the inhibition of c-Met signaling in a gefitinib-resistant HCC827 cell line.73
Patients with acquired resistance to EGFR TKIs were enrolled in a Phase I trial (ClinicalTrials.gov identifier: NCT01121575) to receive dacomitinib combined with crizotinib. The preliminary results showed that toxicity was manageable and the clinical activity was promising.74 A retrospective analysis of 80 advanced NSCLC patients with acquired resistance to first-line EGFR TKIs from People’s Republic of China was conducted. Twenty-three of the 80 patients (28.8%) had c-Met amplifications, including four patients taking crizotinib after resistance to EGFR TKIs. Among the three patients treated with crizotinib plus gefitinib, two attained PR and one attained stable disease.72,75
The preclinical and clinical data showed that c-Met-positive patients would benefit from crizotinib or crizotinib combined with EGFR TKIs and exhibit good tolerance. The major challenge when prescribing crizotinib to c-Met-positive patients is defining which c-Met state is predictive of the effect of crizotinib: c-Met amplification, overexpression, or mutation, and defining the standard detection methods and standard cutoff value of each method.
Conclusion
Crizotinib has played an important role in the development of precise treatments due to its unique antitumor PROFILE. Crizotinib is efficacious in Chinese NSCLC patients with ALK rearrangements and its toxicity is manageable. The application of crizotinib in Chinese c-Met/ROS1-positive NSCLC patients is promising but prospective clinical trials are required to confirm its efficacy and safety. ALK/c-Met/ROS1-positive patients account for approximately 10% of lung cancer cases, meaning about 10% lung cancer patients could benefit from crizotinib. The major challenge for prescribing crizotinib in clinical practice is establishing convenient diagnostic techniques for the detection of ALK/c-Met/ROS1. This review of crizotinib provides a reference for integrating genetic information in the classification of lung cancer and other tumors in the near future. Cancers might be classified as ALKoma, EGFRoma, and RAFoma and treated with ALK TKIs, EGFR TKIs, and RAF TKIs, respectively.
Acknowledgments
This study was supported by the following (1) Guangdong Provincial Key Laboratory of Lung Cancer Translational Medicine (grant no 2012A061400006); (2) special fund for research in the public interest from National Health and Family Planning Commission of People’s Republic of China (grant no 201402031); and (3) research fund from Guangzhou Science and Technology Bureau (grant no 2011Y2-00014).
Author contributions
Fei-Yu Niu and Yi-Long Wu designed the outline, searched literature, drafted and critically revised the manuscript, and approved the final manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Tan W, Wilner KD, Bang Y, et al. Pharmacokinetics (PK) of PF-02341066, a dual ALK/MET inhibitor after multiple oral doses to advanced cancer patients. J Clin Oncol. 2010;28(suppl):abstr2596. | ||
Kim D, Ahn M, Yang P, et al. Updated results of a global phase II study with crizotinib in advanced ALK-positive non-small cell lung cancer (NSCLC). Ann Oncol. 2012;23(suppl 9):ix402. | ||
Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385–2394. | ||
Solomon BJ, Mok T, Kim DW, et al; PROFILE 1014 Investigators. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371(23):2167–2177. | ||
Pfizer. A Study of Crizotinib versus Chemotherapy in Previously Untreated ALK Positive East Asian Non-Small Cell Lung Cancer Patients; 2014. Available from: http://clinicaltrial.gov/ct2/show/NCT01639001. Accessed November 24, 2014. [NLM identifier: NCT01639001]. | ||
Cui JJ, Tran-Dubé M, Shen H, et al. Structure based drug design of crizotinib (PF-02341066), a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK). J Med Chem. 2011;54(18):6342–6363. | ||
Christensen JG, Zou HY, Arango ME, et al. Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol Cancer Ther. 2007;6(12 pt 1):3314–3322. | ||
McDermott U, Iafrate AJ, Gray NS, et al. Genomic alterations of anaplastic lymphoma kinase may sensitize tumors to anaplastic lymphoma kinase inhibitors. Cancer Res. 2008;68(9):3389–3395. | ||
Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30(8):863–870. | ||
Tan W, O’Gorman M, Lanzalone S, et al. Pharmacokinetics (PK) of crizotinib (PF-02341066), a dual ALK/C-MET inhibitor, following administration of multiple oral doses to patients with advanced ALK-positive NSCLC in China. J Thorac Oncol. 2012;7(suppl 5):S491. | ||
Ou S-HI, Salgia R, Clark J, et al. Comparison of crizotinib (PF-02341066) pharmacokinetics between Asian and non-Asian patients with advanced malignancies. J Thorac Oncol. 2010;5(suppl 5):S382. | ||
Li C, Alvey C, Bello A, Wilner KD, Tan W. Pharmacokinetics (PK) of crizotinib (PF-02341066) in patients with advanced non-small cell lung cancer (NSCLC) and other solid tumors. J Clin Oncol. 2011;29(suppl):abstre13065. | ||
Wang E, Nickens D, Bello A, Khosravan R, Amantea M, Tan W. Clinical implication of a population pharmacokinetic analysis of xalkori (crizotinib) in 1,182 patients with non-small cell lung cancer (NSCLC) and 32 patients with other solid tumors. J Thorac Oncol. 2013;8(suppl 2):S296. | ||
Li Y, Li Y, Yang T, et al. Clinical significance of EML4-ALK fusion gene and association with EGFR and KRAS gene mutations in 208 Chinese patients with non-small cell lung cancer. PLoS One. 2013;8(1):e52093. | ||
Zhang X, Zhang S, Yang X, et al. Fusion of EML4 and ALK is associated with development of lung adenocarcinomas lacking EGFR and KRAS mutations and is correlated with ALK expression. Mol Cancer. 2010;9:188. | ||
An SJ, Chen ZH, Su J, et al. Identification of enriched driver gene alterations in subgroups of non-small cell lung cancer patients based on histology and smoking status. PLoS One. 2012;7(6):e40109. | ||
Takeuchi K, Choi YL, Soda M, et al. Multiplex reverse transcription-PCR screening for EML4-ALK fusion transcripts. Clin Cancer Res. 2008;14(20):6618–6624. | ||
Koivunen JP, Mermel C, Zejnullahu K, et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res. 2008;14(13):4275–4283. | ||
Barlesi F, Blons H, Beau-Faller M, et al. Biomarkers (BM) France: results of routine EGFR, HER2, KRAS, BRAF, PI3KCA mutations detection and EML4-ALK gene fusion assessment on the first 10,000 non-small cell lung cancer (NSCLC) patients (pts). J Clin Oncol. 2013;31(suppl):abstr8000. | ||
Zhang X, Lu S, Zhang L, et al. The Chinese expert consensus opinion of the diagnosis of ALK-positive NSCLC (2013 version). Chin J Pathol. 2013;42(6):402–406. [Chinese]. | ||
Ying J, Guo L, Qiu T, et al. Diagnostic value of a novel fully automated immunochemistry assay for detection of ALK rearrangement in primary lung adenocarcinoma. Ann Oncol. 2013;24(10):2589–2593. | ||
Demidova I, Barinov A, Savelov N, et al. Immunohistochemistry, fluorescence in situ hybridization, and reverse transcription-polymerase chain reaction for the detection of anaplastic lymphoma kinase gene rearrangements in patients with non-small cell lung cancer: potential advantages and methodologic pitfalls. Arch Pathol Lab Med. 2014;138(6):794–802. | ||
Wang J, Cai Y, Dong Y, et al. Clinical characteristics and outcomes of patients with primary lung adenocarcinoma harboring ALK rearrangements detected by FISH, IHC, and RT-PCR. PLoS One. 2014;9(7):e101551. | ||
Mino-Kenudson M, Chirieac LR, Law K, et al. A novel, highly sensitive antibody allows for the routine detection of ALK-rearranged lung adenocarcinomas by standard immunohistochemistry. Clin Cancer Res. 2010;16(5):1561–1571. | ||
Minca EC, Portier BP, Wang Z, et al. ALK status testing in non-small cell lung carcinoma: correlation between ultrasensitive IHC and FISH. J Mol Diagn. 2013;15(3):341–346. | ||
Wynes MW, Sholl LM, Dietel M, et al. An international interpretation study using the ALK IHC antibody D5F3 and a sensitive detection kit demonstrates high concordance between ALK IHC and ALK FISH and between evaluators. J Thorac Oncol. 2014;9(5):631–638. | ||
Guangdong Association of Clinical Trials. A Prospective Epidemiologic Study of ALK-Positive NSCLC in China (C-TALK); 2014. Available from: http://clinicaltrial.gov/ct2/show/NCT02042105. Accessed November 24, 2014. [NLM identifier: NCT02042105]. | ||
Wallander ML, Geiersbach KB, Tripp SR, Layfield LJ. Comparison of reverse transcription-polymerase chain reaction, immunohistochemistry, and fluorescence in situ hybridization methodologies for detection of echinoderm microtubule-associated proteinlike 4-anaplastic lymphoma kinase fusion-positive non-small cell lung carcinoma: implications for optimal clinical testing. Arch Pathol Lab Med. 2012;136(7):796–803. | ||
Li Y, Pan Y, Wang R, et al. ALK-rearranged lung cancer in Chinese: a comprehensive assessment of clinicopathology, IHC, FISH and RT-PCR. PLoS One. 2013;8(7):e69016. | ||
Wu YC, Chang IC, Wang CL, et al. Comparison of IHC, FISH and RT-PCR methods for detection of ALK rearrangements in 312 non-small cell lung cancer patients in Taiwan. PLoS One. 2013;8(8):e70839. | ||
Zhang YG, Jin ML, Li L, et al. Evaluation of ALK rearrangement in Chinese non-small cell lung cancer using FISH, immunohistochemistry, and real-time quantitative RT-PCR on paraffin-embedded tissues. PLoS One. 2013;8(5):e64821. | ||
Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13(10):1011–1019. | ||
Shaw AT, Yeap BY, Solomon BJ, et al. Overall survival in patients with advanced non-small cell lung cancer harboring concomitant EGFR mutations and ALK rearrangements: a cohort study. J Clin Oncol. 2014;32(suppl):abstre19010. | ||
Cheng Y, Zhu J. Clinical characteristics of ALK-positive advanced NSCLC patients and the clinical study of crizotinib 2014. Oral presented at: the Chinese Society of Clinical Oncology; September 17–21; 2013; Xiamen. [Chinese]. | ||
Loren CE, Englund C, Grabbe C, Hallberg B, Hunter T, Palmer RH. A crucial role for the anaplastic lymphoma kinase receptor tyrosine kinase in gut development in Drosophila melanogaster. EMBO Rep. 2003;4(8):781–786. | ||
Bazigou E, Apitz H, Johansson J, et al. Anterograde Jelly belly and Alk receptor tyrosine kinase signaling mediates retinal axon targeting in Drosophila. Cell. 2007;128(5):961–975. | ||
Blackhall F, Shaw A, Jänne P, et al. Crizotinib safety profile in elderly and non-elderly patients with advanced ALK+ non-small cell lung cancer. Poster presented at: the European Cancer Congress; September 27; 2013; Amsterdam. | ||
Frampton JE. Phase 2 data for crizotinib (PF-02341066) in ALK-positive advanced non-small cell lung cancer (NSCLC): profile 1005. J Thorac Oncol. 2011;6(suppl 2):S411. | ||
Ou S-HI, Kim D-W, Camidge DR, et al. Crizotinib therapy for patients with advanced ROS1-rearranged non-small cell lung cancer (NSCLC). J Thorac Oncol. 2013;8(suppl 2):S295. | ||
Cao Y, Xiao G, Qiu X, Ye S, Lin T. Efficacy and safety of crizotinib in patients with advanced c-MET-amplified non-small cell lung cancer (NSCLC). J Clin Oncol. 2014;32(suppl):abstr8001. | ||
Schnell P, Safferman AZ, Bartlett CH, Tang Y, Wilner KD. Clinical presentation of hepatotoxicity-associated crizotinib in ALK-positive (ALK+) advanced non-small cell lung cancer (NSCLC). J Clin Oncol. 2012;30(suppl):abstr7598. | ||
Hida T, Shi Y, Ahn M-J, et al. Exploratory subgroup analysis of crizotinib efficacy and safety in Asian and non-Asian patients with advanced ALK-positive non-small cell lung cancer (NSCLC) enrolled in a global phase II study. J Thorac Oncol. 2012;7(suppl):5. | ||
Ou S-HI, Tang Y, Polli A, Wilner KD, Schnell P. Characterization of heart rate (HR) changes during crizotinib treatment: a retrospective analysis of 1,053 ALK+ NSCLC patients. J Clin Oncol. 2014;32(suppl):abstre13065. | ||
Doebele RC, Pilling AB, Aisner DL, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res. 2012;18(5):1472–1482. | ||
Katayama R, Shaw AT, Khan TM, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung cancers. Sci Transl Med. 2012;4(120):120ra117. | ||
Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med. 2014;370(13):1189–1197. | ||
Nakagawa K, Hida T, Seto T, et al. Antitumor activity of alectinib (CH5424802/RO5424802) for ALK-rearranged NSCLC with or without prior crizotinib treatment in bioequivalence study. J Clin Oncol. 2014;32(suppl):abstr8103. | ||
Gettinger SN, Bazhenova L, Salgia R, et al. Updated efficacy and safety of the ALK inhibitor AP26113 in patients (pts) with advanced malignancies, including ALK+ non-small cell lung cancer (NSCLC). J Clin Oncol. 2014;32(suppl):abstr8047. | ||
Ou SH, Jänne PA, Bartlett CH, et al. Clinical benefit of continuing crizotinib beyond initial disease progression in patients with advanced ALK-positive non-small cell lung cancer. J Thorac Oncol. 2013;8(suppl 2):S294. | ||
Morris SW, Kirstein MN, Valentine MB, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science. 1994;263(5151):1281–1284. | ||
Griffin CA, Hawkins AL, Dvorak C, Henkle C, Ellingham T, Perlman EJ. Recurrent involvement of 2p23 in inflammatory myofibroblastic tumors. Cancer Res. 1999;59(12):2776–2780. | ||
De Paepe P, Baens M, van Krieken H, et al. ALK activation by the CLTC-ALK fusion is a recurrent event in large B-cell lymphoma. Blood. 2003;102(7):2638–2641. | ||
Jazii FR, Najafi Z, Malekzadeh R, et al. Identification of squamous cell carcinoma associated proteins by proteomics and loss of beta tropomyosin expression in esophageal cancer. World J Gastroenterol. 2006;12(44):7104–7112. | ||
Debelenko LV, Raimondi SC, Daw N, et al. Renal cell carcinoma with novel VCL-ALK fusion: new representative of ALK-associated tumor spectrum. Mod Pathol. 2011;24(3):430–442. | ||
Lin E, Li L, Guan Y, et al. Exon array profiling detects EML4-ALK fusion in breast, colorectal, and non-small cell lung cancers. Mol Cancer Res. 2009;7(9):1466–1476. | ||
Mano H. ALKoma: a cancer subtype with a shared target. Cancer Discov. 2012;2(6):495–502. | ||
Butrynski JE, D’Adamo DR, Hornick JL, et al. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N Engl J Med. 2010;363(18):1727–1733. | ||
Gambacorti-Passerini C, Messa C, Pogliani EM. Crizotinib in anaplastic large-cell lymphoma. N Engl J Med. 2011;364(8):775–776. | ||
Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131(6):1190–1203. | ||
Rimkunas VM, Crosby KE, Li D, et al. Analysis of receptor tyrosine kinase ROS1-positive tumors in non-small cell lung cancer: identification of a FIG-ROS1 fusion. Clin Cancer Res. 2012;18(16):4449–4457. | ||
Pan Y, Zhang Y, Li Y, et al. ALK, ROS1 and RET fusions in 1139 lung adenocarcinomas: a comprehensive study of common and fusion pattern-specific clinicopathologic, histologic and cytologic features. Lung Cancer. 2014;84(2):121–126. | ||
Clavé S, Gimeno J, De Muga S, et al. ROS1 rearrangements and copy number alterations in NSCLC patients: high frequency of ROS1 deletions. Ann Oncol. 2014;25(suppl 4):iv566. | ||
Go H, Kim DW, Kim D, et al. Clinicopathologic analysis of ROS1-rearranged non-small-cell lung cancer and proposal of a diagnostic algorithm. J Thorac Oncol. 2013;8(11):1445–1450. | ||
Mescam-Mancini L, Lantuéjoul S, Moro-Sibilot D, et al. Detection of ROS1 translocations in triple-negative lung adenocarcinomas. J Clin Oncol. 2013;31(suppl):abstr8099. | ||
Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371(21):1963–1971. | ||
Li A, Yang J, Zhang X, Zhou Q, Wu Y. Targeting de novo cMET overexpression in advanced non-small cell lung cancer. Ann Oncol. 2014;25(suppl 4):iv68. | ||
Li A, Gao HF, Wu YL. Targeting the MET pathway for potential treatment of NSCLC. Expert Opin Ther Targets. 2014;23:1–12. | ||
Lou NN, Yang J, Zhang X, Chen H, Wu Y. De novo MET overexpression coexisting with oncogenic drivers in advanced non-small cell lung cancer. Ann Oncol. 2014;25(suppl 4):iv462. | ||
Yang JJ, Zhang XC, Su J, et al. Lung cancers with concomitant EGFR mutations and ALK rearrangements: diverse responses to EGFR-TKI and crizotinib in relation to diverse receptors phosphorylation. Clin Cancer Res. 2014;20(5):1383–1392. | ||
Yamaguchi N, Lucena-Araujo AR, Nakayama S, et al. Dual ALK and EGFR inhibition targets a mechanism of acquired resistance to the tyrosine kinase inhibitor crizotinib in ALK rearranged lung cancer. Lung Cancer. 2014;83(1):37–43. | ||
Yang JJ, Chen HJ, Yan HH, et al. Clinical modes of EGFR tyrosine kinase inhibitor failure and subsequent management in advanced non-small cell lung cancer. Lung Cancer. 2013;79(1):33–39. | ||
Landi L, Minuti G, D’Incecco A, Cappuzzo F. MET overexpression as a promising therapeutic target in non-small cell lung cancer with acquired resistance to EGFR TKIs. J Clin Oncol. 2014;32(suppl):abstract 19047. | ||
Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316(5827):1039–1043. | ||
Janne PA, Shaw AT, Giaccone G, et al. Phase I trial of irreversible pan-ERBB inhibitor dacomitinib (DAC) in combination with ALK/MET inhibitor crizotinib (CRIZ) in previously treated advanced non-small cell lung cancer (NSCLC). Ann Oncol. 2012;23(suppl 9):ix423. | ||
Gou L, Wu Y, Yang J, Zhang X. Targeting c-MET overexpression for acquired resistance to EGFR TKIs. Ann Oncol. 2014;25(suppl 4):iv450. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


