Back to Journals » Cancer Management and Research » Volume 10
Peroxiredoxin 1 silencing inhibited the growth and promoted apoptosis of pancreatic cancer cells via targeting FOXO3 gene
Authors Sun X, Kong L, Li B, Zhang Y, Yang H
Received 14 June 2018
Accepted for publication 7 September 2018
Published 26 October 2018 Volume 2018:10 Pages 5019—5026
DOI https://doi.org/10.2147/CMAR.S177243
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Antonella D'Anneo
Xianchun Sun,1 Lingting Kong,2 Bingshu Li,2 Yan Zhang,2 Haiyan Yang2
1Department of No. 2 Gastrointestinal Surgery, The Affiliated Yantai Yuhuangding Hospital of Qingdao University, Yantai 264000, China; 2Department of Emergency, Yantaishan Hospital, Yantai 264000, China
Objective: Our study aimed to investigate the interaction between peroxiredoxin 1 (Prx1) and forkhead box O3 (FOXO3) and to explore the role of PI3K/AKT pathway in the development of pancreatic cancer.
Material and methods: Human pancreatic normal cells HPDE6-C7 and pancreatic cancer cells PANC-1 were randomly divided into control group, Prx1-silencing (si-Prx1) group, Prx1/FOXO3 dual-silencing (si-Prx1/FOXO3) group, and negative control group. Cell proliferation assay, clone formation assay, and cell apoptosis assay were performed to investigate the effects of Prx1 silencing and FOXO3 silencing on the proliferation and apoptosis ability of pancreatic cancer cells. qRT-PCR and Western blot were performed to study the Prx1 and FOXO3 mRNA in the two cells and FOXO3 protein expression in PANC-1 cells.
Result: We found Prx1 silencing could inhibit growth and promote apoptosis of PANC-1 cells. And Prx1 silencing could decrease the Prx1 mRNA level and increase FOXO3 mRNA level. To further explore the role of Prx1 in PI3K/AKT, we study the cell proliferation and apoptosis ability after adding the PI3K inhibitor and PI3K activator. We observed that PI3K inhibitor could inhibit tumor cell growth and promote cell apoptosis. And PI3K inhibitor also downregulated Prx1 protein expression.
Conclusion: We concluded that the Prx1 silencing inhibited the growth and promoted apoptosis of pancreatic cancer cells via modulation of PI3K/AKT pathway by targeting FOXO3 gene.
Keywords: Prx1, PI3K/AKT pathway, proliferation, apoptosis
Introduction
Pancreatic cancer is a kind of malignant tumor developed from the cells in the pancreas. Although pancreatic cancer can be divided into many different subtypes according to the pathological features, pancreatic adenocarcinoma, as the dominant type, accounts for about 85% of all the cases.1 As one of the main causes of cancer-related death, about 200,000 patients died of pancreatic cancer worldwide every year.2 In China, the mortality rate of pancreatic cancer almost reaches the incidence due to the poor prognosis.3 Although a variety of treatment strategies, including chemotherapy, radiotherapy, and so on, have been developed for pancreatic cancer, surgical resection is still the standard treatment for this disease. Patients with pancreatic cancer are usually diagnosed at advanced stage and only less than 20% of them can be treated with surgery.4 Previous studies have shown that the occurrence of pancreatic cancer is significantly affected by a variety of factors including male gender, smoking, aging, imbalanced diet structure, obesity, diabetes mellitus, and so on.5 However, our understanding on the pathogenesis of pancreatic cancer is still limited. The development of pancreatic cancer is a complex process with multiple genetic factors involved. Therefore, the in-depth studies on the mechanism of pancreatic cancer at gene level may facilitate the identification of novel targets for the treatment of pancreatic cancer.
Peroxiredoxin-1 (Prx1) is a kind of antioxidant enzymes that can reduce alkyl hydroperoxides and hydrogen peroxide.6 As an enzyme that can combat oxidative stress, Prx1 is proved to be involved in the development of a variety of human diseases.7 Previous studies have shown that Prx1 can play a role as tumor suppressor gene, while in other types of cancers, the overexpression of Prx1 can promote the progression of tumor.8 It has been shown that the expression level of Prx1 is upregulated in patients with pancreatic cancer, and the elevated expression level of Prx1 may serve as a marker for the diagnosis and prognosis of pancreatic cancer.9 However, the molecular mechanism of the function of Prx1 in pancreatic cancer is still unclear. Forkhead box O3 (FOXO3) is a member of the O subclass of transcription factor forkhead family, which is featured by a distinct forkhead DNA-binding domain. The downregulation of FOXO3 was observed in the development of various human cancers and has been proven to be involved in tumorigenesis.10 However, functionality of FOXO3 in pancreatic cancer still has not been well studied.
Asano et al reported that the PI3K/AKT pathway is significantly active in pancreatic cancer cells.11 Activation of the PI3K/AKT pathway may result in increased tumor cell proliferation and the inhibition of tumor cell apoptosis.12,13AKT plays an important role in cell viability and proliferation.12,13 PI3K triggers production of phospholipids that anchor AKT to the plasma membrane where its activation by other protein kinases can occur. In 2010, Roy et al reported that the inhibition of the PI3K/AKT pathway leads to the decreased pancreatic cancer cell proliferation, increased cell apoptosis, and cell cycle arrest.14 Therefore, we speculated that Prx1 may play a role through PI3K/AKT pathway in pancreatic cancer.
Materials and methods
Cell lines and grouping
Human pancreatic normal cells HPDE6-C7 and pancreatic cancer cells PANC-1 were purchased from ATCC (Manassas, VA, USA). Cells were cultured with DMEM medium (Solarbio, Beijing) containing 10% heat-inactivated FBS, 100 kU/L benzylpenicillin, and 100 mg/L streptomycin (Solarbio, Beijing, China) in incubator (37°C, 5% CO2). Cells were harvested during logarithmic phase for subsequent experiments. PANC-1 cells were divided into four groups including control group, Prx1-silencing (si-Prx1) group, Prx1/FOXO3 dual-silencing (si-Prx1/FOXO3) group, and negative control (NC) group. Cells in control group were without any treatment, cells in NC group were treated with negative control lentivirus vectors, cells in si-Prx1 group were treated with Prx1 gene-silencing lentivirus vectors, and cells in si-Prx1/FOXO3 group were treated with Prx1 gene-silencing lentivirus vectors and FOXO3-silencing lentivirus vectors. To verify the effect of Prx1 gene on PTEN/PI3K/AKT pathway, we set up three groups, including si-Prx1 group (the PANC-1 cells were transfected with Prx1-siRNA), PI3K inhibitor group (the untransfected PANC-1 cells were treated with PI3K inhibitor), and si-Prx1 + PI3K activator group (the PANC-1 cells were transfected with Prx1-siRNA followed by treating with PI3K activator).
Prx1-silencing and Prx1/FOXO3 dual-silencing vectors
Prx1 siRNA, FOXO3 siRNA, and lentivirus vectors were purchased from Shanghai GenePharm Pharmaceutical Technology Co., Ltd.
Cell transfection
The PANC-1 cells in logarithmic growth phase were collected and adjusted to 3×105/mL. Then the cells were seeded into 12-well plate and transfected with the corresponding vectors. The specific transfection process was carried out in accordance with the Lipofectamine 2000 kit instructions.
qRT-PCR
Total RNA was extracted from cells using RNeasy Plus kit (Qiagen, Valencia, CA, USA). Reverse transcription was performed using a high-capacity cDNA transcription kit (Applied Biosystems, Waltham, MA, USA). PCR reaction system was prepared using SYBR Green Master Mix (Applied Biosystems, San Diego, CA, USA). Primers used in PCR reaction were: 5′-ACAGCCGTTGTCAATGGAGAG-3′ (forward) and 5′-ACGTCGTGAAATTCGTTAGCTT-3′ (reverse) for Prx1; 5′-CGGACAAACGGCTCACTCT-3′ (forward) and 5′-GGACCCGCATGAATCGACTAT-3′ (reverse) for FOXO3; and 5′-GAAGGTGAAGGTCGGAGTC-3′ (forward) and 5′-GAAGATGGTGATGGGATTTC-3′ (reverse) for GAPDH. Ct values were processed using 2−ΔΔCt method, and the relative expression level of each gene was normalized to endogenous control GAPDH.
MTT assay
Cells in logarithmic growth phase were collected and seeded into 96-well plate. After incubation of 24 hours, medium was removed, and 100 µL of MTT (5 mg/mL, FuHeng Biology, China) was added into each well. After incubation at 37°C in dark for 4 hours, MTT solution was removed and 150 µL of DMSO was added and incubated for 10 minutes. OD values at 570 nm were measured using VersaMax Microplate Reader (Molecular Devices, Sunnyvale, CA, USA).
Flow cytometry
Apoptosis was detected by Annexin V-PI apoptosis detection kit (BD Biosciences, Franklin Lakes, NJ, USA). The posttransfected PANC-1 cells were collected, centrifuged at 1,000 rpm for 5 minutes, and adjusted into 2×106 cells/mL. Then the cells were washed three times by precooled 1× PBS solution and suspended in 300 µL 1× binding buffer. Five microliters of Annexin V-FITC were added into the above cell suspension and incubated for 15 minutes at 37°C in the dark. Then, 5 µL PI solution and 190 µL 1× binding buffer was added and immediately detected. The absorbance was analyzed by flow cytometry (Beckman Coulter, Brea, CA, USA).
Western blot
Protein samples were quantified by BCA method. Protein (30 µg) was mixed with loading buffer and denatured, followed by electrophoresis and transmembrane to polyvinylidene difluoride membrane (Merck, Darmstadt, Germany). Membranes were blocked with 5% skim milk at room temperature for 2 hours. After that, primary antibodies including anti-Prx1 (ab211292, 1:1,000, Abcam, Cambridge, UK), anti-FOXO3 (SAB2107951, 1:1,000, Sigma-Aldrich, St. Louis, MO, USA), anti-PI3K (GW21071, 1:500, Sigma-Aldrich), anti-p-PI3K (#SAB1305578, 1:1,000, Sigma-Aldrich), anti-Akt (SAB4500797, 1:1,000, Sigma-Aldrich), anti-p-Akt (#9271, 1:1,000, Cell Signaling Technology Danvers, MA, USA), and anti-GAPDH (ab37168, 1:1,000, Abcam) were used to incubate with the corresponding overnight at 4°C. After washing, membranes were incubated with goat antirabbit LgG (H + L) secondary antibody (1:1,000, Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd, Beijing, China). After washing, ECL detection reagent (Sigma-Aldrich, USA) was added to detect the signal. Relative expression level of each protein was normalized to endogenous control GAPDH using Image J software (https://imagej.nih.gov/ij/).
Statistical analysis
SPSS19.0 statistical software was used. Data were expressed as mean ± SD. Comparisons between two groups were performed by related sample t-test. The statistical comparison among multiple groups was carried out by one-way ANOVA followed by LSD test. P<0.05 was considered to be statistically significant.
Results
The level of Prx1 and FOXO3 in HPDE6-C7 and PANC-1 cells
As seen from Figure 1A, there was higher expression of Prx1 mRNA in PANC-1 cells than in HPDE6-C7 (P<0.01). The level of Prx1 mRNA in PANC-1 cells was ~2.4-fold of that in HPDE6-C7 cells. However, FOXO3 mRNA exhibited decreased level in PANC-1 cells (Figure 1B).
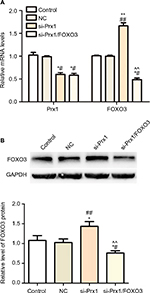
The level of Prx1 and FOXO3 in PANC-1 cells transfected with Prx1 siRNA
As seen from Figure 2A, PANC-1 cells transfected with Prx1 siRNA showed a downregulated level of Prx1 mRNA (P<0.05), while the FOXO3 expression was upregulated after Prx1 siRNA transfection as compared to control and NC groups (P<0.01). In addition, both Prx1 gene-silencing lentivirus vectors and FOXO3-silencing lentivirus vectors were transfected into PANC-1 cells. Then, we could observe a decreased level of Prx1 mRNA as compared to control and NC groups (P<0.05), and there was no significant difference between si-Prx1 group and si-Prx1/FOXO3 group. Furthermore, we found that there was a lower expression of FOXO3 mRNA in si-Prx1/FOXO3 group than control, NC, and si-Prx1 groups (P<0.05 or P<0.01).
The Western blot results indicated that there was no significant difference in FOXO3 protein level between control and NC groups (Figure 2B). Prx1 mRNA silencing could enhance the level of FOXO3 protein, and there was a lower expression of FOXO3 protein in si-Prx1/FOXO3 group compared with that in si-Prx1 group (P<0.01).
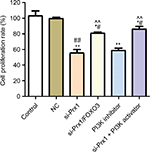
Prx1 siRNA transfection inhibited proliferation of PANC-1 cells through PI3K/AKT pathway
Cell proliferation rate was detected by MTT assay to explore the effect of Prx1 mRNA silencing on PANC-1 cells growth (Figure 3). Compared with control group and NC group, transfection of Prx1 siRNA could significantly inhibit the cell growth (P<0.01). However, Prx1 mRNA and FOXO3 mRNA dual silencing could increase the cell proliferation rate as compared to si-Prx1 group (P<0.01). In addition, we added the PI3K inhibitor and activator to explore the effect of blocking or activating PI3K/AKT pathway on tumor cell proliferation ability. And we found that PI3K inhibitor significantly decreased the cell proliferation (P<0.01), but PI3K activator increased the decreased cell proliferation rate induced as compared to that in control, NC, and si-Prx1 groups (P<0.05 or P<0.01).
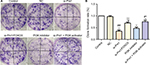
Consistent with the results in Figure 3, clone formation experiment (Figure 4) also indicated that Prx1 siRNA transfection could inhibit the cell proliferation ability. And blocking PI3K/AKT pathway could also decrease the tumor cell growth.
Prx1 siRNA transfection increased PANC-1 cell apoptosis through PI3K/AKT pathway
As seen from Figure 5, there was a higher apoptosis rate (14.2%) in si-Prx1 group than in control group and NC group. Further silencing FOXO3 mRNA could inhibit the cell apoptosis with a significant difference compared with si-Prx1 group (P<0.01). Moreover, we studied the effect of PI3K inhibitor on the cell apoptosis and found that there was increased apoptosis rate (15.2%) in PI3K inhibitor group and a lower apoptosis rate (6.7%) in si-Prx1 + PI3K activator group.
The expression of the related protein in PI3K/AKT pathway
It can be seen from Figure 6 that both Prx1 siRNA and PI3K inhibitor could significantly downregulate Prx1 protein level (P<0.05), while PI3K activator could upregulate the level of Prx1 protein. And FOXO3 protein exhibited an opposite trend. In addition, Prx1 siRNA and PI3K inhibitor could inactivate the PI3K/AKT pathway, while PI3K activator could increase the PI3K and AKT phosphorylation.
Discussion
Although Prx1 plays a role as tumor suppressor gene in some types of malignant tumors, expression level of Prx1 is usually upregulated in the majority types of tumors, and the increased level of Prx1 usually indicates poor treatment outcomes and prognosis.8,15 In the study of lung cancer, Jiang et al reported that the expression level of Prx1 was increased in tumor tissue and the enhanced level of Prx1 was responsible for the increased tumor malignancy.16 During the development of lung cancer, level of Prx-1 in plasma is usually increased by ROS, and the increased plasma level of Prx-1 is proved to accurately predict lung cancer.16 In 2015, Gong F et al reported that Prx1 was found to be overexpressed in esophageal squamous cell carcinoma, and Prx1 can participate in the development of esophageal squamous cell carcinoma through the interactions with mTOR/p70S6K signaling pathway.8 In another study, Prx-1 was shown to be able to promote the migration and invasion of tumor cells by regulating the process of epithelial-to-mesenchymal transition during oral carcinogenesis.17 Due to the unique expression pattern in tumor tissue, Prx-1 has been used as a biological marker for the diagnosis of prognosis of various human cancers. Consistent with previous studies, in this study, Prx-1 miRNA silencing significantly reduced the proliferation and promoted apoptosis of pancreatic cancer cell lines. Our results in this study and the findings in previous studies both revealed that Prx-1 can participate in the development of pancreatic cancer by promoting or at least maintaining tumor cell proliferation and inhibiting tumor cell apoptosis.
FOXO3 is a transcription factor that plays pivotal role in various physiological and biochemical processes in the body.18 The reduced expression level of FOXO3 is significantly correlated with tumorigenesis,19 indicating the function of FOXO3 as a tumor suppressor in the development of human cancers. Expression level of FOXO3 was significantly reduced during colorectal cancer progression, and the decreased expression level of FOXO3 may potentially serve as a biomarker for this disease.20 Therefore, FOXO3 and Prx-1 play opposite roles in the development of various human cancers. However, the interactions between FOXO3 and Prx-1 in pancreatic cancer still have been reported. It has been reported that FOXO3 can regulate the expression of peroxiredoxin III, which is a member of peroxiredoxin family, in human cardiac fibroblasts.21 In this study, Prx-1 silencing significantly increased the expression level of FOXO3. FOXO3 overexpression significantly reduced the proliferation and promoted the apoptosis of pancreatic cancer, further confirming the role of FOXO3 as tumor suppressor in pancreatic cancer. These data suggested that Prx-1 and FOXO3 play opposite role in the development of pancreatic cancer by affecting tumor cell proliferation and apoptosis.
Additionally, we also explored the effect of PI3K/AKT pathway on tumor cell growth and apoptosis. From the previous studies,22–25 we concluded that the activation of the PI3K/AKT pathway can increase the proliferation of tumor cells and inhibit the apoptosis of cells, which is consistent with our study. In our study, we added PI3K inhibitor or PI3K activator to explore the effect of PI3K/AKT pathway. We found that inhibition of PI3K/AKT pathway by PI3K inhibitor could lead to the enhanced proliferation rate. Furthermore, we studied whether Prx1 mRNA silencing inhibited the growth and promoted apoptosis of pancreatic cancer cells via PI3K/AKT pathway. We found that PI3K inhibitor supplement not only inhibited phosphorylation of PI3K and AKT but also decreased the Prx1 protein level. And when PI3K activator was added after silencing Prx1 mRNA, we found a higher level of Prx1 protein. The above results indicated that Prx1 mRNA silencing inhibited the growth and promoted apoptosis of pancreatic cancer cells via PI3K/AKT pathway.
Conclusion
In conclusion, Prx1 silencing can significantly reduce the proliferation and increase the apoptosis rate of pancreatic cancer cells. Meanwhile, Prx1 silencing can increase the expression level of FOXO3 in pancreatic cancer cells. In addition, we also explored the effect of PI3K/AKT pathway and found that Prx1 siRNA transfection could inhibit the activation of PI3K/AKT pathway. Therefore, we concluded that Prx1 and FOXO3 can negatively regulate the expression of each other to play opposite roles in pancreatic cancer through PI3K/AKT pathway. Our study provided the basis for future-related studies.
Disclosure
The authors report no conflicts of interest in this work.
References
Mcguire S. World Cancer Report 2014. Geneva, Switzerland: World Health Organization, International Agency for Research on Cancer, WHO Press, 2015. Adv Nutr. 2016;7(2):418–419. | ||
Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. | ||
Chen W, Zheng R, Zuo T, Zeng H, Zhang S, He J. National cancer incidence and mortality in China, 2012. Chin J Cancer Res. 2016;28(1):1–11. | ||
Sharma C, Eltawil KM, Renfrew PD, Walsh MJ, Molinari M. Advances in diagnosis, treatment and palliation of pancreatic carcinoma: 1990–2010. World J Gastroenterol. 2011;17(7):867–897. | ||
Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378(9791):607–620. | ||
Neumann CA, Cao J, Manevich Y. Peroxiredoxin 1 and its role in cell signaling. Cell Cycle. 2009;8(24):4072–4078. | ||
El Eter E, Al-Masri AA. Peroxiredoxin isoforms are associated with cardiovascular risk factors in type 2 diabetes mellitus. Braz J Med Biol Res. 2015;48(5):465–469. | ||
Gong F, Hou G, Liu H, Zhang M. Peroxiredoxin 1 promotes tumorigenesis through regulating the activity of mTOR/p70S6K pathway in esophageal squamous cell carcinoma. Med Oncol. 2015;32(2):455. | ||
Cai CY, Zhai LL, Wu Y, Tang ZG. Expression and clinical value of peroxiredoxin-1 in patients with pancreatic cancer. Eur J Surg Oncol. 2015;41(2):228–235. | ||
Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7(11):847–859. | ||
Asano T, Yao Y, Zhu J, Li D, Abbruzzese JL, Reddy SA. The PI 3-kinase/Akt signaling pathway is activated due to aberrant PTEN expression and targets transcription factors NF-kappaB and c-Myc in pancreatic cancer cells. Oncogene. 2004;23(53):8571–8580. | ||
Yamamoto S, Tomita Y, Hoshida Y, et al. Prognostic significance of activated Akt expression in pancreatic ductal adenocarcinoma. Clin Cancer Res. 2004;10(8):2846–2850. | ||
Jiang H, Fan D, Zhou G, Li X, Deng H. Phosphatidylinositol 3-kinase inhibitor (LY294002) induces apoptosis of human nasopharyngeal carcinoma in vitro and in vivo. J Exp Clin Cancer Res. 2010;29:34. | ||
Roy SK, Srivastava RK, Shankar S. Inhibition of PI3K/AKT and MAPK/ERK pathways causes activation of FOXO transcription factor, leading to cell cycle arrest and apoptosis in pancreatic cancer. J Mol Signal. 2010;5:10. | ||
Goncalves K, Sullivan K, Phelan S. Differential expression and function of peroxiredoxin 1 and peroxiredoxin 6 in cancerous MCF-7 and noncancerous MCF-10A breast epithelial cells. Cancer Invest. 2012;30(1):38–47. | ||
Jiang H, Wu L, Mishra M, Chawsheen HA, Wei Q. Expression of peroxiredoxin 1 and 4 promotes human lung cancer malignancy. Am J Cancer Res. 2014;4(5):445–460. | ||
Niu W, Zhang M, Chen H, et al. Peroxiredoxin 1 promotes invasion and migration by regulating epithelial-to-mesenchymal transition during oral carcinogenesis. Oncotarget. 2016;7(30):47042–47051. | ||
Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96(6):857–868. | ||
Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7(11):847–859. | ||
Bullock MD, Bruce A, Sreekumar R, et al. FOXO3 expression during colorectal cancer progression: biomarker potential reflects a tumour suppressor role. Br J Cancer. 2013;109(2):387–394. | ||
Chiribau CB, Cheng L, Cucoranu IC, Yu YS, Clempus RE, Sorescu D. FOXO3A regulates peroxiredoxin III expression in human cardiac fibroblasts. J Biol Chem. 2008;283(13):8211–8217. | ||
Liu D, You P, Luo Y, Yang M, Liu Y. Galangin induces apoptosis in MCF-7 human breast cancer cells through mitochondrial pathway and phosphatidylinositol 3-Kinase/Akt inhibition. Pharmacology. 2018;102(1–2):58–66. | ||
Zhao S, Wang L, Zhang C, et al. Inhibitor of growth 3 induces cell death by regulating cell proliferation, apoptosis and cell cycle arrest by blocking the PI3K/AKT pathway. Cancer Gene Ther. 2018;106. | ||
Li H, Zhang Y, Hai J, et al. Knockdown of TRIM31 suppresses proliferation and invasion of gallbladder cancer cells by down-regulating MMP2/9 through the PI3K/Akt signaling pathway. Biomed Pharmacother. 2018;103:1272–1278. | ||
Gu QZ, Nijiati A, Gao X, et al. TROP2 promotes cell proliferation and migration in osteosarcoma through PI3K/AKT signaling. Mol Med Rep. 2018;18(2):1782–1788. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.