Back to Journals » OncoTargets and Therapy » Volume 10
Peripheral venous blood neutrophil-to-lymphocyte ratio predicts survival in patients with advanced gastric cancer treated with neoadjuvant chemotherapy
Authors Chen L , Zuo YJ, Zhu LH, Zhang YX, Li S, Ma F, Han Y, Song HJ, Xue YW
Received 14 February 2017
Accepted for publication 5 April 2017
Published 17 May 2017 Volume 2017:10 Pages 2569—2580
DOI https://doi.org/10.2147/OTT.S134716
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yao Dai
Li Chen,1 Yanjiao Zuo,1 Lihua Zhu,2 Yuxin Zhang,3 Sen Li,1 Fei Ma,4 Yu Han,5 Hongjiang Song,1 Yingwei Xue1
1Department of Gastrointestinal Surgery, Harbin Medical University Cancer Hospital, Harbin Medical University, Harbin, Heilongjiang, 2Department of Pathogen Biology, School of Basic Medical Sciences, North China University of Science and Technology, Tangshan, Hebei, 3Department of General Surgery, Mudanjiang First People’s Hospital, Mudanjiang, 4Department of Breast Surgery, 5Department of Gastrointestinal Oncology, Harbin Medical University Cancer Hospital, Harbin Medical University, Harbin, Heilongjiang, People’s Republic of China
Background: Accurate and useful predictors of gastric carcinoma treated with neoadjuvant chemotherapy are lacking at present. We aim to explore the potential prognostic significance of the neutrophil-to-lymphocyte ratio (NLR) in advanced gastric cancer receiving S-1 plus oxaliplatin (SOX) or oxaliplatin and capecitabine (XELOX) regimen.
Methods: We enrolled 91 patients with advanced gastric cancer treated with neoadjuvant chemotherapy from August 2008 to September 2015. The peripheral venous blood samples were collected before neoadjuvant chemotherapy. The NLR was divided into two groups: low NLR <2.17 group and high NLR ≥2.17 group. Univariate analysis on disease-free survival (DFS) and overall survival (OS) were generated using the Kaplan–Meier method and compared using the log-rank test. Prognostic factors were assessed by univariate analyses, and the independent prognostic factors were evaluated using multivariate analysis (Cox’s proportional-hazards regression model).
Results: The univariate analysis showed that median DFS and median OS were worse for high NLR values than low NLR values before neoadjuvant chemotherapy (median DFS: 19.97 and 26.87 months, respectively, P=0.299; median OS: 25.83 and 29.73 months, respectively, P=0.405). Multivariate analysis showed that the NLR before neoadjuvant chemotherapy was not an independent prognostic factor for DFS and OS. However, median DFS and median OS were worse for high neutrophil values than for low neutrophil values (median DFS: 21.03 and 26.87 months, respectively, P=0.396; median OS: 24.43 and 29.37 months, respectively, P=0.534); for low lymphocyte values than for high lymphocyte values before neoadjuvant chemotherapy (median DFS: 22.33 and 26.87 months, respectively, P=0.624; median OS: 26.37 and 27.93 months, respectively, P=0.584). Nevertheless, patients with low NLR had better 1-year, 3-year, and 5-year DFS and OS rates.
Conclusion: NLR may serve as a cheap and convenient prognostic indicator in gastric carcinoma patients receiving SOX or XELOX neoadjuvant chemotherapy. Low NLR may help the doctors to take efficient treatment measures for gastric cancer.
Keywords: advanced gastric cancer, neoadjuvant chemotherapy, disease-free survival, overall survival, neutrophil-to-lymphocyte ratio
Introduction
Gastric carcinoma is one of the most malignant tumors, severely influencing the physical and mental health of people, and the second leading major cause of cancer-related mortality worldwide.1,2 The latest publication data from the World Health Organization (WHO) and International Arctic Research Center (IARC) indicate that the incidence of gastric cancer is 952,000 worldwide and 405,000 in China, accounting for 42.6% of the global incidence.3 In China, most of the patients are diagnosed in advanced stages of gastric carcinoma, ~10% patients are at the early stage of gastric cancer, and the 5-year survival rate is 10%–30%.4 Gastric resection with D2 lymph node dissection is the mainstay of treatment in advanced gastric carcinoma. Many randomized trials have indicated that adjuvant chemoradiotherapy has been a part of the combined modality therapy of advanced gastric cancer.5–7 Therefore, it is of significance to explore actively the potential prognostic factor in gastric cancer.
Neoadjuvant chemotherapy has, for several decades, been proven to benefit patients with advanced gastric cancer. It has been reported that neoadjuvant chemotherapy may decrease the tumor staging and volume, increase the R0 resection rate without increasing surgical morbidity and mortality compared with those undergoing surgical treatment alone.8 Nevertheless, there is no internationally generally acknowledged standard neoadjuvant chemotherapy regimen for patients with advanced gastric carcinoma. For the past few years, novel chemotherapeutics have been emerging prominently. Although there are numerous neoadjuvant chemotherapy regimens for gastric cancer treatment, the S-1 plus oxaliplatin (SOX) and oxaliplatin and capecitabine (XELOX) regimens are the commonly used.9,10 In Asia, neoadjuvant chemotherapy regimen with SOX or XELOX delivered at R0 surgical resection with D2 lymph node dissection has shown remarkably improved survival for patients with locally advanced gastric carcinoma compared to those treated with surgery alone.11 For the sake of improving the survival outcome and providing better treatment measures, it is important to find some accurate and sensitive tumor indicators.12
Although there are some immunological and histological biomarkers that may influence the prognosis of patients with gastric carcinoma, these largely depend on the primary tumor specimens and are often time consuming and expensive; this limits their use in clinical practice.13,14 Tumor and inflammation are relationships dependent on each other.15 Inflammation is an essential component of the tumor microenvironment, and the changes in inflammatory cells might influence tumor progression, such as neoplastic cell proliferation, migration, invasion, collapse of antitumor immunity, metastasis, and so forth.16,17 Tumor–inflammation interaction might represent a possible therapeutic target for cancer treatment. At the time of diagnosis and treatment, peripheral blood tests can reflect the tumor inflammatory conditions. The peripheral blood parameters, including white blood cell, neutrophil, lymphocyte, monocyte, and platelet counts, as well as the neutrophil-to-lymphocyte ratio (NLR), derived NLR (d-NLR), platelet-to-lymphocyte ratio (PLR), and monocyte-to-lymphocyte ratio (MLR), are the systemic inflammatory response indicators that have been widely proposed as prognostic factors for many malignancies.18,19
To our knowledge, the NLR has been previously identified as a useful predictive factor in gastric cancer.20,21 Nevertheless, the NLR is reported rarely in patients receiving neoadjuvant chemotherapy for advanced gastric carcinoma, especially the SOX or XELOX regimen.22,23 This study was aimed at evaluating the prognostic significance of NLR in patients with advanced gastric carcinoma receiving neoadjuvant chemotherapy such as SOX or XELOX regimen.
Materials and methods
The study was approved by the ethics committee of Harbin Medical University Cancer Hospital, Harbin Medical University, China, and complied with the standards of the Declaration of Helsinki. Prior to the research, written informed consent was obtained from all patients.
Patient selection
This retrospective analysis included data from 91 patients with stage II/III gastric carcinoma and treated with neoadjuvant chemotherapy in the Department of Gastrointestinal Surgery, Harbin Medical University Cancer Hospital, Harbin Medical University, between August 2008 and September 2015. All advanced gastric cancer cases were confirmed in accordance with pathological evidence, and the clinical stage was determined as II/III according to the tumor–node–metastasis (TNM) staging system.24 The treatment regimens of patients with advanced gastric cancer were obtained from the patients’ history. The inclusion criteria included the following: 1) patients with locally advanced gastric cancer were confirmed in accordance with pathological evidence; 2) Eastern Cooperative Oncology Group (ECOG) performance status ≥2, Karnofsky performance status (KPS) ≥80; 3) patients with life expectancy ≥3 months; 4) no previous chemotherapy, radiotherapy, targeted therapy, and so forth. Exclusion criteria included the following: 1) patients with distant metastases; 2) patients with diabetes mellitus, hypertension, atherosclerotic heart diseases, and other acute or chronic diseases; 3) patients with serious complications, such as intestinal obstruction, active bleeding, enterobrosis, and obvious infections; 4) patients having received a blood product transfusion within 1 month before neoadjuvant chemotherapy.
Treatment protocols
SOX regimen: on the first day, oxaliplatin (130 mg/m2) was administered by intravenous infusion in 500 mL of 5% glucose over a period of 2 h. From the first day to the 14th day, S-1 (60 mg, twice daily [bid]) was administered as oral (per os [po]) XELOX regimen: on the first day, oxaliplatin (130 mg/m2) was administered by intravenous infusion in 500 mL of 5% glucose over a period of 2 h. From the first day to the 14th day, capecitabine (1,500 mg, bid) was administered by the po route. A cycle of the two regimens lasted for 3 weeks.
Response evaluation
Response rates were evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines.25 The clinical response was divided into four groups: complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The clinical objective response rate was defined as CR and PR, and nonclinical response was defined as SD or PD.
Peripheral venous blood sample
Peripheral venous blood samples were taken at the time of diagnosis before neoadjuvant chemotherapy. These samples were obtained from patients with empty stomach. Hematological parameters were analyzed by XE-2100 hematology analyzer (Sysmex, Kobe, Japan).
Follow-up
After surgery, all cases were followed regularly every 3–6 months for the first and second years, every 6–12 months from the third to the fifth years, then annually and until death. The patients were followed regularly every year thereafter with laboratory tests, multislice computed tomography (CT), and gastroscopy. Disease-free survival (DFS) was defined as the time from surgery to relapse (local recurrence and distant metastases). Overall survival (OS) was defined as the time from surgery to death by any cause or the last follow-up. The last follow-up date was December 3, 2016.
Statistical analysis
The optimal cutoff levels were decided using receiver operating characteristic (ROC) curve analyses. The areas under the curve (AUCs) were used to assess the predictive value. The categorical variables were described by frequencies and percentages (%) and then compared using the chi-square test or Fisher’s exact test. The continuous variables were described by the mean value ± standard error and were compared using the Student’s t-test. Survival curves were generated using the Kaplan–Meier and log-rank tests. Univariate analyses were used to assess the prognostic factors. Multivariate analysis (Cox’s proportional-hazards regression model) was used to evaluate independent prognostic factors. Hazard ratio (HR) and 95% confidence interval (95% CI) were used as common measures to assess relative risk. These analyses were performed using SPSS software (version 17.0, SPSS Inc, Chicago, IL, USA). Alpha was set at 0.05, and P<0.05 was found to be of statistical significance.
Results
Patient demographic and clinicopathological characteristics
The AUC of NLR was 0.570, and the optimal cutoff value by ROC was 2.17. The patients were categorized into two groups: low NLR <2.17 group and high NLR ≥2.17 group. Table 1 shows the distribution of the demographic and clinicopathological characteristics of the 91 patients in the two groups divided according to the NLR. The males and females numbered 70 and 21, respectively; the median age was 57 years, with the range being 32–73 years; the median body mass index (BMI) was 22.32, and the range was 17.06–34.08. The number of patients with each of the ABO blood type (A, B, O, and AB) was 23, 32, 27, and 9, respectively. Moreover, 35 patients received the SOX regimen and 56 patients received the XELOX regimen. The radical resection cases (R0, R1, and R2) were 51, 21, and 19, respectively. All 91 patients underwent gastrectomy, 52 underwent distal gastrectomy, 6 underwent proximal gastrectomy, and 33 underwent total gastrectomy. The differentiation of tumors in these patients was as follows: 54 patients showed poorly differentiated, 32 patients moderately differentiated, and 5 patients well differentiated tumors. Tumors were located in the upper one-third (n=11), middle one-third (n=31), and the lower one-third (n=49) parts of the stomach, respectively. In terms of pathology, 64 patients had adenocarcinoma, 10 patients showed mucinous carcinoma, 12 patients had signet ring cell carcinoma, and 5 patients had other types. Of these patients, 54 were human epidermal growth factor receptor (HER)2 negative (0–+), and 37 were HER2-positive (++–+++). A median neutrophil count of 3.70 (range: 1.06–11.46), and a median lymphocyte count of 1.68 (range: 0.70–3.91). The overall response, CR, and PR were 76.9%, 5.5%, and 71.4%, respectively. A low baseline NLR (low NLR <2.17) correlated with improved demographic and clinicopathological characteristics, including age (χ2=3.963, P-value =0.046), gender (χ2=5.275, P-value =0.022), neutrophil count (χ2=26.381, P-value <0.001), and lymphocyte count (χ2=6.920, P-value =0.009).
Prognostic variables for DFS and OS
The median DFS was 23.73 months (range: 1.17–93.87 months), and the median OS was 26.87 months (range: 4.03–96.00 months) (Figures 1 and 2). The factors predicting high DFS were R0 resection, pathological N0 stage, pathological tumor in situ (Tis)/I stage. In multivariate Cox regression analysis, factors predicting improved DFS were R0 resection (P-value <0.001, HR: 3.084, 95% CI: 2.001–4.753), pathological N0 stage (P-value =0.002, HR: 4.289, 95% CI: 1.682–10.937), and pathological Tis/I stage (P-value <0.001, HR: 2.782, 95% CI: 1.829–4.233) (Table 2). Factors predicting high OS were R0 resection, pathological N0 stage, and pathological Tis/I stage. In multivariate Cox regression analysis, factors predicting high OS were R0 resection (P-value <0.001, HR: 2.494, 95% CI: 1.730–3.595), pathological N0 stage (P-value =0.002, HR: 4.263, 95% CI: 1.680–10.815), and pathological Tis/I stage (P-value <0.001, HR: 3.401, 95% CI: 1.949–4.746) (Table 3).
  | Figure 1 Disease-free survival of 91 patients with gastric cancer. |
  | Figure 2 Overall survival of 91 patients with gastric cancer. |
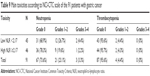
Meanwhile, we found that neutrophil count, lymphocyte count, and NLR before neoadjuvant chemotherapy had no statistical significance using the cutoff values of 3.70×109/L, 1.68×109/L, and 2.17 for DFS (P-value =0.510, 0.644, and 0.550, respectively) and OS (P-value =0.941, 0.621, and 0.625, respectively) in univariate analysis (Tables 2 and 3). However, median DFS and median OS were worse for high NLR values than for low NLR values before neoadjuvant chemotherapy (Figures 3 and 4). Nevertheless, median DFS and median OS were worse for high neutrophil values than for low neutrophil values (Figures 5 and 6) and for low lymphocyte values than for high lymphocyte values before neoadjuvant chemotherapy (Figures 7 and 8) (Table 4).
  | Figure 3 Disease-free survival in relation to NLR. |
  | Figure 4 Overall survival in relation to NLR. |
  | Figure 5 Disease-free survival in relation to neutrophil count. |
  | Figure 6 Overall survival in relation to neutrophil count. |
  | Figure 7 Disease-free survival in relation to lymphocyte count. |
  | Figure 8 Overall survival in relation to lymphocyte count. |
Tables 5 and 6 show the characteristics of the 91 patients before neoadjuvant chemotherapy: patients with low NLR and low neutrophil count had better median DFS and OS (median DFS: 26.87 and OS: 29.73 months, respectively) values; patients with low NLR and high lymphocyte count had better median DFS and OS values (median DFS: 36.93 and OS: 39.33 months, respectively).
  | Table 5 Clinical and laboratory characteristics as well as disease-free survival of the 91 patients with gastric cancer by NLR |
  | Table 6 Clinical and laboratory characteristics as well as overall survival of the 91 patients with gastric cancer by NLR |
Among the 91 patients with gastric cancer, the 1-year, 3-year, and 5-year DFS rates were 75.8%, 23.1%, and 7.7%; the 1-year, 3-year, and 5-year OS rates were 87.9%, 26.4%, and 11.0%, respectively. We also found that the patients with low NLR <2.17 had better 1-year, 3-year, and 5-year DFS and OS rates (Table 7).
  | Table 7 One-year, 3-year, and 5-year DFS and OS rates of the 91 patients with gastric cancer |
The common hematologic toxicities after neoadjuvant chemotherapy of the 91 patients with gastric cancer were National Cancer Institute Common Toxicity Criteria (NCI-CTC) grades 1 and 2 anemia, leukopenia, neutropenia, and thrombocytopenia in 33/91 (36.3%), 18/91 (19.8%), 21/91 (23.1%), and 4/91 (4.4%), respectively (Tables 8 and 9). We found that NLR before neoadjuvant chemotherapy had no significance on toxicities of the 91 patients with gastric cancer using the cutoff value of 2.17 (Tables 8 and 9).
Discussion
Gastric carcinoma is one of the common types of malignant tumors worldwide. Although mortality and morbidity rates of gastric carcinoma have declined over the past several decades, the disease still has a poor prognosis and leads to hundreds of thousands of deaths annually.26 Over the past 2 decades, multiple therapies, including radiotherapy, adjuvant chemotherapy, neoadjuvant chemotherapy, perioperative chemotherapy, and targeted therapy, have improved survival and quality of life for these gastric cancer patients.27
Cancer and inflammation are closely connected and have implications for prevention and treatment. Inflammation contributes to tumor proliferation, migration, invasion, angiogenesis, and so forth.28,29 Various studies have suggested that a systemic inflammatory response is associated with a poor prognosis in many malignancies.30–35 Using the cellular components of a systemic inflammatory response in peripheral venous blood for predicting survival has received increased attention. Nevertheless, the mechanisms are still ambiguous and poorly understood. Clinical and epidemiological studies have shown the connection between gastric carcinoma and inflammation.36 There is growing interest in a clinical interpretation of the relation between inflammation and tumor cells, resulting in the establishment of novel biomarkers of cancer to evaluate the prognostic significance. For selecting the optimal treatment regimen for individuals, accurate and useful predictors are needed. To our knowledge, the association of NLR values with DFS and OS in patients receiving neoadjuvant chemotherapy with SOX or XELOX regimen has been studied rarely.
We analyzed the relationship between NLR and clinicopathological characteristics in patients with advanced gastric carcinoma. We found that low NLR correlated with improved demographic and clinicopathological characteristics, including age, gender, neutrophil count, and lymphocyte count. In univariate and multivariate Cox regression analyses, factors predicting improved DFS and OS were R0 resection, pathological N0 stage, and pathological Tis/I stage. We also found that the neutrophil count, lymphocyte count, and NLR had no significance using the cutoff values of 3.70×109/L, 1.68×109/L, and 2.17 for DFS and OS in univariate analysis. However, median DFS and median OS were worse for high NLR values than for low NLR values before neoadjuvant chemotherapy (median DFS: 19.97 and 26.87 months; median OS: 25.83 and 29.73 months), and this is consistent with the results of other authors.37–39 At the same time, median DFS and median OS were worse for high neutrophil values than for low neutrophil values (median DFS: 21.03 and 26.87 months; median OS: 24.43 and 29.37 months), as well as for low lymphocyte values than for high lymphocyte values before neoadjuvant chemotherapy (median DFS: 22.33 and 26.87 months; median OS: 26.37 and 27.93 months).
Meanwhile, we found that the patients with low NLR and low neutrophil count had better median DFS and OS (median DFS: 26.87 and OS: 29.73 months); the patients with low NLR and high lymphocyte count had better median DFS and OS (median DFS: 36.93 and OS: 39.33 months). A study by Eo et al40 suggested that low monocyte count and high lymphocyte count had better 5-year DFS and OS rates. We also found that the 1-year, 3-year, and 5-year DFS and OS rates in the low NLR group were higher than in the high NLR group. Although the NLR values before neoadjuvant chemotherapy lost their independent prognostic significance for DFS or OS, they still provided fundamental information for clinical practice. Jin et al41 reported that NLR had statistical significance with PFS, but not with OS, in multivariate analysis. Another study by el Aziz38 suggested that NLR lost its independent prognostic significance for PFS, but had significance with OS, in multivariate analysis.
Various studies have indicated that elevated NLR is associated with poor survival in many tumors, and the association has not been clear.42–45 The tumor microenvironment inhabited by inflammatory cells is important in carcinogenesis, promoting cancer growth, invasion, tumor cell proliferation, and migration.46 The neutrophils inhibit the immune system via restraining the cytolytic activity of immune cells and influence the tumor environment, thus probably contributing to stimulating tumor angiogenesis and progression.47 The lymphocyte is known to play a significant role in defense of tumor cells by inducing cytotoxic cell death and suppressing tumor cell proliferation and migration.48 Furthermore, patients with lymphocyte infiltration may have a better prognosis than those with no infiltration.49 In the development of gastric cancer, chronic inflammation may be caused by Helicobacter pylori, and it may be a critical risk factor for gastric carcinoma.50 The mechanism of NLR responses to tumors may be explained as decrease in the number of lymphocytes and increase in the number of neutrophils. Hence, the NLR, calculated based on both the neutrophil and the lymphocyte counts, may be a good marker reflecting the degree of tumor progression and predict prognosis.
To the best of our knowledge, the significance of NLR values’ association with DFS and OS in gastric carcinoma receiving neoadjuvant chemotherapy is reported rarely. This research suggests that the NLR may be used in the prediction of prognosis in advanced gastric carcinoma. It is crucial to take into consideration the high gastric cancer morbidity and unbalanced medical condition in China, and thus, these cheap, noninvasive, and convenient biomarkers may be beneficial with regard to the prevention and treatment of gastric cancer. Therefore, better understanding of hematologic parameters can help identify new targets for individual treatment. Thus, this study may provide important information for clinical practice.
In summary, SOX and XELOX regimens were well tolerated by all patients who received it. Our study explains the reason for the NLR enhancing tumor progression, and low NLR may be a more favorable prognostic factor. The differences in the cutoff values of NLR among these studies may be attributable to the differences in the cumulative number of patients and the disease stage among the studies. Whether the cutoff value of 2.17 for NLR used in our study is correct requires further investigation.
Conclusion
It is believed that the NLR may be a cheap and convenient prognostic indicator in gastric carcinoma patients receiving neoadjuvant chemotherapy. Low NLR may help doctors to take efficient treatment measures for gastric cancer. However, more studies are needed to assess changes in inflammatory markers in larger groups of patients with advanced gastric carcinoma.
Disclosure
The authors report no conflicts of interest in this work.
References
Schuhmacher C, Reim D, Novotny A. Neoadjuvant treatment for gastric cancer. J Gastric Cancer. 2013;13(2):73–78. | ||
Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. | ||
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. | ||
Mackenzie M, Spithoff K, Jonker D. Systemic therapy for advanced gastric cancer: a clinical practice guideline. Curr Oncol. 2011;18(4):e202–e209. | ||
Manoharan V, Lee S, Chong S, et al. Serial imaging using [18F] Fluorodeoxyglucose positron emission tomography and histopathologic assessment in predicting survival in a population of surgically resectable distal oesophageal and gastric adenocarcinoma following neoadjuvant therapy. Ann Nucl Med. 2017;31(4):315–323. | ||
Li S, Li B, Wang J, et al. Identification of sensitivity predictors of neoadjuvant chemotherapy for the treatment of adenocarcinoma of gastroesophageal junction. Oncol Res. 2017;25(1):93–97. | ||
Neves Filho EH, de Sant’Ana RO, Nunes LV, Pires AP, da Cunha MD. Histopathological regression of gastric adenocarcinoma after neoadjuvant therapy: a critical review. APMIS. 2017;125(2):79–84. | ||
Park SC, Chun HJ. Chemotherapy for advanced gastric cancer: review and update of current practices. Gut Liver. 2013;7(4):385–393. | ||
Wang X, Wang ML, Zhou LY, Lu XY, Yang JF, Yu HG. Randomized phaseII study comparing paclitaxel with S-1 vs. S-1 as first-line treatment in patients with advanced gastric cancer. Clin Transl Oncol. 2013;15(10):836–842. | ||
Bang YJ, Kim YW, Yang HK, et al. CLASSIC trial investigators. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379(9813):315–321. | ||
Quéro L, Guillerm S, Hennequin C. Neoadjuvant or adjuvant therapy for gastric cancer. World J Gastrointest Oncol. 2015;7(8):102–110. | ||
Pang W, Lou N, Jin C, et al. Combination of preoperative platelet/lymphocyte and neutrophil/lymphocyte rates and tumor-related factors to predict lymph node metastasis in patients with gastric cancer. Eur J Gastroenterol Hepatol. 2016;28(5):493–502. | ||
Kim KH, Kwon HC, Oh SY, et al. Clinicopathological significance of ERCC1, thymidylate synthase and glutathione S-transferase P1 expression for advanced gastric cancer patients receiving adjuvant 5-Fu and cisplatin chemotherapy. Biomarkers. 2011;16(1):74–82. | ||
Shirai O, Ohmiya N, Taguchi A, et al. P53, P21 and P73 gene polymorphisms in gastric carcinoma. Hepatogastroenterology. 2010;57(104):1595–1601. | ||
Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–1081. | ||
Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. | ||
Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. | ||
Borsig L, Wolf MJ, Roblek M, Lorentzen A, Heikenwalder M. Inflammatory chemokines and metastasis-tracing the accessory. Oncogene. 2014;33(25):3217–3224. | ||
Feng F, Sun L, Zheng G, et al. Low lymphocyte-to-white blood cell ratio and high monocyte-to-white blood cell ratio predict poor prognosis in gastric cancer. Oncotarget. 2017;8(3):5281–5291. | ||
Lian L, Xia YY, Zhou C, et al. Application of platelet/lymphocyte and neutrophil/lymphocyte ratios in early diagnosis and prognostic prediction in patients with resectable gastric cancer. Cancer Biomark. 2015;15(6):899–907. | ||
Wang SC, Chou JF, Strong VE, et al. Pretreatment neutrophil to lymphocyte ratio independently predicts disease-specific survival in resectable gastroesophageal junction and gastric adenocarcinoma. Ann Surg. 2016;263(2):292–297. | ||
Kunisaki C, Takahashi M, Ono HA, et al. Inflammation-based prognostic score predicts survival in patients with advanced gastric cancer receiving biweekly docetaxel and S-1 combination chemotherapy. Oncology. 2012;83(4):183–191. | ||
Goh BK, Chok AY, Allen JC Jr, et al. Blood neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios are independent prognostic factors for surgically resected gastrointestinal stromal tumors. Surgery. 2016;159(4):1146–1156. | ||
Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17(12):3077–3079. | ||
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RESIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. | ||
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. | ||
Brenkman HJ, Haverkamp L, Ruurda JP, van Hillegersberg R. Worldwide practice in gastric cancer surgery. World J Gastroenterol. 2016;22(15):4041–4048. | ||
Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493–e503. | ||
Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. | ||
Koh CH, Bhoo-Pathy N, Ng KL, et al. Utility of pre-treatment neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as prognostic factors in breast cancer. Br J Cancer. 2015;113(1):150–158. | ||
Stotz M, Pichler M, Absenger G, et al. The preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancer. Br J Cancer. 2014;110(2):435–440. | ||
Tanoglu A, Karagoz E, Yiyit N, Berber U. Is combination of neutrophil to lymphocyte ratio and platelet lymphocyte ratio a useful predictor of postoperative survival in patients with esophageal squamous cell carcinoma? Onco Targets Ther. 2014;7:433–434. | ||
Gu L, Ma X, Li H, et al. Prognostic value of preoperative inflammatory response biomarkers in patients with sarcomatoid renal cell carcinoma and the establishment of a nomogram. Sci Rep. 2016;6:23846–23856. | ||
Xia W-K, Liu Z-L, Shen D, Lin Q-F, Su J, Mao W-D. Prognostic performance of pre-treatment NLR and PLR in patients suffering from osteosarcoma. World J Surg Oncol. 2016;14:127–135. | ||
Bagante F, Tran TB, Postlewait LM, et al. Neutrophil-lymphocyte and platelet-lymphocyte ratio as predictors of disease specific survival after resection of adrenocortical carcinoma. J Surg Oncol. 2015;112(2):164–172. | ||
Lochhead P, El-Omar EM. Gastric cancer. Br Med Bull. 2008;85:87–100. | ||
Kim JH, Han DS, Bang HY, Kim PS, Lee KY. Preoperative neutrophil-to-lymphocyte ratio is a prognostic factor for overall survival in patients with gastric cancer. Ann Surg Treat Res. 2015;89(2):81–86. | ||
el Aziz LM. Blood neutrophil–lymphocyte ratio predicts survival in locally advanced cancer stomach treated with neoadjuvant chemotherapy FOLFOX 4. Med Oncol. 2014;31(12):311–316. | ||
Lee S, Oh SY, Kim SH, et al. Prognostic significance of neutrophil lymphocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with FOLFOX chemotherapy. BMC Cancer. 2013;13:350. | ||
Eo WK, Jeong DW, Chang HJ, et al. Absolute monocyte and lymphocyte count prognostic score for patients with gastric cancer. World J Gastroenterol. 2015;21(9):2668–2676. | ||
Jin H, Zhang G, Liu X, et al. Blood neutrophil-lymphocyte ratio predicts survival for stages III-IV gastric cancer treated with neoadjuvant chemotherapy. World J Surg Oncol. 2013;11:112–122. | ||
Lee SM, Russell A, Hellawell G. Predictive value of pretreatment inflammation-based prognostic scores (neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and lymphocyte-to-monocyte ratio) for invasive bladder carcinoma. Korean J Urol. 2015;56(11):749–755. | ||
Jiang C, Hu WM, Liao FX, et al. Elevated preoperative neutrophil-to-lymphocyte ratio is associated with poor prognosis in gastrointestinal stromal tumor patients. Onco Targets Ther. 2016;9:877–883. | ||
Kim SH, Lee HW, Go SI, Lee SI, Lee GW. Clinical significance of the preoperative platelet count and platelet-to-lymphocyte ratio (PLT-PLR) in patients with surgically resected non-small cell lung cancer. Oncotarget. 2016;7(24):36198–36206. | ||
Ho CL, Lu CS, Chen JH, Chen YG, Huang TC, Wu YY. Neutrophil/lymphocyte ratio, lymphocyte/monocyte ratio, and absolute lymphocyte count/absolute monocyte count prognostic score in diffuse large B-cell lymphoma: useful prognostic tools in the rituximab era. Medicine (Baltimore). 2015;94(24):e993. | ||
Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13(11):759–771. | ||
Jung MR, Park YK, Jeong O, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts poor survival following resection in late stage gastric cancer. J Surg Oncol. 2011;104(5):504–510. | ||
Milne K, Alexander C, Webb JR, et al. Absolute lymphocyte count is associated with survival in ovarian cancer independent of tumor-infiltrating lymphocytes. J Transl Med. 2012;10:33. | ||
Kim JW, Nam KH, Ahn SH, et al. Prognostic implications of immunosuppressive protein expression in tumors as well as immune cell infiltration within the tumor microenvironment in gastric cancer. Gastric Cancer. 2016;19(1):42–52. | ||
Matsumoto Y, Marusawa H, Kinoshita K, et al. Helicobacter pylori infection triggers aberrant expression of activation-induced cytidine deaminase in gastric epithelium. Nat Med. 2007;13(4):470–476. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.