Back to Journals » ClinicoEconomics and Outcomes Research » Volume 9
Persistence, switch rates, drug consumption and costs of biological treatment of rheumatoid arthritis: an observational study in Italy
Authors Degli Esposti L , Favalli EG , Sangiorgi D , Di Turi R, Farina G, Gambera M, Ravasio R
Received 18 March 2016
Accepted for publication 4 October 2016
Published 21 December 2016 Volume 2017:9 Pages 9—17
DOI https://doi.org/10.2147/CEOR.S108730
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Giorgio Colombo
Luca Degli Esposti,1 Ennio Giulio Favalli,2 Diego Sangiorgi,1 Roberta Di Turi,3 Giuseppina Farina,4 Marco Gambera,5 Roberto Ravasio,6
1CliCon S.r.l. – Health, Economics & Outcomes Research, Ravenna, 2Department of Rheumatology, Istituto Ortopedico Gaetano Pini, Milan, 3Local Pharmaceutical and Supplementary Assistance Unit, Roma Local Health Authority D, Rome, 4Internal Management Control Unit – Pharmaceutical Spending Control Sector, Caserta Local Health Authority, Caserta, 5Local Pharmaceutical Service, Bergamo Local Health Authority, Bergamo, 6Health Publishing & Services Srl, Milan, Italy
Objectives: The aim of this analysis was to provide an estimate of drug utilization indicators (persistence, switch rate and drug consumption) on biologics and the corresponding costs (drugs, admissions and specialist care) incurred by the Italian National Health Service in the management of adult patients with rheumatoid arthritis (RA).
Methods: We conducted an observational retrospective cohort analysis using the administrative databases of three local health units. We considered all patients aged ≥18 years with a diagnosis of RA and at least one biologic drug prescription between January 2010 and December 2012 (recruitment period). Persistence was defined as maintenance over the last 3 months of the follow-up period of the same biological therapy administered at the index date. A switch was defined as the presence of a biological therapy other than that administered at the index date during the last 3 months of the follow-up period. Hospital admissions (with a diagnosis of RA or other RA-related diagnoses), specialist outpatient services, instrumental diagnostics and pharmaceutical consumption were assessed.
Results: The drug utilization analysis took into account only biologics with at least 90 patients on treatment at baseline (adalimumab n=144, etanercept n=236 and infliximab n=94). In each year, etanercept showed better persistence with initial treatment than adalimumab or infliximab. Etanercept was characterized by the lowest number of patients increasing the initial drug consumption (2.6%) and by the highest number of patients reducing the initial drug consumption (10.5%). The mean cost of treatment for a patient persisting with the initial treatment was €12,388 (€14,182 for adalimumab, €12,103 for etanercept and €11,002 for infliximab). The treatment costs for patients switching from initial treatment during the first year of follow-up were higher than for patients who did not switch (€12,710 vs. €11,332).
Conclusion: Persistence, switch rate and drug consumption seem to directly influence treatment costs. In subjects not persisting with initial treatment, other health care costs were approximately three times higher than for persistent patients. This difference could suggest a positive effect on the quality of life for persistent patients. Etanercept showed the highest persistence with treatment.
Keywords: rheumatoid arthritis, biologic drugs, real-life clinical conditions
Introduction
Rheumatoid arthritis (RA) is a chronic, progressive autoimmune disease that causes severe articular damage and functional impotence in the affected joints.1,2 RA is characterized by persistent synovitis, which usually involves the peripheral joints. Synovial inflammation leads to cartilage destruction and bone erosion, with consequent joint deformation and disability. The rate of RA progression varies from one patient to another, but both joint damage and functional loss occur at the very early stages of the disease.3,4 As the condition occasionally involves other organs, with severe complications due to respiratory, cardiovascular and infectious diseases, patients with RA have a higher rate of mortality than the general population.5,6
RA is estimated to affect 0.5–1% of the adult population worldwide, with a higher prevalence among females (ratio 2:1).1,2 Its prevalence appears to be influenced by the geographic area and is higher in northern Europe (the Netherlands 1–1.5% and Scandinavian Peninsula 3%) than in southern Europe (Spain 0.5%). In the United States and Japan, its prevalence is 1% and 0.2%, respectively.7,8 Two observational studies have estimated the prevalence of RA in Italy, reporting rates of 0.33% in the Liguria region9 and 0.31% in Lombardy.
Although RA management includes non-pharmacological measures and non-steroidal anti-inflammatory drugs and/or glucocorticoids, the mainstay of therapy is disease-modifying anti-rheumatic drugs (DMARDs).10 In order to optimize response and minimize long-term disability, in recent years, the therapeutic approach to RA has been based on early use of DMARDs.11,12 In this regard, inhibitors of tumor necrosis factor-α (TNF-α) have significantly improved treatment of RA, with demonstrated efficacy even in patients not responding to conventional DMARDs.13–15
Although the clinical aspects (efficacy and tolerability) that, even if fundamental in choosing a pharmacological treatment, are not pertinent to this analysis, the parameters of drug utilization that best characterize the use of a pharmacological treatment are adherence, compliance and persistence. For example, non-adherence affects the success of treatment, remission and disease severity. In RA patients, long-term treatment persistence is important to decide whether a drug should be used in routine clinical practice.16 The impact on consumption of health care resources related to pharmacological therapy is primarily determined by a reduction in its efficacy.17,18 Poor persistence is usually associated with high consumption of resources and treatment costs.19,20
The aim of this analysis was to provide an estimate of drug utilization indicators (persistence, switch rate and drug consumption) on biologics and the corresponding costs (drugs, admissions and specialist care) incurred by the Italian National Health Service (NHS) in the management of adult patients with RA.
Methods
Data sources
This study was based on the administrative databases of Italian Local Health Authorities (LHAs), based in Lombardy, Lazio and Campania, which include ~1.5 million health-assisted individuals. For each LHA, an administrative database is available that includes data (pharmaceutical expenditure, admissions, services, etc.) that describe a “contact” (ie, predefined and classified in a univocal manner) between a patient and a facility of the social and health care system. Therefore, it is possible to trace each service provided to the patient who initiated a contact.
Data were collected from the three administrative databases on demographic aspects, consumption and costs of the health care resources administered to the patients considered herein. More specifically, the patient register provided information on demographic characteristics (date of birth, gender, date of death, exemption code, etc.). From hospital discharge records, complete information was available on hospital admissions (ordinary and day hospital) that were classified in accordance with the International Classification of Diseases, IX Revision, Clinical Modification (ICD-9-CM). In addition to patient’s hospital stay, the register also details each hospitalization: admitting unit, diagnoses (main and secondary), interventions and discharge unit. Moreover, based on the diagnosis-related groups (DRGs), the amount the LHA pays to the facility providing the service is also recorded. The pharmaceutical register includes all drug prescriptions to LHA patients (for drugs reimbursed by the Italian NHS). For each prescription, the register provides the date, marketing authorization code, active substance, medicinal product anatomical, therapeutic and chemical classification code (ATC code), number of packs and cost per pack. The register for specialist ambulatory care includes all specialist services (laboratory tests, instrumental tests and visits) provided to patients and paid by the Italian NHS. For each of these services, the register reports the date it was provided, along with the code, description and unit cost. Finally, the medical exemption register contains all disease exemptions; this database was our source for identifying diagnoses and comorbidities in the sample (to be integrated with information from hospital admissions and drug consumption).
Each patient was given a progressive number to anonymize the data extracted, which was in full compliance with the Personal Data Privacy Act (Law 196/03) and the resolution issued by the Italian Personal Data Privacy Authority on March 1, 2012, and published in the Official Gazette no. 72 on March 26, 2012. The patient anonymization process was performed by LHA staff at their offices. Informed consent is not required by the LHA ethics committees for using encrypted retrospective information. This study was notified to the local ethics committee in each participating LHA (Bergamo, Lombardy; Roma D, Lazio; Caserta, Campania) and the LHA ethics committees approved the study.
Cohort definition
This observational retrospective study considered all patients aged ≥18 years with a diagnosis of RA and at least one biologic drug prescription between January 2010 and December 2012 (recruitment period). Diagnosis of RA was based on the hospitalization with a primary or secondary diagnosis of RA (ICD-9-CM code 714) and/or specific exemption codes. The prescription date of the first biological drug during the inclusion period represented the “index date” for each patient. From the index date onward, each patient was observed for at least 1 year (follow-up period).
In order to improve the representativeness of data, the drug utilization analysis took into account only biologics with a significant volume of prescriptions. Assuming a mean relative variability (coefficient of variation) of ~50% for the analyzed parameters, our sample size would make it possible to achieve an estimated precision (in terms of relative estimate error) of no less than 10% (with a 95% confidence interval [95% CI] level).
The clinical characteristics of the patients enrolled in this study were also investigated in the 12 months before the index date.
The presence of previous use of DMARDs and biologics agents was also evaluated. Patients who were already under treatment with biologic agents before the index date were defined established. Comorbidities were measured using the Charlson Comorbidity Index (CCI); the sum of weights related to each condition (ie, myocardial infarction, cancers, diabetes and ulcer) identified through treatments and hospitalizations. All comorbidities were evaluated in the 12-month period before the index data; the CCI score reflects a patient’s overall health status.
Drug utilization indicators
Patients were classified as persistent if they were still on treatment with the index drug during the last 3 months of the follow-up period. A switch, on the other hand, was defined as the presence of a biological therapy other than that administered at the index date during the follow-up period. Drug consumption, escalation or reduction was evaluated as the change in the average dose prescribed between the two following prescriptive intervals during the period of observation. Dose escalation or reduction was defined as having two consecutive prescriptions with an average weekly dose 30% greater or lower than the initial average weekly dose, according to previously published methods.21,22
Costs of treatment
Health care consumption refers to the period 2010-2013. RA-related hospitalization (with a diagnosis of RA or other RA-related diagnoses), specialist outpatient services, instrumental diagnostics and pharmaceutical consumption were assessed. Specifically, the other RA-related diagnoses were uveitis (ICD-9-CM code: 364), fusion of metacarpophalangeal or interphalangeal joint spaces (ICD-9-CM code: 718), carpal tunnel syndrome (ICD-9-CM code: 354), pneumoconiosis or interstitial fibrosis (ICD-9-CM code: 515), cardiac conduction abnormalities or pericarditis or cardiomyopathy (ICD-9-CM codes: 420, 425), heart failure (ICD-9-CM code: 428), cardiac conduction abnormalities (ICD-9-CM code: 427), respiratory failure (ICD-9-CM code: 518) and anemia (ICD-9-CM codes: 280–285). The costs of each service/drug were calculated with reference to the time it was provided. Drugs were priced according to the NHS’s purchase price. Hospitalizations were calculated using the DRG tariffs (version 24). DRG tariffs represent the reimbursement levels of the NHS to health care providers. This made it possible to calculate the mean annual cost of treatment for each patient.
For the first year of follow-up, the mean annual costs of treatment per patient were calculated separately for each pharmacological consumption indicator:
- mean costs per patient with or without a switch from the initial treatment;
- mean costs per patient with or without drug consumption escalation (≥30%) compared to the initial one;
- mean costs per patient with or without drug consumption reduction (≥30%) compared to the initial one.
The cost analysis was conducted from the perspective of the NHS. The currency reference used was the Euro (€).
Statistical analysis
Continuous variables are expressed as mean ± standard deviation (SD), whereas categorical variables are reported as absolute numbers and percentages. The significance of differences between raw/processed data was verified using a Student’s t-test (two samples) or chi-square test, as appropriate.
A generalized linear regression model was performed to identify the associations between total health care costs during the follow-up period and age, sex, CCI, drug dose escalation, drug dose reduction and switching among biologic agents.
A multivariable logistic regression model was performed to estimate the odds ratios (ORs) and 95% CIs and to examine predictors of persistence to therapy and switching among biologic agents.
The model was used to identify differences in factors associated with persistence and switching among biologic therapies, adjusting for covariates such as age, male sex, CCI, drug dose escalation and drug dose reduction.
A p-value <0.05 was considered statistically significant. Statistical analyses were performed using Microsoft® Excel® for Windows® (Microsoft Corporation, Seattle, WA, USA) and SPSS® version 13.0 for Windows® (SPSS Inc., Chicago IL, USA).
Results
Overall, 564 patients met the inclusion criteria and were considered in the analysis. Subjects treated at baseline with adalimumab, etanercept, or infliximab accounted for 85% of the sample (474 of 564 patients) (Figure 1).
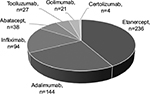  | Figure 1 Patient distribution by biologic drug at baseline. |
Only three biologics satisfied the inclusion criteria for this study: 1) adalimumab (n=144 patients), 2) etanercept (n=236 patients) and 3) infliximab (n=94 patients).
Patients’ characteristics and utilization of prior health care resources are shown in Table 1. Mean age was 53.4 years (SD ±13.0) for infliximab, 52.6 years (SD ±14.1) for etanercept and slightly higher for adalimumab 56.5 years (SD ±13.0); the majority of patients were female.
The percentage of subjects for whom DMARD therapy was previously used was 88.3% for infliximab, 81.3% for adalimumab and slightly lower for etanercept (76.3%). Patients who were already under treatment with biologic agents before the index date were almost the same for adalimumab and etanercept (43.8% and 44.5%, respectively) and slightly higher for infliximab (60.6%).
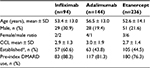
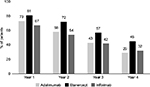
Figure 2 shows persistence data for the three biologics, calculated for the first year of treatment and, when available, for the second, third and fourth years of follow-up. In each year, etanercept showed better persistence with initial treatment than adalimumab or infliximab. In the first year, 81% of patients treated with etanercept were persistent with initial therapy, compared to 73% for adalimumab and 67% for infliximab. At 4 years, 45% of patients were persistent with etanercept treatment vs. 32% for infliximab and 29% for adalimumab. Etanercept showed a lower switch rate from initial therapy at 1 year (11%) and 2 years (7%) vs. adalimumab (18% and 11%, respectively) and infliximab (15% and 13%, respectively) (Figure 3).
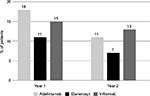
When assessing drug consumption variation, calculated for subjects persisting with initial treatment during the first year of follow-up, etanercept was characterized by the lowest number of patients increasing the initial drug consumption (2.6%) and by the highest number of patients reducing the initial drug consumption (10.5%) (Figures 4 and 5). With infliximab, no patient reduced the initial drug consumption, which was increased in ~20% of patients on infliximab during the first year of follow-up (Figures 4 and 5). For adalimumab, the percentage of patients increasing or reducing the initial drug consumption was the same (6.9%) (Figures 4 and 5).
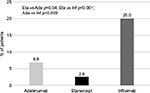  | Figure 4 Percentage of patients with drug consumption escalation. Abbreviations: Eta, etanercept; Ada, adalimumab; Inf, infliximab. |
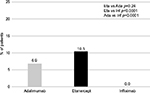  | Figure 5 Percentage of patients with drug consumption reduction. Abbreviations: Eta, etanercept; Ada, adalimumab; Inf, infliximab. |
These results were partially confirmed in the logistic regression analysis of the probability of remaining persistent and switch to another biologic drugs as a function of patient characteristics and their index therapy (Table 2). Model results show that in the RA sample, as compared to etanercept users, adalimumab users were less likely to be persistent (OR 0.51; 95% CI: 0.31–0.82) and more likely to switch their index biologic drugs (OR 2.06; 95% CI: 1.10–3.86) during follow-up period. In addition, patients who had increased their initial drug dose were more likely to switch their index biologic (OR 2.75; 95% CI: 1.40–5.38) during follow-up period. Other patient factors in the model were not significant predictors of persistence or switching among biologic agents.
Costs of treatment
The mean cost of treatment for a patient diagnosed with RA and persisting with the initial treatment during the first year of follow-up was calculated on the entire sample (n=474). The mean cost was €12,388, of which €12,096 was for pharmacological therapy and €292 for other health care costs (hospitalizations, specialist care, etc.). The mean cost of the three biologics was €14,182 for adalimumab (drugs: €13,816; other health care consumption: €366), €12,103 for etanercept (drugs: €11,823; other health care consumption: €280) and €11,002 for infliximab (drugs: €10,874; other health care consumption: €128 [excluding infusion-related costs]) (Figure 6).
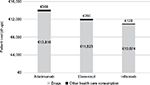  | Figure 6 Costs for the three biologics investigated*. Notes: *Excluding infusion costs for infliximab. |
The treatment costs for patients switching from initial treatment during the first year of follow-up were higher than for patients who did not switch (€12,710 vs. €11,332). Obviously, the costs of treatment were seen to increase with increasing drug consumption (€12,949 vs. €11,352) and to decrease with drug consumption reduction (€10,503 vs. €11,680) (Table 3). It is noteworthy that, for patients not persistent with their initial drug, other health care costs (hospitalizations, specialist care, etc.) were significantly higher than those for persistent patients (€1,088 vs. €375) (Figure 7).
  | Table 3 Mean annual costs of treatment per indicator of pharmacological consumption. |
These findings were partially confirmed by multivariate analysis (Table 4).
  | Table 4 Cost of illness, generalized linear model Note: β, beta coefficients. Abbreviations: 95% CI, 95% confidence interval; CCI, Charlson Comorbidity Index. |
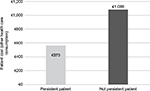  | Figure 7 Costs for other health care consumption (excluding drugs). |
Discussion and conclusion
This analysis was based on information from the administrative databases of three Italian LHAs covering the 4-year period 2010–2013. Therefore, it was possible to create a large sample of RA patients who had been treated with a biological therapy. In addition to the mean costs of treatment, we assessed indicators of consumption of biological drugs, such as persistence, switch rate and drug consumption. Attempts were also made to identify relationships between these consumption indicators and costs of treatment.
The features of treatment (eg, persistence, switch rate and drug consumption) seem to directly influence its costs. In subjects not persisting with initial treatment, other health care costs (hospitalizations, specialist services, etc.; €1,088) were approximately three times higher than for persistent patients (€375). This difference could suggest a positive effect on the quality of life for persistent patients. Etanercept showed the highest persistence with treatment; at the end of the 4-year follow-up, the number of patients persisting with etanercept treatment was ~1.4 and 1.6 times higher than for infliximab and adalimumab, respectively. This finding is consistent with the results of two important Italian registries, GISEA (Italian Early Arthritis study)23 and LORHEN (Lombardy Rheumatology Network),24 and with another study that, based on a local registry, estimated persistence with therapy at 12 years of the three most commonly used TNF inhibitors for the treatment of RA, showing that the risk of discontinuation for adalimumab (hazard ratio [HR] 2.35 [95% CI 1.73–3.21]) and infliximab (HR 1.55 [95% CI 1.14–2.11]) was significantly greater than for etanercept.25
Patients switching as early as the first year of follow-up had higher treatment costs than patients who do not switch (€11,332 vs. €12,710). This finding suggests that clinical conditions were likely deteriorating (higher consumption of medicinal products) and/or patients had switched to a different pharmacological therapy that was more expensive than the previous. Herein, etanercept had the lowest switch rate to another therapy in the first and second years of follow-up.
Comparing the three biologics, in the first year of follow-up, etanercept had the lowest percentage of patients showing a drug consumption increase (≥30% of initial dose) and the highest percentage of patients with a drug consumption reduction (≥30% of initial dose). Taking into account that for biologic drugs assessed in the study dose adjustments are observed in some patients, our findings show that there is a difference between the actual and theoretical costs of treatment.
This significant difference may be due to a number of reasons. One explanation might be the imbalanced distribution of baseline disease activity of the RA patients included in this study. Our findings might support the hypothesis that RA patients with pre-index DMARD or biologic drugs use might have higher severe disease. As a result, their disease may be managed more aggressively, and motivation for adhering to therapy may be greater.
Consumption data and costs estimated in this analysis should be interpreted in the light of some limitations. It was not possible to assign important clinical data such as the initial disease activity, a specific RA severity score, treatment’s efficacy and clinical decision to administer a given biologic agent (ie, high-risk patient), which were not retrievable from the data set given their administrative scope. Consequently, the analysis on the three biologics (adalimumab, etanercept and infliximab) could be characterized by patient recruitment bias, since the three groups we compared could have been not perfectly homogeneous in terms of disease severity. Another limitation is that the reasons for switching and low persistence to treatment, as well as the reasons for drug dose escalation or reduction, are not recorded in the databases. In addition, unlike adalimumab and etanercept, infliximab is given as an intravenous infusion; this may not only influence patient selection but is also likely to have an impact on costs that these administrative databases may not include. One study, conducted from a different perspective, estimated that the cost of a single infusion (including materials and health care professionals) was €43.40.26
Finally, it should be remembered that these results, since they refer to three Italian LHAs, are not exhaustive of RA treatment and costs; however, they suggest the opportunity to improve the resource allocation strategy of the Italian NHS. In reality, better knowledge of pre-existing prescription patterns for RA and greater awareness of the economic burden of the disease could stimulate planning of health care interventions aimed at improving public health services for the management of this disease.
Acknowledgment
This research was made possible by an unconditional grant from Pfizer Italy.
Disclosure
The authors report no conflicts of interest in this work.
References
Gibofsky A. Epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis: a synopsis. Am J Manag Care. 2014;20(7 suppl):S128–S135. | ||
Gibofsky A. Overview of epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis. Am J Manag Care. 2012;18(13 suppl):S295–S302. | ||
Singh JA, Furst DE, Bharat A, et al. 2012 Update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res. 2012;64(5):625–639. | ||
Kvien TK. Epidemiology and burden of illness of rheumatoid arthritis. Pharmacoeconomics. 2004;22(Suppl 1):1–12. | ||
De Maria AN. Relative risk of cardiovascular events in patients with rheumatoid arthritis. Am J Cardiol. 2002;89(6A):33D–38D. | ||
Lubeck DP. Patient-reported outcomes and their role in the assessment of rheumatoid arthritis. Pharmacoeconomics. 2004;22(Suppl 1):27–38. | ||
Cerra C, Ravasio R, Polcaro F. Il costo dell’Artrite Reumatoide: l’esperienza dell’ASL della Provincia di Pavia. Giornale Italiano di Health Technol Assess. 2009;2(3):111–117. | ||
Shichikawa K, Inoue K, Hirota S, et al. Changes in the incidence and prevalence of rheumatoid arthritis in Kamitonda, Wakayama, Japan, 1965–1996. Ann Rheum Dis. 1999;58:751–756. | ||
Cimmino MA, Parisi A, Moggiana G, et al. Prevalence of rheumatoid arthritis in Italy: the Chiavari study. Ann Rheum Dis. 1998;57:315–318. | ||
Smolen JS, Landewe R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis. 2010;69:964–975. | ||
Nell VP, Machold KP, Ebrel G, et al. Benefit of very early referral and very early therapy with disease-modifying anti-rheumatic drugs in patients with early rheumatoid arthritis. Rheumatology. 2004;43:906–914. | ||
Emery P, Breedveld FC, Dougados M, et al. Early referral recommendation for newly diagnosed rheumatoid arthritis: evidence-based development of a clinical guide. Ann Rheum Dis. 2002;61:290–297. | ||
Kuek A, Hazleman BL, Östor AJK. Immune mediated inflammatory diseases (IMIDs) and biologic therapy: a medical revolution. Postgrad Med J. 2007;83:251–260. | ||
Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358:903–911. | ||
Caporali R, Conti F, Alivernini S, et al. Recommendations for the use of biologic therapy in rheumatoid arthritis: update from the Italian Society for Rheumatology I. Efficacy. Clin Exp Rheumatol. 2011;29(Suppl 66):S7–S14. | ||
Markenson JA, Gibofsky A, Palmer WR, et al. Persistence with anti-tumor necrosis factor therapies in patients with rheumatoid arthritis: observations from the RADIUS registry. J Rheumatol. 2011;38(7):1273–1281. | ||
Cramer JA, Silverman S. Persistence with bisphosphonate treatment for osteoporosis: finding the root of the problem. Am J Med. 2006;119(4 Suppl 1):S12–S17. | ||
World Health Organization. Adherence to Long-Term Therapies: Evidence for Action. Geneva: World Health Organization; 2003. | ||
Hughes D, Cowell W, Koncz T, Cramer J; International Society for Pharmacoeconomics and Outcomes Research Economics of Medication Compliance Working Group. Methods for integrating medication compliance and persistence in pharmacoeconomic evaluations. Value Health. 2007;10(6):498–509. | ||
Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–47. | ||
Bonafede MM, Gandra SR, Fox KM, Wilson KL. Tumor necrosis factor blocker dose escalation among biologic naïve rheumatoid arthritis patients in commercial managed-care plans in the 2 years following therapy initiation. J Med Econ. 2012;15(4):635–643. | ||
Darkow T, Chastek B, Rosenblatt L, Trivedi D, Hebden T, Henk H, Liu F. Dose Escalation among rheumatoid arthritis patients treated with infliximab or abatacept: comparison in claims data. Arthritis Rheum. 2011;63(10):1221. | ||
Iannone F, Gremese E, Atzeni F, et al. Longterm retention of tumor necrosis factor-α inhibitor therapy in a large italian cohort of patients with rheumatoid arthritis from the GISEA registry: an appraisal of predictors. J Rheumatol. 2012;39(6):1179–1184. | ||
Marchesoni A, Zaccara E, Gorla R, et al. TNF-alpha antagonist survival rate in a cohort of rheumatoid arthritis patients observed under conditions of standard clinical practice. Ann NY Acad Sci. 2009;1173(1):837–846. | ||
Favalli EG, Biggioggero M, Pregnolato F, Becciolini A, Penatti AE, Marchesoni A, Meroni PL. The 12-year retention rate of the first-line TNF-inhibitor in the treatment of rheumatoid arthritis: real-life data from a local registry. Arthritis Care Res (Hoboken). Epub 2015 Nov 10. | ||
Favalli EG, Marchesoni A, Colombo GL, et al. Pattern of use, economic burden and vial optimization of infliximab for rheumatoid arthritis in Italy. Clin Exp Rheumatol. 2008;26:45–51. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.