Back to Journals » Journal of Pain Research » Volume 12
Personalized needle modification for CT-guided percutaneous infrazygomatic radiofrequency ablation of the maxillary nerve through the foramen rotundum in order to treat V2 trigeminal neuralgia
Authors Huang B, Yao M , Liu Q, Chen Y, Ni H , Li Z, Xie K , Fei Y, Li L
Received 1 March 2019
Accepted for publication 8 July 2019
Published 26 July 2019 Volume 2019:12 Pages 2321—2329
DOI https://doi.org/10.2147/JPR.S207297
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael Schatman
Bing Huang,1 Ming Yao,1 Qianying Liu,1 Yajing Chen,1 Huadong Ni,1 Zhang Li,1 Keyue Xie,1 Yong Fei,1 Langping Li2
1Department of Anesthesiology and Pain Medical Center, First Affiliated Hospital of Jiaxing University, Jiaxing 314001, People’s Republic of China; 2Department of Anesthesiology, Ruijin Hospital Luwan Branch, Shanghai Jiao Tong University School of Medicine, Shanghai 200020, People’s Republic of China
Background: The computed tomography (CT)-guided radiofrequency ablation (RFA) of the maxillary nerve (V2) via foramen rotundum (FR) approach has been reported to offer the highest rates of pain relief in V2 trigeminal neuralgia (TN). However, the access to FR may be obstructed by the greater wing of the sphenoid bone.
Objectives: We report on an optimized CT-guided percutaneous infrazygomatic of maxillary nerve through the foramen rotundum (FR) to treat V2 trigeminal neuralgia (TN) using personalized RFA needles based on patient’s individual CT-image parameters.
Patients and methods: 176 patients with isolated V2 TN were included. If the entry of the percutaneous needle into the FR canal was blocked by the greater wing of the sphenoid bone, straight RFA needles was bent at the tip with an angle α (the angle between the straight line from the external opening of FR to the skin entry point and the long axis of the FR canal). The maxillary nerve RFA was performed after confirmation with electrophysiological tests. Pain relief in the V2 territory and TN recurrence rate were followed for up to 60 months.
Results: Fifty-two patients (29.55%) required needle bending. The maxillary nerve thermal RFA resulted in analgesia in the V2 territory without affecting the V1 or V3 zone. TN recurrence rate at 6, 12, 24, 36, 48 and 60 months was 2.55%, 7.64%, 17.20%, 24.41%, 30.28% and 33.77%, respectively.
Conclusion: The personalized needle modification technique for maxillary nerve RFA through FR is safe and effective to treat V2 TN.
Keywords: trigeminal neuralgia, maxillary nerve, foramen rotundum, radiofrequency ablation
Introduction
Trigeminal neuralgia (TN) is a severe painful condition characterized by touch-evoked unilateral brief shock-like paroxysmal pain in one or more divisions (V1, V2, or V3) of the trigeminal nerve.1,2 TN pain can be very refractory to a number of therapies, including medications, nerve blocks, and microvascular decompression surgeries.3 Percutaneous radiofrequency thermocoagulation or ablation (RFA) has been reported to offer the highest rates of pain relief in TN.4 A number of research groups, amongst them ourselves, have reported that the computed tomography (CT)-guided RFA of the maxillary nerve (V2) via foramen rotundum (FR) approach can achieve selective analgesia to treat V2 TN.5,6 FR is a bony canal of 3 mm in diameter and 3–6 mm in length. The long axis of the canal runs in an obliquely posterosuperior to anteroinferior direction,7 which may form an angle with the percutaneous needle trajectory. In addition, the external opening of the FR is located in the upper portion of the pterygopalatine fossa. Access to FR may be obstructed by the greater wing of the sphenoid bone. Under these circumstances, a straight RFA needle may not be able to reach FR, yielding a less satisfactory ablative effect.5 To address this issue, we have designed a modified RFA needle technique based on individual CT scans to improve the access of the FR canal for the treatment of V2 TN.
Methods
Design: retrospective chart review
Patient characteristics
From November 2012 to March 2017, 176 patients with primary isolated V2 TN were included (Figure 1). This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the First Affiliated Hospital of Jiaxing University. All cases were conducted in the same institution. The diagnosis of TN was confirmed according to the International Headache Society guidelines.8 Potential secondary causes of TN were ruled out. There were 74 males and 102 females patients with a mean age of 66.9±9.84 years (range 32–101 years). Seventy-seven patients had TN on the left side and 99 patients on the right side. The mean duration of TN pain was 2.4 years (range 0.5–13 years) and the mean pre-procedural numerical rating scale (NRS) pain score was 7.1 (range 6–10). All patients failed to respond to multiple pharmacological treatments and responded to diagnostic maxillary nerve block with >50% pain relief. Patients were counseled with risk and benefits of the RFA procedure and all patients signed a written informed consent (Table 1).
 |
Table 1 Patient baseline characteristics (n=176) |
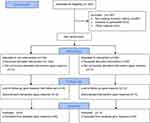 |
Figure 1 Flow diagram. |
CT-guided percutaneous RFA of V2 nerve through FR
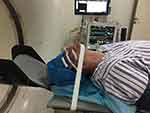
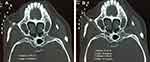
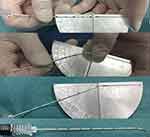
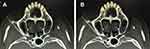
Patient was positioned in the supine position and under monitored anesthesia care. Access of FR was achieved as we described previously with individual modification.5 In short terms, a positioning grid was placed over the cheek of the affected side (Figure 2) and a semi-coronal CT scan was conducted. The specific CT-image frame (Figures 3, 5 and 6) that simultaneously captures the FR and its external and internal opening was used to establish the needle trajectory. The built-in ruler of the CT instrument was used to measure the distance from the needle entry point to the external opening of FR. Using the CT instruments image processing software we drew a line 1 from the midpoint of the FR canal (puncture target) against the lateral wall of the maxillary sinus to the surface of the skin (needle entry point). If line 1 passes through the ipsilateral greater wing of the sphenoid bone (Figure 3A), line 2 is drawn from the external opening of the FR to the skin entry point. The angle α between line 2 and the long axis of the FR canal (the degree at which the RFA needle is to be bent) and the angle between line 2 and the sagittal plane (puncture angle β1) were measured. The length of the segment of line 2 serves as puncture depth (Figure 3B). Then, the tip of a 10 cm 22 gauge straight RFA probe is inserted into a 16G sterilized piercing needle for about 5–8 mm and the RFA probe is bent to the degree of α (Figure 4). In this way, the RF probe becomes the patient’s individualized RFA needle. The skin and subcutaneous tissues were anesthetized with 2 mL of 1% lidocaine using a 27G intradermal needle. Under CT guidance, the personalized RFA needle was inserted infrazygomatically and advanced based on predetermined parameters, including angle, path, and depth to reach the external opening of FR anterosuperiorly (Figure 5A). The needle was then turned inferoposteriorly using the individually made needle curvature to enter the FR canal (Figure 5B). If line 1 does not pass through the sphenoid bone, it can be set as the puncture path using the unmodified straight RFA needle at the angle between line 1 and the sagittal plane (puncture angle β2) (Figure 6).
After confirming that there was no evidence of blood, cerebral spinal fluid, or paresthesia, a sensory test was performed by stimulating the RFA probe at 100 Hz with pulse width of 500 msec to generate paresthesia concordant to the patient’s usual TN pain at 0.1–0.5 V.9 A motor test was then performed by stimulating the probes at 2 Hz and 0.1–0.5 V to confirm that the probe was not in proximity to other adjacent nerves, 0.5 mL of 1% lidocaine was then injected 2 mins prior to ablation. Subsequently, continuous RFA was performed at 90°C for 120 s under intravenous propofol anesthesia. During the treatment, blood pressure, heart rate, electrocardiogram, and blood oxygen saturation were closely monitored. Rescue medications and equipment such as atropine, lidocaine, epinephrine, and airway devices were made immediately available. Oxygen was given via the nasal cannula. If the patient’s blood pressure rose by more than 20% of the baseline, Urapidil was administered at increments of 12.5 mg intravenously. After the procedure, the patient was transferred to the post-procedure recovery room where the NRS score was evaluated and recorded. The patient’s vital signs were monitored for at least four additional hours before being discharged. The intraoperative and postoperative complications were recorded and the immediate and long-term outcomes were evaluated during follow-up.
Outcomes
The NRS score before and after the procedure was assessed. The bending angle (α value), puncture depth, puncture angle (β value), puncture time, and number of CT scans for the RFA were documented. V2 TN recurrence rate, defined as number of patient with returning V2 TN after RFA/total number of follow-up patients, was tracked for up to 48 months. Complications and adverse effects were recorded.
Statistical analysis
Data were presented as mean±standard deviations and analyzed using SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA). Comparison of continuous data was performed by Student’s t-test. P-values lower than 0.05 were considered statistically significant.
Results
Infrazygomatic percutaneous access to the FR canal was obstructed by the greater wing of the sphenoid in 52 of the 176 (29.55%) patients recruited in this study. The RFA needle was bent at an angle of 14–31º (19.6±4.3º) to facilitate the entry into the FR canal. In the other 124 patients, the RFA needle route was not blocked by the sphenoid wing so the access to the FR canal was gained using a straight probe. Sensory test at 0.1–0.5 mA produced paresthesia in the V2 territory. All patients obtained more than 95% V2 TN pain reduction in NRS score after RFA. There was no numbness in the territory of V1 or V3. The puncture depth, angle, and puncture time of the bent needle group and the straight needle group are compared in Table 2. The puncture time and number of CT scans in the bent needle group were significantly greater than those in the straight needle group (P<0.05). Patients with V2 TN recurrence received repeated RFA. V2 TN total recurrence rate (Table 3) was 2.55%, 7.64%, 17.20%, 24.41%, 30.28%, and 33.77% at 6, 12, 24, 36, 48, and 60 months, respectively, while the recurrence rate of intervention group was 2.2%, 6.8%, 20.45%, 28.2%, 30%, and 33.3%. There was no statistical difference between the two groups.
 |
Table 2 The bending angle (α value), puncture depth, puncture angle (β value), puncture time, and number of CT scans |
 |
Table 3 The V2 TN recurrence rate (patients or recurrence rate of intervention group) |
There were no intracranial hemorrhage, trigeminal nerve V1 or V3 nerve injuries, cerebrovascular accidents, infections, or deaths recorded. All patients in the study suffered from varying degrees of numbness in the original pain area after treatment. There were 23 cases (non-intervention group/intervention group 19/4) of facial hematoma which resolved within 3 days after treatment with ice-bag compressions. Thirty-six patients of 167 (20%) required an average dose of 37.5 mg Urapidil to help control an increase in blood pressure of more than 20% of the baseline. No significant decrease in heart rate occurred during the procedure.
Discussion
We reported a personalized needle modification technique for percutaneous infrazygomatic CT-guided maxillary nerve RFA to treat isolated V2 TN. TN is one of the most common causes of facial pain and the treatment requires a multidisciplinary approach.10 For patients who could not tolerate or are not willing to undergo open craniotomy microvascular decompression surgery, percutaneous RFA provides sustained pain relief.9,11–13 A systematic review evaluated 166 studies and concluded that, comparing to glycerol rhizolysis, balloon compression of the trigeminal ganglion, and stereotactic radiosurgery, the RF thermocoagulation offers the highest rate of complete pain relief.4 The key for a successful RFA is the accurate placement of RFA electrode alongside the affected nerve. Traditionally, TN can be treated with RFA of the trigeminal Gasserian ganglion which can be accessed through the foramen ovale (FO) using the Härtel technique.14 CT fluoroscopy can be used to improve the visualization and access of FO.15,16 However, the FO approach is not optimal to treat sub-branch TN. It is often difficult to distinguish between V2 and V3 or V1 nerve once the needle passes the external opening of FO.5 Consequently, it often requires frequent adjustment of position of the needle to identify the V2 nerve. Performing these adjustments may increase the risk of dural puncture, infection, headache, and intracranial bleeding.17 Unintentional lesioning of V3 or V1 may result in masseter muscle weakness or paralysis, diminished or absent corneal reflex, and keratitis.9,13,18,19 Duration of pain relief for isolated V2 TN was significantly shorter than for V3 TN using the FO RFA technique20 likely because the V2 nerve is less successfully ablated using the FO approach. To address these concerns for isolated V2 TN, we amongst others have developed the FR approach at the pterygopalatine fossa to selectively block the V2 nerve.5,6,21 The V2 trigeminal nerve begins at the Gasserian ganglion and passes through the FR to exit the cranium.7 Blocking the V2 nerve at this peripheral (ie, FR) location reduced risks of abovementioned complications related to the FO technique.5 As we performed more cases of V2 block/RFA using the FR approach, we have encountered a new challenge due to anatomical variations among individual patients. In some patients, percutaneous infrazygomatic access of FR was obstructed by the greater wing of the sphenoid bone (2). To overcome this obstacle, we have adapted the bent needle technique based on individual CT measurements and gained access to the FR canal. This individualized bent needle technique (with personalized angle for each patient) was required in 52 of 176 (29.55%) patients with isolated V2 TN. The average bending angle was 14–31º (19.6±4.3º), suggesting that a commercial RF probe bent at 20º would have a great potential for percutaneous infrazygomatic FR access under CT-guidance. The procedure time required by the bent needle technique was about 5 mins longer than using the straight needle (23.53±6.14 mins Vs 19.42±4.03 mins, P<0.05). On the other hand, the individualized bent needle technique allowed precise needle positioning in the FR canal and subsequently a highly selective RFA of the V2 nerve. The recurrence rate of V2 TN in our study at 24 months was 17.20%, lower than that (28.3%) reported in RFA of Gasserian ganglion,12 suggesting again that a selective V2 RFA is preferable to the ganglion RFA in treating V2 TN.
Traditionally, pulsed RF (PRF) has been used to treat TN.22 PRF is a nondestructive ablation that generates short bursts of RF current at 42°C with long pauses between bursts to allow heat to dissipate in the target tissue.23 The advantage of using PRF is probably due to the fact that the smaller demyelinated C fibers are selectively lesioned under a lower temperature without motor ablation. Chua et al recommended performing PRF prior to thermal RF for the purpose of avoiding the disturbing of sensory paresthesia and masseter paralysis.24 However, a recent randomized controlled trial comparing conventional thermal RF with PRF showed that conventional RF provided significant pain reduction and patient satisfaction improvement in patients with idiopathic TN whereas PRF failed to achieve these effects.25 Another randomized prospective study reported that better outcome was observed in the thermal treatment as compared to pulsed RF treatment of the Gasserian ganglion.26 Based on these findings, we performed the selective V2 RFA at 90°C for 120 s. All patient had immediate TN pain relief after the procedure and the V2 TN recurrence rate at 36 months in our cohort was lower than that reported in the studies using 75°C for 4 mins (24.41% vs 36%),21 indicating that the temperature utilized for RFA may play an important role in patients with TN. Another study indicated that different temperature may cause different cardiovascular responses during RFA of trigeminal nerves.27 Significant elevation of the mean arterial blood pressure was observed in all patient studied (n=48) during RFA of the trigeminal ganglion using the FO approach. The correlation between the RFA stimulus and magnitude of the pressor was positive when the temperature used for RFA was below 75°C but it became negative when the temperature was above 75°C. These findings may indicate that responses of the trigeminocardiac reflex during trigeminal RFA are temperature dependent.28 We performed V2 RFA at 90°C and about 20% of our patients did require Urapidil to help control the elevation of blood pressure during the procedure. However, no significant tachycardia or bradycardia occurred during the RFA. We want to emphasize that vasoactive medications should be made immediately available during RFA of trigeminal nerves.
Conclusion
Nearly 30% of V2 TN patients in our cohort required the personalized needle-bending technique for percutaneous RFA of the maxillary nerve due to the obstruction of FR access by the ipsilateral greater wing of the sphenoid bone. We have optimized the maxillary RFA through the FR approach with precise localization of the entry of the FR canal. Highly selective thermocoagulation (90°C for 120 s) of the V2 nerve using this personalized technique is a safe and effective treatment and provides sustained pain relief and lower recurrence rate of isolated V2 TN pain.
Acknowledgments
The authors would like to thank Dr Daniel Lockwood from the Department of Diagnostic Radiology at the Cleveland Clinic, Cleveland OH, USA for the valuable discussion on the CT images. This work was funded by the National Natural Science Foundation of China (81341035), Natural Science Foundation of Zhejiang Province (LY16H090016 and LY17H090019), the Construction Project of Anesthesiology Discipline Special Disease Center in Zhejiang North Region (201524), Cultivation of High-level Innovative Health Talents of Zhejiang Province (2012-RC-22), Zhejiang Medicine and Health Program (2016ZDA018), Professional Cultivation of Health and Family Planning System in Huangpu District of Shanghai Municipal (2019GG02). The abstract of this paper was presented at the 2019 AAPM Annual Meeting named “Personalized Needle Modification in CT-Guided Percutaneous Infrazygomatic Radiofrequency Ablation of the Maxillary Nerve through Foramen Rotundum to Treat V2 Trigeminal Neuralgia” as a poster presentation with interim findings. The abstract was published in Pain Medicine, Volume 20, Issue 3, March 2019.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Maarbjerg S, Di Stefano G, Bendtsen L, Cruccu G. Trigeminal neuralgia - diagnosis and treatment. Cephalalgia. 2017;37(7):648–657. doi:10.1177/0333102416687280
2. Truini A, Galeotti F, Cruccu G. New insight into trigeminal neuralgia. J Headache Pain. 2005;6:237–239. doi:10.1007/s10194-005-0195-9
3. Obermann M. Treatment options in trigeminal neuralgia. Ther Adv Neurol Disord. 2010;3:107–115. doi:10.1177/1756285609359317
4. Lopez BC, Hamlyn PJ, Zakrzewska JM. Systematic review of ablative neurosurgical techniques for the treatment of trigeminal neuralgia. Neurosurgery. 2004;54:973–982. discussion 82–3. doi:10.1227/01.neu.0000114867.98896.f0
5. Huang B, Yao M, Feng Z, et al. CT-guided percutaneous infrazygomatic radiofrequency neurolysis through foramen rotundum to treat V2 trigeminal neuralgia. Pain Med. 2014;15:1418–1428. doi:10.1111/pme.12440
6. Wan Q, Zhang D, Cao X, Zhang Y, Zhu M, Zuo W. CT-guided selective percutaneous radiofrequency thermocoagulation via the foramen rotundum for isolated maxillary nerve idiopathic trigeminal neuralgia. J Neurosurg. 2018;128:211–214. doi:10.3171/2016.9.JNS152520
7. Sepahdari AR, Mong S. Skull base CT: normative values for size and symmetry of the facial nerve canal, foramen ovale, pterygoid canal, and foramen rotundum. Surg Radiol Anat. 2013;35:19–24. doi:10.1007/s00276-012-1001-4
8. Headache classification committee of the International Headache Society (IHS) the international classification of headache disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211. doi:10.1177/0333102417738202
9. Emril DR, Ho KY. Treatment of trigeminal neuralgia: role of radiofrequency ablation. J Pain Res. 2010;3:249–254. doi:10.2147/JPR.S14455
10. Heinskou T, Maarbjerg S, Rochat P, Wolfram F, Jensen RH, Bendtsen L. Trigeminal neuralgia–a coherent cross-specialty management program. J Headache Pain. 2015;16:66. doi:10.1186/s10194-015-0550-4
11. Taha JM, Tew JM
12. Sanders M, Henny CP. Results of selective percutaneous controlled radiofrequency lesion for treatment of trigeminal neuralgia in 240 patients. Clin J Pain. 1992;8:23–27.
13. Kanpolat Y, Savas A, Bekar A, Berk C. Percutaneous controlled radiofrequency trigeminal rhizotomy for the treatment of idiopathic trigeminal neuralgia: 25-year experience with 1,600 patients. Neurosurgery. 2001;48:524–532. discussion 32–4. doi:10.1097/00006123-200103000-00013
14. Härtel F. Über die intrakranielle Injektion Behandlung der Trigeminusneuralgie. Med Klin. 1914;10:582–584.
15. Koizuka S, Saito S, Sekimoto K, Tobe M, Obata H, Koyama Y. Percutaneous radio-frequency thermocoagulation of the gasserian ganglion guided by high-speed real-time CT fluoroscopy. Neuroradiology. 2009;51:563–566. doi:10.1007/s00234-009-0541-8
16. Gusmao S, Oliveira M, Tazinaffo U, Honey CR. Percutaneous trigeminal nerve radiofrequency rhizotomy guided by computerized tomography fluoroscopy. Technical note. J Neurosurg. 2003;99:785–786. doi:10.3171/jns.2003.99.4.0785
17. Ward L, Khan M, Greig M, Dolin SJ. Meningitis after percutaneous radiofrequency trigeminal ganglion lesion. Case report and review of literature. Pain Med. 2007;8:535–538. doi:10.1111/j.1526-4637.2006.00199.x
18. Zheng S, Wu B, Zhao Y, et al. Masticatory muscles dysfunction after CT-guided percutaneous trigeminal radiofrequency thermocoagulation for trigeminal neuralgia: a detailed analysis. Pain Pract. 2015;15:712–719. doi:10.1111/papr.12247
19. Rath GP, Dash HH, Bithal PK, Goyal V. Intracranial hemorrhage after percutaneous radiofrequency trigeminal rhizotomy. Pain Pract. 2009;9:82–84. doi:10.1111/j.1533-2500.2008.00246.x
20. Kosugi S, Shiotani M, Otsuka Y, et al. Long-term outcomes of percutaneous radiofrequency thermocoagulation of gasserian ganglion for 2nd- and multiple-division trigeminal neuralgia. Pain Pract. 2015;15:223–228. doi:10.1111/papr.12163
21. Xue T, Yang W, Guo Y, Yuan W, Dai J, Zhao Z. 3D image-guided percutaneous radiofrequency thermocoagulation of the maxillary branch of the trigeminal nerve through foramen rotundum for the treatment of trigeminal neuralgia. Medicine (Baltimore). 2015;94:e1954. doi:10.1097/MD.0000000000000874
22. Van Zundert J, Brabant S, Van de Kelft E, Vercruyssen A, Van Buyten JP. Pulsed radiofrequency treatment of the gasserian ganglion in patients with idiopathic trigeminal neuralgia. Pain. 2003;104:449–452.
23. Bogduk N. Pulsed radiofrequency. Pain Med. 2006;7:396–407. doi:10.1111/j.1526-4637.2006.00210.x
24. Chua NH, Halim W, Beems T, Vissers KC. Pulsed radiofrequency treatment for trigeminal neuralgia. Anesth Pain Med. 2012;1:257–261. doi:10.5812/aapm.3493
25. Erdine S, Ozyalcin NS, Cimen A, Celik M, Talu GK, Disci R. Comparison of pulsed radiofrequency with conventional radiofrequency in the treatment of idiopathic trigeminal neuralgia. Eur J Pain. 2007;11:309–313. doi:10.1016/j.ejpain.2006.04.001
26. Elawamy A, Abdalla EEM, Shehata GA. Effects of pulsed versus conventional versus combined radiofrequency for the treatment of trigeminal neuralgia: a prospective study. Pain Physician. 2017;20:E873–E81.
27. Meng Q, Zhang W, Yang Y, Zhou M, Li X. Cardiovascular responses during percutaneous radiofrequency thermocoagulation therapy in primary trigeminal neuralgia. J Neurosurg Anesthesiol. 2008;20:131–135. doi:10.1097/ANA.0b013e3181628305
28. Schaller B, Sandu N, Filis A, Buchfelder M. Trigemino cardiac reflex examination G. Cardiovascular responses during percutaneous radiofrequency thermocoagulation therapy in primary trigeminal neuralgia: an explanation of the trigeminocardiac reflex? J Neurosurg Anesthesiol. 2008;20:270. doi:10.1097/ANA.0b013e3181817b50
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.