Back to Journals » Clinical, Cosmetic and Investigational Dentistry » Volume 12
Peripheral Adenomatoid Odontogenic Tumor — A Rare Cause of Gingival Enlargement: A Case Report with CBCT Findings
Authors Sadasivan A , Ramesh R , Kurien NM
Received 6 May 2020
Accepted for publication 26 June 2020
Published 21 July 2020 Volume 2020:12 Pages 297—304
DOI https://doi.org/10.2147/CCIDE.S261308
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Christopher E. Okunseri
Arun Sadasivan,1 Roshni Ramesh,2 Nikhil M Kurien3
1Department of Periodontics, Sree Mookambika Institute of Dental Sciences, Kulashekaram, Tamil Nadu, India; 2Department of Periodontics, Government Dental College, Thrissur, Kerala, India; 3Department of Oral and Maxillofacial Surgery, PMS College of Dental Science and Research, Trivandrum, Kerala, India
Correspondence: Arun Sadasivan
Department of Periodontics, Sree Mookambika Institute of Dental Sciences, Kulashekaram, Tamil Nadu, India
Tel +91 9847246961
Email [email protected]
Introduction: Adenomatoid odontogenic tumor (AOT) is an uncommon benign odontogenic lesion with varied clinical and histological presentation. It has slow growth potential and a low recurrence rate. The tumor is mainly seen in females in the second decade of life, predominantly affecting the maxilla and associated most often with unerupted canine teeth, earning the epithet “two-thirds tumor”. There are three variants: intrafollicular, extrafollicular, and peripheral. The peripheral or extra osseous type is a rare form that arises in gingival tissue.
Case Presentation: This article describes a case of AOT in a 10-year-old girl who presented with gingival enlargement in relation to the maxillary left central incisor. Interestingly, intraoral periapical radiography did not show any significant findings. However, cone-beam computed tomography of the site revealed significant bone loss in the area. A surgical excision was done. Histopathological examination revealed features of AOT. Based on clinical, radiographic, and histological evidence, a diagnosis of peripheral AOT (PAOT) was made.
Conclusion: PAOT is a rare disease entity in children that mimics gingival swelling, and may often be misdiagnosed by dentists. With literature still ambiguous on the origin of the tumor and biological course, it becomes imperative to examine any gingival swelling in children with a proper clinical examination, periapical radiography, and if necessary cone-beam computed tomography. Excision and histopathological evaluation will help in confirming the exact disease condition.
Keywords: peripheral adenomatoid odontogenic tumour, gingival enlargement, maxillary, CBCT
Introduction
Adenomatoid odontogenic tumor (AOT) is a relatively rare, slowly growing benign epithelial tumor of odontogenic origin that has been known by varied nomenclature, such as adenoameloblastoma, ameloblastic adenomatoid tumor, epithelioma adenomatoid, adamantinoma, or teratomatous odontoma. It was in 1969 that Philipsen and Birn coined the term “adenomatoid odontogenic tumor”.1,2 This terminology was included in the World Health Organization (WHO) histological typing of odontogenic tumors, jaw cysts, and allied lesions in 1971. The 1971 WHO classification had reservations in terming AOT a neoplasm; however, in 2005 the WHO redefined AOT as a tumor composed of the odontogenic epithelium, presenting with a variety of histoarchitectural patterns, embedded in mature connective tissue stroma, and characterized by slow and progressive growth.3,4
AOT was classified as a benign epithelial odontogenic tumor in the 2017 WHO histological classification of odontogenic and maxillofacial bone tumors.5 It is a relatively rare odontogenic neoplasm, accounting for about 2.3%−13% of all odontogenic tumors.6–10 However, in a retrospective study on the clinical and epidemiological profile of AOT, Philipsen et al in 2007 analysed 1,082 cases of AOT and reported the relative frequency of AOT to be 0.6%–38.5%.1 AOT is thought to originate from the remnants of the dental lamina or enamel organ.11,12 It is also known as “two-thirds tumor”, as two-thirds of AOTs occur in the maxilla, two-thirds in young females, two-thirds of cases are associated with impacted teeth, and two-thirds of the teeth affected are canines.13,14
There are three clinicopathological variants of AOT: follicular, extrafollicular, and peripheral. The follicular and extrafollicular variants account for almost 97.7% of all AOTs, and present as intrabone tumors.1 Another variant is peripheral manifestation, which originates at a distance from the tooth germ and is rarely seen. Peripheral AOT (PAOT) presents as a gingival growth with a significant predominance in females, is seen more in the anterior maxilla, and has primary involvement of incisors. The peripheral type is the rarest of these tumors, constituting only 2.3% of all AOTs.1 PAOT demonstrates similar histological characteristics to their intraosseous counterparts, but occur solely in the soft tissue covering the tooth-bearing portion of the jaws. They are also known by other terms, such as extraosseous odontogenic tumors, soft-tissue odontogenic tumors, or odontogenic tumors of the gingiva.15,16
Radiography in follicular AOT shows a well-defined, circumscribed, and unilocular radiolucency associated with unerupted teeth, often mimicking a dentigerous cyst. The extrafollicular variant presents a well-defined unilocular radiolucency around or between the roots of erupted teeth, with varying degrees of calcification.17 Since PAOT occurs in the soft tissue covering the tooth-bearing bone, radiography may be inconclusive. In some cases, resorption of the cortical bone or displacement of teeth can be seen.18,19 The histopathological picture of AOT is of epithelial cells arranged in strands of spindle-shaped cells in a whorl-like manner, as well as cuboidal epithelial cells arranged in a duct-like pattern. Sometimes, calcification may be present as non-specific or cementum-like globules or amyloid-like homogeneous eosinophilic material. The tumor is usually surrounded by a well-developed connective-tissue capsule, and may present as a solid mass or as numerous small cystic spaces.12,20 As AOTs are most often encapsulated, it makes it easy to separate the lesion from adjacent anatomic structures, and thus the treatment of choice is conservative surgical removal through either simple curettage or enucleation. Recurrence has been described, but it is exceedingly rare.21
In accordance with the CARE (compassion to self and others, adaptability, resourcefulness, and emotional well-being) criteria, we report a case of PAOT in the anterior region of the maxilla in a 10-year-old female child. The clinical examination and radiographic features are described. The lesion was surgically removed, and histopathological examination confirmed the diagnosis of PAOT, which is a rare variant of AOT.
Case Presentation
A 10-year-old female patient reported to a private dental clinic with a chief complaint of a slowly growing, painless swelling of 6 months’ duration in the maxillary anterior region. On general examination, she was apparently healthy. Medical and family history did not reveal anything of significance. On extraoral examination, no abnormalities were detected.
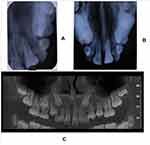
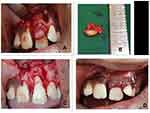
Intraoral examination revealed a solitary firm nontender enlargement (1×0.5 cm) of marginal and attached gingiva covering the cervical third of the tooth in relation to a palatally displaced 21 (Figure 1). It was a slowly growing swelling. The colour of the lesion was pale pink. The lesion was asymptomatic with no pain, bleeding on probing, or pus exudation. There were deep periodontal pockets (>10 mm) in the buccal aspect of 21. Intraoral periapical and occlusal radiographs did not reveal any significant findings (Figure 2).
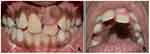 |
Figure 1 (A) Labial view of gingival enlargement in relation to maxillary left central incisor (21) (B) Gingival enlargement has displaced 21 palatally. |
Based on the clinical findings of deep pockets and with radiography showing no findings, it was decided to conduct cone-beam computed tomography (CBCT) of the region to get a three-dimensional view of the lesion. CBCT revealed a well-defined corticate radiolucency on the buccal aspect of 21 (Figure 3). The lesion extended from the alveolar crest up to the middle third of the root. It displaced tooth 21 palatally. The superior margin of the lesion was connected to the alveolar crest. There was thinning and expansion of the buccal cortical plate. No abnormality was seen in relation to the crown of 21. A single root with single-root canal and partially closed apex was noted. No evidence of fracture line or lateral canal was noted in relation to 21. Based on the clinical and radiological findings, a differential diagnosis of pyogenic granuloma, fibroma, peripheral ossifying fibroma, or peripheral giant-cell granuloma was made and excisional biopsy of the lesion planned. Routine blood examinations were done, which were within normal limits. The lesion was carefully enucleated and curettage done under local anesthesia (Figure 4). The tissue was sent for histopathological examination.
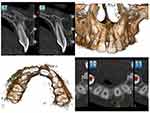 |
Figure 3 CBCT images showing the bone destruction and cortical plate expansion in relation to labial aspect of 21. |
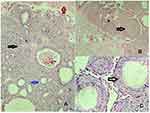
Microscopy analysis showed nodular proliferation of the odontogenic epithelium in nests, whorls, and strands. A few duct-like structures of varying size lined with columnar cells with an eosinophilic rim inside and basophilic inhomogeneous material within the lumen were noted. Trabecular patterns with numerous small ovoid and larger irregular basophilic calcifications and eosinophilic material between spindle and polygonal cells, as well as in the duct-like structures, were seen (Figure 5). Based on the clinical, radiological and histological findings, a confirmatory diagnosis of PAOT was established. The patient was recalled periodically and follow-up examination done till 18 months. Within this period, there was no recurrence of the lesion (Figure 6)
 |
Figure 6 (A) Preoperative clinical picture.; (B) 6 months after surgery; (C) 18 months after surgery. |
Discussion
Ide et al described AOT as a successional tooth-associated lesion that originates during the mixed-dentition stage of children.22 In 2019, Chrcanovic and Gomez did an analysis of 436 publications available in the literature reporting 1,558 cases of AOT, and reported that AOTs were more prevalent in women than men (1.9:1). They reported the mean age of patients as 19±9 years (range 1–82 years). The lesions were more prevalent in the maxilla when compared to the mandible, and at the anterior region in comparison with the posterior region.21 The most commonly thought origin of AOT is said to be from remnants of the dental lamina or enamel organ.11,12 In 2016, Gomes et al reported KRAS p.G12V mutations in a small cohort of AOT cases. However, further research on this somatic mutation is necessary to prove a neoplastic nature for this tumor.23 A strong cytoplasmic expression of β-catenin has been reported in AOT, even though no molecular anomaly has been reported with exon 3 of the CTNNB1 (β-catenin) gene.24 Further studies are needed to confirm the role of specific regulation of β-catenin in the pathogenesis of AOT.
Among the three types of AOT, the peripheral variant is very rare and accounts for only 2.3% of all AOTs. It shows a female predilection of occurrence when compared to males (6.3:1).1 The peak age of incidence is said to be 12 (9–17) years.25 PAOT is found most frequently (88%) in the anterior maxillary gingiva.7,21 In a review of 20 cases of PAOT in 2019, Dwivedi et al reported a preponderance (85% of cases) of anterior maxillary presentation, with age of occurrence 4–27 years and a majority (70%) of cases seen in the second decade. The lesions were more common in females than males (1.85:1). With regard to teeth involved, almost all cases (90%) showed incisor involvement.26 PAOTs occur in soft tissue, which overlays the tooth-bearing areas of jaws. They typically manifest as gingival swellings and have an identical histological presentation as intraosseous lesions.27 PAOT presents as a painless gingival swelling, which often results in a delay in patients seeking treatment. In their review, Chrcanovic and Gomez noted that the lesions were noticed by the patients a mean 13.9±27.5 months (range 0–444 months) before seeking treatment.21 In the present case, too, the findings were similar. A female patient aged 10 years presented with a lesion in the maxillary left central incisor region of a duration of 6 months.
The etiopathogenesis of PAOT is said to be derived from the odontogenic epithelium and its remnants.25 These epithelial remnants are thought to proliferate in response to an unknown stimulus resulting in the lesion.28 PAOT does not present any pathognomonic radiographic findings, but has been reported to present with bone loss, erosion of the labial plate, and deep periodontal pockets.1,17 AOTs are predominantly seen in the anterior maxilla, and for this, conventional radiographic techniques like intraoral periapical radiography and the panoramic technique have limited radiographic interpretation of lesions. Similarly, intralesional calcifications with characteristic patterns, which are a distinctive radiographic feature of AOT, are often missed on conventional radiography. The advantage of CBCT radiography is the availability of multiplanar cross-sectional images in various orientations and an ability to do a three- dimensional reconstruction using a single scan of views of interest. CBCT thus shows a three-dimensional perspective of AOT lesions, shape of tumors, detailed spatial relationship of lesions with adjacent structures, and distribution patterns of radiopaque calcifications, which are critical for radiographic diagnosis of AOT.29 Janavi et al used CBCT in a case of maxillary PAOT, and observed numerous small specks of calcification scattered along the lesion periphery.30 In our case, the lesion was seen as a gingival enlargement attached to the buccal gingiva of the maxillary left central incisor (21) and with palatal displacement of 21. The interesting aspect of this case was that conventional radiographic views failed to show any radiolucent changes, as the cortical bone destruction was on the buccal aspect and only in CBCT images was the severity of the lesion noticeable and cortical expansion seen.
AOT microscopically presents a range of unique and distinctive features. The tumor is delimited by a fibrous capsule that is usually well developed. The primary tumor cells are polygonal, cuboidal, or sometimes spindle-shaped epithelial cells arranged in a characteristic variety of histomorphological patterns.7 They can form duct-like spaces of varying diameter, but this pattern is not predominantly seen. The ducts are lined by a single layer of cuboidal or columnar epithelial cells that have nuclei frequently polarized away from the lumen. Anastomosing strands of basaloid epithelial cells that resemble cell rests of the dental lamina and are arranged in a plexiform, trabecular, or lattice-like configuration are seen. Amorphous homogeneous material that is eosinophilic is usually seen in the core of these rosettes.31 Darkly stained dystrophic calcifications in minute amounts are also observed in histological sections. The presence of cystic areas in AOTs mimicking odontogenic cysts has been reported in the literature.32 A minimal mature connective-tissue stroma is another characteristic feature of AOT, which is often loosely structured and contains thin-walled congested vessels with peripheral hyalinization.32 The histopathology of PAOT is similar to the central variants, with characteristic rosette patterns of odontogenic epithelial cells, duct-like structures lined with cuboidal or columnar cells, and the presence of diffuse calcifications. PAOTs do not usually show encapsulation like the central variants.27,33 The histological findings in this case were consistent with those reported in the literature. The gingival enlargement seen in PAOT can easily be mistaken for other gingival lesions like pyogenic granuloma, peripheral ossifying fibroma, and peripheral giant-cell granuloma. A definitive diagnosis is made only after histopathological examination.
Conservative surgical enucleation and curettage of the lesion is the treatment of choice, because the tumor is well encapsulated and shows benign biological behavior.21 In our case, the lesion was completely enucleated and the defect area curetted. Recurrence of the tumor is very rare, and prognosis is excellent. This case was followed up for 18 months, and no recurrence of the lesion has been seen. It was also seen that the malpositioned maxillary lateral incisor (21) was now correctly positioned in the arch without any orthodontic intervention. Therefore, in this case the timely diagnosis and intervention helped in limiting the destruction of bone that was occurring and was able to correct the malalignment of tooth.
The exact categorisation and nomenclature of the lesion in our case presents a problem. It presented as a gingival swelling, but with CBCT evidence of extensive bone destruction and cortical plate expansion of labial bone. As such, the reported case showed features of both intraosseous and peripheral forms of AOT. The question arises as to whether this was an intrafollicular or extrafollicular AOT of intraosseous origin and assumed a peripheral position during the eruption of the affected tooth or whether it was a peripheral lesion from the beginning. Therefore, we consider this case as one with unidentifiable origin, categorized by Philipsen et al as the hybrid variant.13,14 Similar cases have been reported by Jham et al and Lavanya et al.17,34 Philipsen and Reichart described the PAOT type as one that occurs extraosseously and often manifests as a gingival epulis or fibroma. They also mentioned that this variant may produce erosion of bone.7 We have thus categorized our case PAOT, based on the clinical, radiographic, and histological evidence obtained. The dilemma regarding the categorization of PAOT continues, and could be resolved as more cases are reported and a consensus evolves.
Conclusion
In this case report, we would like to note that a simple gingival enlargement may mask a severe condition in a young child. Therefore, thorough clinical and radiographic examination is a necessity for every gingival enlargement. CBCT can be used when normal radiography fails to provide the necessary diagnostic information. This can definitely improve diagnostic accuracy. This in turn can help the clinician to make informed decisions to formulate better treatment plans to get optimal clinical outcomes.
Abbreviations
AOT, adenomatoid odontogenic tumor; CBCT, cone-beam computed tomography;PAOT, peripheral AOT; WHO, World Health Organization.
Consent for Publication
The authors certify that they obtained written informed consent from the child’s father while treating the child in the private dental clinic. In the form, the child and her parents were told about the disease condition and treatment options available explained to them, and asked for consent for clinical details and images to be reported in journal. They understand that her name will not be published and due efforts will be made to conceal her identity. Approval from Kailas Super Speciality Dental Clinic and Implant Centre, Kollam, Kerala, India was obtained for publishing the case details.
Disclosure
The authors declare that they have no competing interests.
References
1. Philipsen HP, Reichart PA, Siar CH, et al. An updated clinical and epidemiological profile of the adenomatoid odontogenic tumour: a collaborative retrospective study. J Oral Pathol Med. 2007;36(7):383–393. doi:10.1111/j.1600-0714.2007.00536.x.
2. Ide F, Muramatsu T, Ito Y, et al. An expanded and revised early history of the adenomatoid odontogenic tumor. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115(5):646–651. doi:10.1016/j.oooo.2013.01.023
3. Pindborg JJ, Kramer IRH. WHO Histological Typing of Odontogenic Tumours, Jaw Cysts and Allied Lesions.
4. Soluk-Tekkeşin M, Wright JM. The World Health Organization classification of odontogenic lesions: a summary of the changes of the 2017 (4th) Edition. Turk Patoloji Derg. 2018;34(1). doi:10.5146/tjpath.2017.01410
5. Speight PM, Takata T. New tumour entities in the 4th edition of the World Health Organization classification of head and neck tumours: odontogenic and maxillofacial bone tumours. Virchows Arch. 2018;472:331–339. doi:10.1007/s00428-017-2182-3
6. Lawal AO, Adisa AO, Olusanya AA. Odontogenic tumours: a review of 266 cases. J Clin Exp Dent. 2013;5(1):e13–7.
7. Philipsen HP, Reichart PA. Adenomatoid odontogenic tumour: facts and figures. Oral Oncol. 1999;35(2):125–131. doi:10.1016/S1368-8375(98)00111-0
8. Lu Y, Xuan M, Takata T, et al. Odontogenic tumours: A demographic study of 759 cases in Chinese population. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86(6):707–714. doi:10.1016/S1079-2104(98)90208-6
9. Arotiba JT, Ogunbiyi JO, Obiechina AE. Odontogenic tumours: a 15-year review from Ibadan, Nigeria. Br J Oral Maxillofac Surg. 1997;35:363–367. doi:10.1016/S0266-4356(97)90411-3
10. Okada H, Yamamoto H, Tilakaratne WM. Odontogenic tumours in Sri Lanka: analysis of 226 cases. J Oral Maxillofac Surg. 2007;65:875–882. doi:10.1016/j.joms.2006.06.293
11. Friedrich R, Zustin J, Scheuer H. Adenomatoid odontogenic tumour of the mandible.. Anticancer Res. 2010;30(5):1787–1792.
12. Seo WG, Kim CH, Park HS, Jang JW, Chung WY. Adenomatoid odontogenic tumor associated with an unerupted mandibular lateral incisor: a case report. J Korean Assoc Oral Maxillofac Surg. 2015;41:342–345. doi:10.5125/jkaoms.2015.41.6.342
13. Philipsen HP, Samman N, Ormiston IW, Wu PC, Reichart PA. Variants of adenomatoid odontogenic tumour with a note on tumour origin. J Oral Pathol Med. 1992;21:348–352. doi:10.1111/j.1600-0714.1992.tb01363.x
14. Reichart PA, Philipsen HP. Odontogenic Tumours and Allied Lesions. United Kingdom: Quintessence Publishing Co. Ltd; 2004:105–115.
15. Barnes L, Eveson JW, Reichart P, Sidransky D. World Health Organization Classification of Tumours: Pathology and Genetics, Head and Neck tumours.Lyon. IARC Press; 2005.
16. de Matos FR, Nonaka CF, Pinto LP, de Souza LB, de Almeida Freitas R. Adenomatoid odontogenic tumor: retrospective study of 15 cases with emphasis on histopathologic features. Head Neck Pathol. 2012;6(4):430–437. doi:10.1007/s12105-012-0388-x
17. Jham BC, Passos JB, et al. Adenomatoid odontogenic tumor originated in the periodontal ligament. Oral Oncol Extra. 2006;42:268–271. doi:10.1016/j.ooe.2006.05.003
18. Philipsen HP, Nikal H. Adenomatoid odontogenic tumor. Pathology and Genetics of Head and Neck Tumours. Lyon: IARC Press. 2005:304–305
19. Yilmaz N, Acikgoz A, Celebi N, Zengin AZ, Gunhan O. Extrafollicular adenomatoid odontogenic tumor of the mandible: report of a case. Eur J Dent. 2009;3:71–74.
20. Lee SK, Kim YS. Current concepts and occurrence of epithelial odontogenic tumours: I ameloblastoma and adenomatoid odontogenic tumor Korean J Pathol. 2013;47:191–202.
21. Chrcanovic BR, Gomez RS. Adenomatoid odontogenic tumor: an updated analysis of the cases reported in the literature. J Oral Pathol Med. 2019;48:10–16. doi:10.1111/jop.12783
22. Ide F, Mishima K, Kikuchi K, et al. Development and growth of adenomatoid odontogenic tumor related to formation and eruption of teeth. Head Neck Pathol. 2011;5:123–132.
23. Gomes CC, de Sousa SF, de Menezes GH, et al. Recurrent KRAS G12V pathogenic mutation in adenomatoid odontogenic tumours. Oral Oncol. 2016;56:
24. Harnet JC, Pedeutour F, Raybaud H, Ambrosetti D, Fabas T, Lombardi T. Immunohistological features in adenomatoid odontogenic tumor: review of the literature and first expression and mutational analysis of β-catenin in this unusual lesion of the jaws. J Oral Maxillofac Surg. 2013;71:706–713. doi:10.1016/j.joms.2012.10.006
25. Kearns GJ, Smith R. Adenomatoid odontogenic tumour: an unusual case of gingival swelling of a 3-year old patient. Br Dent J. 1996;181:380–382. doi:10.1038/sj.bdj.4809264
26. Dwivedi D, Prabhakar N, Kasetty S, Ahuja R. Peripheral adenomatoid odontogenic tumor in a cloak of an epulis: report of a rare case. BMC Oral Health. 2019;19:81. doi:10.1186/s12903-019-0759-8.
27. Curran AE. Peripheral odontogenic tumours. Oral Maxillofac Surg Clin North Am. 2004;16:399–408. doi:10.1016/j.coms.2004.03.008
28. Motamedi MH, Shafeie HA, Azizi T. Salvage of an impacted canine associated with an adenomatoid odontogenic tumour: a case report. Brit Dent J. 2005;199:89–90. doi:10.1038/sj.bdj.4812522
29. Jiang M, You M, Wang H, Xu L. Characteristic features of the adenomatoid odontogenic tumour on cone beam CT. Dentomaxillofac Radiol. 2014;43(6):20140016. doi:10.1259/dmfr.20140016
30. Janavi BR, Ramnarayan BK, Manasa AM, et al. Peripheral adenomatoid odontogenic tumor of the anterior maxillary gingiva: a rare case report with CBCT findings. Int J Curr Res. 2017;9(9):46890–46895.
31. Lee JK, Lee KB, Hwang BN. Adenomatoid odontogenic tumor: a case report. J Oral Maxillofacial Surg. 2000;58:1161–1164. doi:10.1053/joms.2000.9581
32. Leon JE, Mata GM, Fregnani ER, Carlos-Bregni R, de Almeida OP, Mosqueda-Taylor A. Clinicopathological and immunohistochemical study of 39 cases of Adenomatoid Odontogenic tumor: a multicentric study. Oral Oncol. 2005;41:835–842. doi:10.1016/j.oraloncology.2005.04.008
33. Philipsen HP, Reichart PA, Zhang KH, Nikai H, QX Y. Adenomatoid odontogenic tumor: biologic profile based on 499 cases. J Oral Pathol Med. 1991;20:149–158. doi:10.1111/j.1600-0714.1991.tb00912.x
34. Lavanya N, Rajeshwari MRC, Bharathi R, Shaheen A. Peripheral adenomatoid tumour-Is it really peripheral?: a case report. J Clin Diagn Res. 2013;7(7):1524–1526. doi:10.7860/JCDR/2013/5382.3179
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.