Back to Journals » Infection and Drug Resistance » Volume 14
Persistent Low-Level Viremia is an Independent Risk Factor for Virologic Failure: A Retrospective Cohort Study in China
Authors Li Q, Chen M, Zhao H, Yu F, Yan L, Xiao J, Gao G, Yang D, Zhang F
Received 11 August 2021
Accepted for publication 9 October 2021
Published 2 November 2021 Volume 2021:14 Pages 4529—4537
DOI https://doi.org/10.2147/IDR.S332924
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Qun Li,1,2,* Meiling Chen,2,3 Hongxin Zhao,1,2 Fengting Yu,2,4 Liting Yan,1,2 Jiang Xiao,1,2 Guiju Gao,1,2 Di Yang,1,2 Fujie Zhang1,2,*
1Department of Infectious Diseases, Beijing Ditan Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Clinical Center for HIV/AIDS, Capital Medical University, Beijing, People’s Republic of China; 3The Medical Statistic Department, Beijing Ditan Hospital, Capital Medical University, Beijing, People’s Republic of China; 4Laboratory of Infectious Diseases Center, Beijing Ditan Hospital, Capital Medical University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Fujie Zhang
Clinical and Research Center of Infectious Disease, Beijing Ditan Hospital, Capital Medical University, Beijing, 100015, People’s Republic of China
Tel +86 10 84322581
Email [email protected]
Background: Whether intermittent low-level viremia (iLLV/blip) or persistent low-level viremia (pLLV) increases the risk of virologic failure (VF) in HIV-1 patients is controversial. The objective of this study was to investigate the incidence of blip/pLLV and the association between blip/pLLV and VF in a Chinese antiretroviral therapy cohort.
Methods: HIV-1 patients who underwent antiretroviral therapy (ART) from 2005 to 2018 and had at least two viral load (VL) measurements after a minimum of 6 months ART treatment were included. VF was defined as one or more VL measurements of ≥ 1000 copies/mL. Blip was described as an isolated VL measurement between 50 and 999 copies/mL, and pLLV was defined as two or more consecutive VL measurements between 50 and 999 copies/mL. Blip and pLLV were categorized separately into three groups: 50– 200, 201– 400 and 401– 999 copies/mL. The Cox proportional hazard model was used to explore the association between blip/pLLV and VF.
Results: In total, 8098 participants were enrolled in this long-term cohort study. A 94.3% of the participants were male and among which 77.3% were infected through homosexual transmission. Blip occurred in 4.0% (325/8098) of the patients with an incidence of 0.73 per 100 person-years (/100 PYS) of follow-up (95% CI: 0.71– 0.76), whereas pLLV occurred in 1.3% of the patients (102/8098) with an incidence of 0.23/100 PYS of follow-up (95% CI: 0.21– 0.25). All the three categories of pLLV were associated with VF: pLLV 50– 200 [aHR: 3.82 (1.95– 7.47)], pLLV 201– 400 [aHR: 5.36 (2.35– 12.22)] and pLLV 401– 999 [aHR: 13.51 (8.28– 22.02)]. However, blip is not significantly associated with VF in any category.
Conclusion: Our study suggested that patients with pLLV had an increased risk of subsequent VF. Therefore, if pLLV occurs in patients, monitoring and corresponding measurements must be strengthened to avoid the subsequent VF.
Keywords: HIV-1, blip, persistent low-level viremia, virologic failure
Introduction
Combined antiviral therapy (cART) can effectively inhibit HIV-1 virus replication and reduce HIV-related morbidity and mortality.1 In general, the goal of current HIV-1 treatment is to achieve virologic suppression (VS) in patients receiving cART. Adequate VS could prevent the development of resistance mutation, reduce HIV transmission and improve clinical outcomes.2 Under current guidelines, VS is defined as the viral load (VL) of less than 20–75 copies/mL and is analyzed quantitatively through commercial assays.3 Although most patients adhering to standard ART could achieve VS, the incidence of viral rebounds such as low-level viremia (LLV) occurs frequently.4
LLV has been defined variably in different guidelines. According to the European Acquired Immune Deficiency Syndrome (AIDS) Clinical Society (EACS) guidelines, LLV is defined as the VL between 20 and 50 copies/mL.5 By contrast, the Department of Health and Human Services guidelines (the USA, 2016) have defined LLV as the VL between 50 and 200 copies/mL.6 China adopted the World Health Organisation (WHO) guidelines, according to which LLV is defined as the VL between 50 and 999 copies/mL.7 In addition, LLV is of two types, namely intermittent LLV (iLLV/blip) and persistent LLV (pLLV). Blip refers to an independent LLV with previous and subsequent VL test results less than the standard limit,8 whereas the continual detection of LLV is designated as pLLV.9 The incidence of LLV in different studies has been reported to range from 18% to 34%, which can be attributed to differences in the LLV definition, study design, enrolled population, and VL measurement methods across these studies.10
The impact of blip/pLLV on virologic failure (VF) is contradictory and scientists have paid more attention towards investigating the role of blip/pLLV in HIV-1 patients. In the past debate, several researchers have reported an association between blip and subsequent VF,11,12 whereas others have discovered no correlation between the two aspects.13,14 Previously published studies have reported that pLLV can produce many adverse outcomes. For example, several observational studies have implicated that pLLV could increase the risk of VF.9,15 In one study, pLLV between 50 and 199 copies/mL was associated with deleterious consequences,9 whereas in another study, only the patients with pLLV > 200 copies/mL exhibited an increased risk of subsequent VF.15 Moreover, pLLV could significantly increase the overall immune activation and result in poor immune reconstitution through multiple mechanisms.16 Although the influence of blip/pLLV on VF is controversial, none of the guidelines recommend the treatment strategies for HIV-1 patients experiencing blip/pLLV.
The present study is the first to analyze the effect of blip and pLLV on VF in China. We included a long-term Chinese cohort comprising HIV-1 patients and attempted to investigate the incidence of blip/pLLV and to explore whether different categories of blip/pLLV could predict VF.
Methods
Study Design and Participants
This open, single-centred, and retrospective cohort study included HIV-1 positive patients who received antiretroviral therapy at Beijing Ditan Hospital from 1 January, 2005 to 31 December, 2018. The deadline for follow-up was 25 March, 2021. The enrollment criteria of patients were as following: (1) ≥18 years old; (2) ART-naive at baseline; (3) receiving first-line treatment including two nucleoside reverse transcriptase inhibitors (NRTIs) and a non-nucleoside reverse transcriptase inhibitor (NNRTI) or second-line treatment including two NRTIs and a protease inhibitor (PI) during the follow-up period; (4) having at least two documented VLs after at least 6 months of ART. The treatment effects in all the participants were evaluated every 3 months, and their VL was monitored approximately every 12 months.
Data Collection
Demographic and medical data, including age, gender, HIV transmission route, marital status, HIV-1 RNA VL at baseline, CD4+ cell count at baseline, ART regimen, and HBV/HCV infection, were collected from the database of the national free antiretroviral treatment plan, as described previously.17 Specialized researchers were responsible for managing the data.
Definitions
VF was considered as the primary outcome and defined as one or more VL measurements ≥1000 copies/mL. LLV was defined as at least one VL measurement of 50–999 copies/mL during ART. The definitions mentioned above were based on WHO guidelines.7 LLV is of two types: intermittent LLV (iLLV/blip), which refers to an independent LLV with a previous and subsequent VL of <50 copies/mL,18 and persistent LLV (pLLV), which is defined as two or more consecutive VL between 50 and 999 copies/mL.19 Blip and pLLV were divided into three categories: 50–200, 201–400, and 401–999 copies/mL. If a patient experienced both blip and pLLV during follow-up, only pLLV was considered. However, if a patient experienced either a blip or pLLV episode belonging to a higher category, we analyzed the data according to the higher category.
Analysis
Descriptive statistics are presented as medians with interquartile ranges (IQRs) for continuous variables and as counts with proportions for categorical variables. The VF and without VF groups were compared using the χ2 test or Fisher’s exact test. A Chi-square test was used to compare the VF incidence between the blip and pLLV groups. The Cox proportional hazard model was used to assess the association between blip/pLLV and VF. A sensitivity analysis was performed to exclude patients with blip from no pLLV patients, and another was performed to exclude patients with pLLV from no blip patients. The model was adjusted for age, gender, HIV transmission route, marital status, HBV/HCV infection, ART regimen, HIV-1 RNA VL and CD4+ cell count at baseline. Kaplan–Meier curves were drawn for the entire study population. Time to event VF was estimated from the date of ART initiation to the date of VF. Statistical Package for the Social Sciences software (SPSS 22.0) was applied and a p-value of less than 0.05 was considered statistically significant.
Ethics and Consent
All included patients provided a written informed consent form to allow their clinical data to be used in our study. Furthermore, the study was approved by the Ethics Committee of Beijing Ditan Hospital of Capital Medical University (approval number: 2021-022-01) and conformed to the Declaration of Helsinki.
Results
Baseline Characteristics
Between 1 January, 2005, and 31 December, 2018, a total of 8430 patients received a positive diagnosis of HIV-1 and underwent ART at Beijing Ditan Hospital. Of the total, 8152 patients underwent ≥2 VL measurements after receiving ART for at least 6 months. A few patients (n = 54) who were using integrase inhibitors were excluded. In total, 8098 patients were enrolled in this long-term cohort study (Figure 1). The median follow-up time was 70 (IQR: 45–89) months after ART initiation, and the patients enrolled in this study were followed up to 44,314 person-years in total.
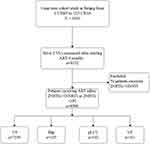 |
Figure 1 Study flow chart. Abbreviations: HAART, highly active antiretroviral therapy; pLLV, persistent low-level viremia; VS, virologic suppression; VF, virologic failure. |
Most of the participants (94.3%) were male, with a median age of 39 years [IQR: 31–44] at HIV diagnosis. 77.3% of the participants were infected with HIV-1 through homosexual transmission. Most of the patients (59.8%) who were enrolled in this cohort started ART between 2015 and 2018. Most of the participants (97.7%) received ART with 2NRTIs + 1NNRTI, whereas a few participants (2.3%) were administered 2NRTIs + 1PI. At baseline, the median HIV-1 RNA VL and CD4+ cell count were 170,051 copies/mL [IQR: 15,562–135,796] and 288 cells/µL [IQR: 160–389], respectively. Baseline characteristics are summarised in Table 1.
 |
Table 1 Demographic and Clinical Characteristics |
Incidence of Blip/pLLV and VF
Of the 8098 patients, 90.9% (7358/8098) patients had sustained VS during the entire follow-up period. 5.3% (427/8098) patients experienced LLV with an incidence of 0.96 per 100 person-years (/100 PYS) during the follow-up period (95% CI: 0.94–0.99). Among the 5.3% patients who experienced LLV, 4.0% (325/8098) experienced blip with an incidence of 0.73/100 PYS of follow-up (95% CI: 0.71–0.76) and 1.3% (102/8098) experienced pLLV with an incidence of 0.23/100 PYS of follow-up (95% CI: 0.21–0.25). VF was exhibited by 3.9% (313/8098) of the patients, with an incidence of 0.71/100 PYS of follow-up (95% CI: 0.68–0.73).
Prevalence of LLV in the range of 51–199 copies/mL (82.4% in blip vs 45.1% in pLLV) was the highest compared with that in the 201–400 copies/mL (10.2% in blip vs 21.6% in pLLV) and 401–999 copies/mL (7.4% in blip vs 33.3% in pLLV) ranges. After a consistent follow-up of patients with blip and pLLV, we observed that VF occurred in 34.3% (35/102) of patients with pLLV and 2.5% (8/325) of patients with blip. The proportion of VF in pLLV patients was significantly higher than that in blip patients, and the chi-square test of the two groups revealed a significant difference (P<0.001).
Risk Factors Associated with VF
Both blip and pLLV were divided into three categories: 50–200, 201–400 and 401–999 copies/mL, respectively. We observed that pLLV 50–200 copies/mL was associated with VF in both univariate [HR: 5.38 (2.77–10.46)] and multivariate analysis [aHR: 4.12 (2.16–7.84)]. pLLV 201–400 copies/mL was also associated with VF in both univariate [HR: 7.54 (3.35–16.98)] and multivariate analysis [aHR: 4.96 (2.17–11.32)]. Additionally, pLLV 401–999 copies/mL was associated with VF in univariate [HR: 15.78 (9.78–25.46)] and multivariate analysis [aHR: 15.03 (9.42–23.98)] (Table 2). In a sensitivity analysis excluding blip from no pLLV, we found that pLLV 51–200, 201–400, and 401–999 copies/mL were also associated with VF. We found that blip 50–200, 201–400, and 401–999 copies/mL were not associated with VF in both univariate and multivariate analysis, respectively (Table 2). Furthermore, in the sensitivity analysis excluding pLLV from no blip patients, and we found that blip 50–200, 201–400, and 401–999 copies/mL were also not associated with VF.
 |
Table 2 Risk Factors Associated with VF by Dividing Blip or pLLV into Different Groups |
In multivariate analysis, several other factors were also found to be associated with VF. Intravenous drug users [aHR: 2.21 (1.29–3.79)] and former plasma donors [aHR: 4.82 (2.35–10.39)] exhibited an increased risk of VF compared with patients who had homosexual transmission. HIV-1 RNA VL > 100,000 copies/mL [aHR: 1.65 (1.21–2.26)] at baseline was a risk factor for VF. Patients whose CD4+ T cell count <50 cells/uL [aHR: 2.12 (1.45–3.09)] and 50–199 cells/uL [aHR: 2.07 (1.49–2.89)] at baseline also had an increased risk of VF. Age, gender, marriage, ART regimens, and HBV/HCV infection were not associated with VF (Table 2).
Kaplan–Meier Analysis
Kaplan–Meier plots of VF stratified by blip and pLLV category are shown in Figure 2. Based on the Kaplan–Meier curves, differences in VF risk among different pLLV categories are shown in Figure 2A (P < 0.01). With an increase in the viremia level among different pLLV categories, the rate of VF also increased. However, no significant difference in the risk of VF among different blip categories was observed, as shown in Figure 2B (P = 0.901). A higher rate of VF was not associated with a higher viremia level in the blip category.
 |
Figure 2 (A) Virologic failure rates of different pLLV categories. (B) Virologic failure rates of different blip categories. |
Discussion
This study is the first in China to report the incidence of blip and pLLV in HIV-1 patients on ART and explore the association of blip/pLLV with subsequent VF. In the current study, 4.0% (325/8098) of the patients experienced blip with an incidence of 0.73/100 PYS of follow-up (95% CI: 0.71–0.76) and 1.3% (102/8098) of the patients experienced pLLV with an incidence of 0.23/100 PYS of follow-up (95% CI: 0.21–0.25). All three categories of pLLV (51–200, 201–400, and 401–999 copies/mL) were associated with VF. However, none of the three categories of blip were associated with VF. Our research suggests that patients experiencing pLLV further require new therapeutic measures, such as drug resistance testing, drug concentration monitoring, and compliance assessment, to avoid subsequent VF.
Due to differences in the VF definition and follow-up interval, the incidence of LLV varies greatly across different regions and countries. A report demonstrated that approximately 20% of AIDS patients experienced viral rebound during HAART treatment,14 whereas another study including 17,902 HIV-infected patients from Europe and North America revealed that 6.2% of HIV-infected patients experienced LLV.20 An extensive multi-center survey from South Africa reported that 23% and 26% of HIV-infected patients experienced LLV during the first-line and second-line treatments, respectively.21 Comparison of our results with other studies reveals that the incidence of LLV in our study is relatively low. Furthermore, one research in China reported a significantly higher incidence of LLV than that in our study (38.7%).22 We speculate that this might be due to differences in the frequency of VL monitoring (3–6 months vs 12 months). Additionally, patients generally do not perform VL testing at their own expense because of the economic constraints, which may lead to a low incidence of LLV.
The clinical significance of blip is still debatable. Although studies have demonstrated that blip could cause subsequent VF,8,10,23 others have reported that it is not associated with VF, which is consistent with our study.12,24 In total, 82.4% of blip occurred in the category of 50–200 copies/mL, which implied that blip might be a random change in virology caused by multiple reasons, including measurement errors, influence of various types of blood collection tube used,25 stochastic variations,26 opportunistic infection27 and low drug concentrations in blood.28 In addition, previous studies have revealed that higher CD8+ T cell activation during VS is associated with subsequent blip.16,29 Studies have suggested that the nature of blip is still controversial and should be investigated in further research.
Our research also illustrated that pLLV was associated with VF.9,14,30 In our study, with an increase in viremia levels in different pLLV categories, the risk of VF increased significantly. Compared with pLLV 51–200 copies/mL and pLLV 201–400 copies/mL, the risk of pLLV 401–999 copies/mL increased significantly by nearly three times. The current guidelines do not recommend treatment strategies for HIV-1 patients even after repeated LLV measurements. Research has revealed that pLLV increases the overall immune activation and accelerates the development of systemic drug resistance.29 New antiretroviral drug resistance could occur in HIV-1 patients experiencing pLLV during the first-line therapy,19 which prompted us to evaluate drug resistance test in pLLV patients to provide some guidance to choose a suitable ART regimen. Since the clinical management of pLLV remains a challenge, the current guidelines on pLLV management must be further discussed and updated.
In multivariate analysis, several other factors were associated with VF. This result ties nicely with previous study, wherein intravenous drug users and former plasma donors exhibited an increased risk of VF.2 Furthermore, the current study is consistent with a previous study, which revealed that CD4+ cell count of fewer than 200 cells/µL at baseline was associated with VF.30 The risk of VF in patients with HIV-1 RNA VL > 100,000 copies/mL at baseline was high. Contrary to several other studies showing that the PI-based regimen may result in VF instead of NNRTI-based cART,31,32 we did not observe the association between 2NRTIs + 1PI and VF. However, age, gender, marriage, and HBV/HCV infection were not associated with VF.
The VS rate was 90.9% in our study, which reflected the feasibility of current antiretroviral programmes and guaranteed medical services in our hospital. At the same time, our cohort held up very well, and a few patients died or lost during follow-up. To ensure the completeness and homogeneity of the clinical data, we first designed the research plan and then collected the data. Further, the follow-up information of each patient was managed by specialized researchers, which guaranteed the accuracy of the data. However, the study has some limitations. Firstly, although the sample size of our study was relatively large, it was a single-center study. Large-scale, prospective, and multi-center cohort studies are warranted to verify the present results. Secondly, because it was a retrospective study, we could not assess drug resistance in LLV patients. Thirdly, we did not consider the effect of ART adherence on the incidence of VF owing to the lack of data on ART adherence of patients.
Conclusion
In our study, blip occurred in 3.7% of the patients, and pLLV occurred in 1.2% patients. All three categories of pLLV (51–200, 201–400, and 401–999 copies/mL) were associated with VF. However, blip in any category was not significantly associated with VF. Clinicians must pay attention to patients with pLLV. At the same time, corresponding measures, such as drug resistance testing, drug concentration monitoring, and compliance assessment, should be taken. Finally, considering the lack of recommendations for the management of patients with LLV in current guidelines, our research will provide evidence for updating the guidelines.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation; and took part in drafting, revising or critically reviewing the article. Furthermore, all authors gave final approval for the version to be published; have agreed on the journal to which the article has been submitted.
Funding
This work was funded in part by The National 13th Five-Year Grand Program on Key Infectious Disease Control (2018ZX10302-102 to F.Z., 2018ZX10715-005 to H.Z.), Beijing Municipal Administration of Hospitals’ Ascent Plan (DFL20191802) and Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX202126).
Disclosure
The authors have no conflicts of interest to declare.
References
1. Palella FJ, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338(13):853–860. doi:10.1056/NEJM199803263381301
2. Soriano V. Gene therapy with CRISPR/Cas9 coming to age for HIV cure. AIDS Rev. 2017;19(3):167–172.
3. Leierer G, Grabmeier-Pfistershammer K, Steuer A, et al. Factors associated with low-level viraemia and virological failure: results from the Austrian HIV cohort study. PLoS One. 2015;10(11):e0142923. doi:10.1371/journal.pone.0142923
4. Garcia-Gasco P, Maida I, Blanco F, et al. Episodes of low-level viral rebound in HIV-infected patients on antiretroviral therapy: frequency, predictors and outcome. J Antimicrob Chemother. 2008;61(3):699–704. doi:10.1093/jac/dkm516
5. Ryom L, Boesecke C, Bracchi M, et al. Highlights of the 2017 European AIDS Clinical Society (EACS) Guidelines for the treatment of adult HIV-positive persons version 9.0. HIV Med. 2018;19(5):309–315. doi:10.1111/hiv.12600
6. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. Department of Health and Human Services; 2018.
7. World Health Organization. Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations; 2016.
8. Sorstedt E, Nilsson S, Blaxhult A, et al. Viral blips during suppressive antiretroviral treatment are associated with high baseline HIV-1 RNA levels. BMC Infect Dis. 2016;16:305. doi:10.1186/s12879-016-1628-6
9. Joya C, Won SH, Schofield C, et al. Persistent low-level viremia while on antiretroviral therapy is an independent risk factor for virologic failure. Clin Infect Dis. 2019;69(12):2145–2152. doi:10.1093/cid/ciz129
10. Ryscavage P, Kelly S, Li JZ, Harrigan PR, Taiwo B. Significance and clinical management of persistent low-level viremia and very-low-level viremia in HIV-1-infected patients. Antimicrob Agents Chemother. 2014;58(7):3585–3598. doi:10.1128/AAC.00076-14
11. Moore A, Youle M, Lipman M, et al. Raised viral load in patients with viral suppression on highly active antiretroviral therapy: transient increase or treatment failure? AIDS. 2002;16:615–618. doi:10.1097/00002030-200203080-00013
12. Leierer G, Grabmeier-Pfistershammer K, Steuer A, et al. A single quantifiable viral load is predictive of virological failure in human immunodeficiency virus (HIV)-infected patients on combination antiretroviral therapy: the Austrian HIV cohort study. Open Forum Infect Dis. 2016;3(2):ofw089. doi:10.1093/ofid/ofw089
13. Greub G, Cozzi-Lepri A, Ledergerber B, et al. Intermittent and sustained low-level HIV viral rebound in patients receiving potent antiretroviral therapy. AIDS. 2002;16:1967–1969. doi:10.1097/00002030-200209270-00017
14. Easterbrook P, Ives N, Waters A, et al. The natural history and clinical significance of intermittent viraemia in patients with initial viral suppression to < 400 copies/mL. AIDS. 2002;16:1521–1527.
15. Esber A, Polyak C, Kiweewa F, et al. Persistent low-level viremia predicts subsequent virologic failure: is it time to change the third 90? Clin Infect Dis. 2019;69(5):805–812. doi:10.1093/cid/ciy989
16. Karlsson AC, Younger SR, Martin JN, et al. Immunologic and virologic evolution during periods of intermittent and persistent low-level viremia. AIDS. 2004;18(7):981–989. doi:10.1097/00002030-200404300-00005
17. Zhang FJ. China Free ART Manual. Beijing: Chinese Center for Disease Control and Prevention; 2005.
18. Grennan JT, Loutfy MR, Su D, et al. Magnitude of virologic blips is associated with a higher risk for virologic rebound in HIV-infected individuals: a recurrent events analysis. J Infect Dis. 2012;205(8):1230–1238. doi:10.1093/infdis/jis104
19. Taiwo B, Gallien S, Aga E, et al. Antiretroviral drug resistance in HIV-1-infected patients experiencing persistent low-level viremia during first-line therapy. J Infect Dis. 2011;204(4):515–520. doi:10.1093/infdis/jir353
20. Vandenhende MA, Ingle S, May M, et al.; Antiretroviral Therapy Cohort Collaboration. Impact of low-level viremia on clinical and virological outcomes in treated HIV-1-infected patients. AIDS. 2015;29(3):373–383.
21. Hermans LE, Moorhouse M, Carmona S, et al. Effect of HIV-1 low-level viraemia during antiretroviral therapy on treatment outcomes in WHO-guided South African treatment programmes: a multicentre cohort study. Lancet Infect Dis. 2018;18(2):188–197. doi:10.1016/S1473-3099(17)30681-3
22. Zhang T, Ding H, An M, et al. Factors associated with high-risk low-level viremia leading to virologic failure: 16-year retrospective study of a Chinese antiretroviral therapy cohort. BMC Infect Dis. 2020;20(1):147. doi:10.1186/s12879-020-4837-y
23. Chen GJ, Sun HY, Chang SY, et al. Incidence and impact of low-level viremia among people living with HIV who received protease inhibitor- or dolutegravir-based antiretroviral therapy. Int J Infect Dis. 2021;105:147–151. doi:10.1016/j.ijid.2021.02.045
24. Havlir D, Bassett R, Levitan D, et al. Prevalence and predictive value of intermittent viremia with Combination HIV therapy. JAMA. 2001;286:171–179. doi:10.1001/jama.286.2.171
25. Stosor V, Palella F, Berzins B, et al. Transient viremia in HIV-infected patients and use of plasma preparation tubes. Clin Infect Dis. 2006;41:1671–1674. doi:10.1086/498025
26. Nettles RE, Kieffer TL, Kwon P, et al. Intermittent HIV-1 viremia (Blips) and drug resistance in patients receiving HAART. JAMA. 2005;293(7):817–829. doi:10.1001/jama.293.7.817
27. Jones LE, Perelson AS. Opportunistic infection as a cause of transient viremia in chronically infected HIV patients under treatment with HAART. Bull Math Biol. 2005;67(6):1227–1251. doi:10.1016/j.bulm.2005.01.006
28. Günthard H, Wong J, Spina C, et al. Effect of influenza vaccination on viral replication and immune response in persons infected with human immunodeficiency virus receiving potent antiretroviral therapy. J Infect Dis. 2000;181:522–531. doi:10.1086/315260
29. Taiwo B, Hunt P, Gandhi R, et al. CD8+ T-cell activation in HIV-1–infected patients experiencing transient low-level viremia during antiretroviral therapy. J Acquir Immune Defic Syndr. 2013;63:101. doi:10.1097/QAI.0b013e3182895af4
30. Fleming J, Mathews WC, Rutstein RM, et al. Low-level viremia and virologic failure in persons with HIV infection treated with antiretroviral therapy. AIDS. 2019;33(13):2005–2012. doi:10.1097/QAD.0000000000002306
31. Maldarelli F, Palmer S, King MS, et al. ART suppresses plasma HIV-1 RNA to a stable set point predicted by pretherapy viremia. PLoS Pathog. 2007;3(4):e46. doi:10.1371/journal.ppat.0030046
32. Giuliano M, Nicastri E, Pirillo M, et al. Residual viraemia in subjects with chronic HIV infection and viral load < 50 copies/mL: the impact of highly active antiretroviral therapy. AIDS. 2005;19:1843–1847.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
