Back to Journals » Cancer Management and Research » Volume 13
Perioperative Use of Glucocorticoids and Intraoperative Hypotension May Affect the Incidence of Postoperative Infection in Patients with Gastric Cancer: A Retrospective Cohort Study
Authors Zhang Y , Li S , Yan C, Chen J, Shan F
Received 9 August 2021
Accepted for publication 20 September 2021
Published 8 October 2021 Volume 2021:13 Pages 7723—7734
DOI https://doi.org/10.2147/CMAR.S333414
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Yunxiao Zhang,1 Shuo Li,1 Chao Yan,2 Jiheng Chen,1 Fei Shan2
1Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Department of Anesthesiology, Peking University Cancer Hospital & Institute, Beijing, People’s Republic of China; 2Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), The First Department of Gastrointestinal Surgery, Peking University Cancer Hospital & Institute, Beijing, People’s Republic of China
Correspondence: Fei Shan
Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), The First Department of Gastrointestinal Surgery, Peking University Cancer Hospital & Institute, 52 Fucheng Street, Haidian District, Beijing, 100142, People’s Republic of China
Tel +86 10 88196553
Fax +86 10 88122437
Email [email protected]
Background: In patients undergoing surgical resection for gastric cancer, postoperative complications—in particular, postoperative infections—remain an important problem and can result in delayed recovery and increased postoperative mortality.
Objective: To investigate the association between perioperative anesthesia management and postoperative infectious complications in patients undergoing resection for gastric cancer.
Design: Retrospective cohort study.
Setting: A single-center study performed from April 1, 2015, to June 30, 2018, at Peking University Cancer Hospital.
Patients: Patients who underwent resection for gastric cancer.
Main Outcome Measures: Demographic information, perioperative data (including anesthesia-related data, surgery-related data, and cancer diagnosis), and information on postoperative recovery were recorded. The primary outcome was incidence of postoperative infection; the secondary outcome was length of hospital stay. The associations between perioperative factors and postoperative infectious complications were analyzed using multivariable logistic regression models and the classification tree method.
Results: A total of 880 patients were included in the study; of these, 111 (12.6%) had postoperative infectious complications during hospitalization, including 78 surgical site infections and 62 remote infections. After correction for confounding factors on logistic multivariable analysis, perioperative use of glucocorticoids was associated with a lower incidence of postoperative infection (hazard ratio 0.968, 95% confidence interval 0.939 to 0.997, P=0.029), and intraoperative systolic blood pressure < 90 mmHg for > 10 min was associated with a higher incidence of postoperative infection (hazard ratio 2.112, 95% confidence interval 1.174 to 3.801, P=0.013). In addition, older age, preoperative hypoproteinemia, and total gastrectomy were identified as independent predictors of postoperative infection.
Conclusion: For patients with gastric cancer, perioperative use of glucocorticoids and avoiding intraoperative hypotension may decrease the incidence of postoperative infectious complications.
Keywords: gastric cancer, surgery, glucocorticoids, hypotension, infection
Introduction
Gastric cancer remains one of the most deadly cancers.1 According to the latest global cancer statistics, gastric cancer ranked as the fifth most frequently diagnosed and the third most fatal cancer.2 At present, surgical resection accompanied by systemic adjuvant chemotherapy is the only treatment approach associated with prolonged survival in patients with gastric cancer.3 However, postoperative complications—in particular, postoperative infections—remain an important problem and can lead to delayed recovery and increased postoperative mortality.4,5
Prolonged operative duration,6 older age,7 use of total gastrectomy,8 and body mass index ≥30 kg/m29 are all known predictors of postoperative infectious complications. In recent years, it has been shown that perioperative anesthesia management may affect the occurrence of postoperative complications—in particular, infectious complications.10 For instance, patients who received intravenous propofol anesthesia had a lower incidence of serious postoperative complications and faster asymptomatic recovery after esophageal surgery, compared with patients who received inhalational sevoflurane.11 Another study found that, among 3081 patients who underwent total hip or knee replacement, general anesthesia was associated with a higher risk of surgical site infection, compared with general anesthesia combined with epidural or spinal anesthesia.12 Furthermore, it has been shown that perioperative use of low-dose dexamethasone can reduce the incidence of postoperative infectious complications in patients with pancreatic cancer.13
Studies attempting to identify predictors of infection after gastrointestinal surgery have mainly focused on colorectal surgery, and scant research has been performed on gastric surgery—even more rarely on the association between anesthesia management and postoperative infectious complications after resection for gastric cancer.14,15 As for the important role of anesthesia on patients’ postoperative recovery,10–13 this retrospective cohort study was designed to explore the relationship between perioperative anesthesia management and postoperative in-hospital infectious complications in patients who underwent resection for gastric cancer.
Materials and Methods
This retrospective cohort study was approved by the Clinical Research Ethics Committee of Peking University Cancer Hospital, Beijing, China (approval no. 2018YJZ71, Jie Li, December 25, 2018). Our study was designed and conducted in accordance with the Helsinki declaration. As the study was purely observational, the Ethics Committee of Peking University Cancer Hospital waived the need for written informed patient consent; however, all patients or their family members had provided oral consent to participate in this study before the collection of data.
Participants
Potential participants were screened using the electronic medical record system of the hospital. Eligible patients underwent gastrectomy for gastric cancer at the First Department of Gastrointestinal Surgery, Peking University Cancer Hospital, from April 1, 2015, to June 30, 2018. Exclusion criteria included (1) reoperation for recurrence of gastric cancer, (2) clinical signs of infection before operation, (3) combined diseases requiring long-term glucocorticoid treatment (eg, rheumatoid arthritis, vasculitis, or systemic lupus erythematosus),16 or (4) lack of key clinical data (eg, tumor stage, differentiation grade, or patient follow-up data).
Anesthesia, Surgery, and Perioperative Management
All patients underwent general anesthesia with endotracheal intubation. Anesthesia was induced by intravenous anesthetics (propofol and/or etomidate) and opioids (fentanyl or sufentanil) and maintained with inhalational anesthetics (sevoflurane or isoflurane) and opioids (fentanyl, sufentanil, oxycodone, and/or dezocine). Low-dose glucocorticoids (dexamethasone or methylprednisolone) were administered at the discretion of the anesthesiologist to prevent postoperative nausea and vomiting.17,18 The indications for administration of glucocorticoids were selected in accordance with the consensus guidelines for perioperative nausea and vomiting management.19
All patients received antibiotics (Cefuroxime sodium, 1.5 g per time) 30 mins before operation and when patients went back to the ward. Depending on the location of the tumor, total gastrectomy, proximal gastrectomy, distal gastrectomy, or palliative surgery was performed in accordance with the 2014 Japanese guidelines for the treatment of gastric cancer.20 The method of reconstruction (Roux-en-Y esophagojejunostomy, gastroduodenostomy, gastrojejunostomy, or dual-channel reconstruction) was selected at the discretion of the operating surgeon.
Postoperative patient-controlled analgesia was provided for up to 3 days. Opioids were used for intravenous analgesia. Antiemetics (dexamethasone, 5-HT3 receptor antagonist, and/or metoclopramide) were administered when considered necessary.21 Other perioperative treatments were performed in accordance with routine practice.
Perioperative Data Collection
Patient data were collected from the hospital’s electronic medical record system. Baseline data included age, sex, height, weight, drinking and smoking history, preoperative comorbidities, preoperative laboratory test results, American Society of Anesthesiologists classification, and receipt of preoperative chemotherapy. Anesthesia-related data included anesthetic method, types and doses of anesthetics, intraoperative fluid infusion and blood transfusion, occurrence and duration of intraoperative hypotension (systolic blood pressure <90 mmHg for >10 min), postoperative analgesia, and perioperative receipt of glucocorticoids. Equivalent doses were calculated for glucocorticoids22 and opioids.23–25 Surgery-related data included duration and type of surgery (laparoscopic or open gastrectomy), surgical range (total, distal, or proximal gastrectomy or palliative resection), and estimated intraoperative blood loss. Tumor-related data and postoperative data included pathological diagnoses, degree of tumor differentiation, pathologic tumor-node-metastasis stage of gastric cancer, occurrence of postoperative infectious complications, and length of hospital stay.
Outcomes
The primary outcome was the incidence of postoperative, in-hospital infection, which was defined as any clinical-related infection after gastrectomy and before first discharge. Each specific type of infection was diagnosed in accordance with clinical criteria, recorded in the patient’s clinical history, and confirmed by two independent researchers. Surgical site infections and remote infections were included. The criteria used to define and classify surgical site infections (incision infection, ascites or abscess, and anastomotic fistula combined infection) were established in accordance with the guidelines of the American Committee of Disease Control.26–28 Remote infections included respiratory tract infections, urinary tract infections, catheter-related infections, and primary bacteremia, in accordance with American National Healthcare Safety Network infection criteria.29 The secondary outcome was the length of the hospital stay.
Data Analysis
Continuous data were compared using Student’s t-test (normal distribution) or the Mann–Whitney U-test (nonnormal distribution); categorical data were analyzed using the χ2 test. Missing data were not replaced. Univariable associations between baseline or perioperative variables and postoperative infection were assessed using logistic regression models. Variables with P<0.20 on univariable analyses were included in multivariable models to assess the adjusted association between perioperative anesthesia management and postoperative infection.
We also built a model using a classification tree method. A classification tree is a nonlinear discrimination method that uses a set of independent variables to split a sample into progressively smaller subgroups. The procedure, iterative at each branch of the tree selects the independent variable that has the strongest association with the dependent variable according to a specific criterion.30 In our analysis, the target variable was the postoperative infection rate. The analysis begins with the root node, which contains all the observations in the sample; then the computer program for the classification tree will select another node based on the database, and each node depicted in the classification tree can then be expressed in terms of an “if-then” rule. An individual moves through the tree in the direction determined by the answer to a question at each branch, and the infection rate changes accordingly in order to illustrate the association between the selected independent variables and the dependent variable more clearly.31
Statistical analyses were performed using SPSS 25.0 software (IBM SPSS, Chicago, IL) and the rpart package of R (http://www.r-project.org). Two-sided P<0.05 was considered to indicate statistical significance.
Results
A total of 901 patients underwent surgery for gastric cancer between April 1, 2015, and June 30, 2018. Of these, 21 were excluded after data review, including 10 who underwent reoperations for recurrence of gastric cancer and 11 with clinical signs of infection before operation. In total, 880 patients were enrolled and were ultimately considered in the final analysis (Figure 1).
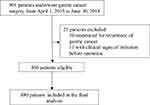 |
Figure 1 Study flow chart. |
Baseline and Perioperative Data
Of the 880 patients included in the analysis, 111 (12.6%) had postoperative infections—78 infections (8.9%) were surgical site infections (14 surgical wound infections, 59 peritoneal effusions or abscess, and 10 anastomotic fistula infections) and 62 (7.0%) were remote infections (46 respiratory tract infections, 7 urinary tract infections, 15 catheter-related infections, and 11 primary bacteremia) (Table 1). Compared with patients without infection, patients with postoperative infection were older (P=0.002); more likely to have preoperative arrhythmia (P=0.026), have preoperative hypoproteinemia (albumin <35 g/L) (P=0.003), have intraoperative hypotension (systolic blood pressure <90 mmHg for >10 min) (P=0.004), and undergo total gastrectomy (P=0.000); and less likely to receive perioperative glucocorticoids (P=0.002). Furthermore, patients with postoperative infection had longer operation duration (P=0.001) and longer length of hospital stay (P=0.000). Other factors analyzed were not statistically significantly different between the two groups (Table 2, Supplemental Tables 1 and 2).
 |
Table 1 Infection Sites of Postoperative, in-Hospital Infections (N=880) |
 |
Table 2 Baseline and Perioperative Variables |
Univariable Analysis of Risk Factors for Postoperative Infection
Univariable analysis identified 12 factors that may be related to postoperative infection during hospitalization (P<0.20), including age, sex, history of coronary heart disease, history of hypertension, history of arrhythmia, preoperative hypoproteinemia, surgical resection range, operative duration, estimated intraoperative blood loss, intraoperative crystalloids infusion, dose of perioperative glucocorticoids, and intraoperative systolic blood pressure <90 mmHg for >10 min (Table 3). There was no collinearity among the above factors.
 |
Table 3 Risk Factors for Early Postoperative Infection After Elective Gastric Cancer Surgery: Univariable and Multivariable Logistic Regression Analyses |
Multivariable Analysis of Risk Factors for Postoperative Infection
After correction for confounding factors by logistic multivariable analysis, perioperative use of glucocorticoids was associated with a lower incidence of postoperative infection (hazard ratio 0.968, 95% confidence interval 0.939 to 0.997, P=0.029), and intraoperative systolic blood pressure <90 mmHg for >10 min was associated with a higher incidence of postoperative infection (hazard ratio 2.112, 95% confidence interval 1.174 to 3.801, P=0.013). Among the other factors, older age, preoperative hypoproteinemia, and total gastrectomy were independent predictors of postoperative infection (Table 3).
Classification Tree
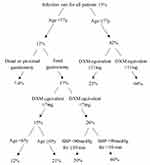
We also used a classification tree to further illustrate the effect of the five independent predictors on postoperative infectious complications. As shown in Figure 2, four of the five factors were selected by the computer program for the classification tree: age, range of gastrectomy, dexamethasone equivalent, and presence of intraoperative hypotension. Beginning with the root node, the infection rate for all patients in the cohort was 13%. The first node depicted was age, with an infection rate of 42% for patients aged ≥77 years and 12% for patients aged <77 years. For patients aged ≥77 years, if the dose of perioperative dexamethasone equivalent was ≥11 mg, the rate of infection decreased to 23%; if the dose of perioperative dexamethasone equivalent was <11 mg, the rate of infection increased to 64%. For patients aged <77 years, total gastrectomy, dexamethasone equivalent <7 mg, and systolic blood pressure <90 mmHg for >10 min increased the infection rate, whereas distal or proximal gastrectomy, dexamethasone equivalent ≥7 mg, and systolic blood pressure <90 mmHg for ≤10 min decreased the infection rate.
Discussion
In this retrospective study comprising 880 patients with gastric cancer, the incidence of postoperative infection was consistent with rates in previous reports.6,32,33 After correction for confounding factors by logistic multivariable analysis, perioperative use of glucocorticoids was associated with a lower incidence of infectious complications, and intraoperative systolic blood pressure <90 mmHg for >10 min was associated with a higher incidence of postoperative infectious complications.
Perioperative glucocorticoids are commonly used to prevent postoperative nausea and vomiting associated with general anesthesia.19 However, the perioperative use of glucocorticoids has been a matter of controversy.34,35 Concerns exist that the use of glucocorticoids may lead to peripheral insulin resistance, and postoperative hyperglycemia may be a risk factor for postoperative infection.34 However, these studies mainly focused on the long-term use of glucocorticoids,35,36 and more-recent studies have observed favorable outcomes with the use of glucocorticoids even in long-term use cases.37 For instance, a randomized controlled study from 2016 found that perioperative application of hydrocortisone for 3 days reduced the incidence of postoperative complications in patients undergoing pancreatoduodenectomy.37 What is more, in a retrospective cohort study from 2018, patients undergoing resection for pancreatic cancer received a single antiemetic dose of dexamethasone during the perioperative period, which was associated with a lower incidence of postoperative infectious complications.13 In line with the previous studies, the present study found that perioperative use of an antiemetic dose of glucocorticoids was associated with a lower incidence of postoperative infections in patients who underwent gastrectomy. Similarly, a randomized controlled study including patients undergoing hepatectomy also found that a single dose of methylprednisolone before surgery reduced postoperative infectious and systemic complications and shortened the length of the hospital stay.38
The anti-inflammatory effect of glucocorticoids may be the mechanism responsible for reducing the incidence of postoperative infectious complications. Studies have confirmed that perioperative dexamethasone is associated with a lower rate of positive systemic inflammatory syndrome in patients after selective surgery for colorectal cancer.39 C-reactive protein level has been proved to be a predictor of postoperative infectious complications,14,40 and a recent study by Laaninen et al found that patients receiving hydrocortisone treatment had lower plasma C-reactive protein levels.37 Another retrospective analysis based on propensity score matching in patients with colorectal cancer also found that the use of dexamethasone to prevent postoperative nausea and vomiting was associated with lower plasma C-reactive protein levels on postoperative day 3.39
Adequate blood pressure is required for suitable wound perfusion. Some studies have suggested that there is an important association between intraoperative hypotension and surgical site infections.15,41,42 In the present study, we found that systolic blood pressure <90 mmHg for >10 min was associated with an increased incidence of infectious complications during hospitalization in patients who underwent surgical resection for gastric cancer. Similarly, another retrospective study including patients with colon cancer also found that intraoperative hypotension was an independent predictor of postoperative infections.43 However, there may be some doubt about how intraoperative hypotension can influence postoperative infections, as the duration of intraoperative hypotension is usually short, owing to timely intervention by the anesthetist. Actually, Abdelmalak et al had shown that, although the duration of intraoperative hypotension was short, it was the lowest tissue oxygen saturation that was associated with severe complications, not time-weighted average oxygenation.44 This is consistent with the findings from more-recent studies, which have observed that even transient hypotension during operation is associated with myocardial injury, acute kidney injury, and death.45,46 A retrospective study including patients with colon cancer also found that postoperative time-weighted average arterial pressure was not associated with postoperative surgical site infections, whereas lowest mean arterial pressure was.47
Regarding the definition of “intraoperative hypotension,” some studies use the absolute reduction of mean arterial pressure, others use the absolute reduction of systolic arterial pressure,48 and still others use the proportion of reduction based on baseline blood pressure, such as the traditional 20% reduction.49 The data on hypotension collected in the present study were based on the absolute decrease in systolic blood pressure. Some studies have shown that, at least for the relationship between hypotension and myocardial and renal injury, the absolute decrease in blood pressure and the decrease ratio of baseline values are both predictive of postoperative infections.50
Our other main findings—that preoperative albumin <35 g/L, older age and total gastrectomy were independent predictors of increased incidence of postoperative infection in patients who underwent radical resection for gastric cancer—are consistent with the findings of previous studies.7,8,51 For instance, a retrospective study of 1798 patients who underwent gastrectomy for gastric adenocarcinoma found that prealbumin concentrations were useful predictors of short-term postoperative outcomes after gastrectomy.51 Another randomized controlled trial involving 685 patients found that advanced age was identified as a risk factor for superficial incisional surgical site infections after gastrectomy.7 A retrospective study of 407 patients also showed that total gastrectomy is an independent risk factor for organ/space SSI after gastric surgery.8
The limitations of our study include its retrospective design, which may have introduced selection bias, and its single-center nature, limiting the generalizability of the results. What is more, all cases received inhalational anesthesia and no case received combined epidural block during general anesthesia in our patients. This limited our ability to detect any effects of total intravenous anesthesia and epidural block on the outcome of gastric patients, as suggested by previous studies.11,12 Finally, previous studies reported that body mass index ≥30 kg/m2, perioperative ventilator setting, inspiratory oxygenation fraction, temperature, blood transfusion and postoperative extubation/not extubation were also risk factors for postoperative infection,9,52–56 but only 25 (2.8%) cases in our patients with body mass index ≥30 kg/m2 and 12 (1.4%) cases received blood transfusion, and the present study did not collect data about perioperative ventilator setting, inspiratory oxygenation fraction, temperature, or postoperative extubation/not extubation, which also limited our ability to detect the effect of these factors on the outcome of gastric patients.
Conclusions
For patients with gastric cancer, perioperative use of glucocorticoids and avoiding intraoperative hypotension may decrease the incidence of postoperative infectious complications. Considering the widespread perioperative use of glucocorticoids and the high incidence of intraoperative hypotension, prospective studies are urgently needed to clarify their effects on infections after gastric cancer surgery.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396(10251):635–648. doi:10.1016/S0140-6736(20)31288-5
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
3. Song Z, Wu Y, Yang J, Yang D, Fang X. Progress in the treatment of advanced gastric cancer. Tumor Biol. 2017;39(7):1010428317714626. doi:10.1177/1010428317714626
4. Tu RH, Lin JX, Li P, et al. Prognostic significance of postoperative pneumonia after curative resection for patients with gastric cancer. Cancer Med. 2017;6(12):2757–2765. doi:10.1002/cam4.1163
5. Xiao H, Xiao Y, Quan H, Liu W, Pan S, Ouyang Y. Intra-abdominal infection after radical gastrectomy for gastric cancer: incidence, pathogens, risk factors and outcomes. Int J Surg. 2017;48:195–200. doi:10.1016/j.ijsu.2017.07.081
6. Jeong SJ, Ann HW, Kim JK, et al. Incidence and risk factors for surgical site infection after gastric surgery: a multicenter prospective cohort study. Infect Chemother. 2013;45(4):422–430. doi:10.3947/ic.2013.45.4.422
7. Endo S, Tsujinaka T, Fujitani K, et al. Risk factors for superficial incisional surgical site infection after gastrectomy: analysis of patients enrolled in a prospective randomized trial comparing skin closure methods. Gastric Cancer. 2016;19(2):639–644. doi:10.1007/s10120-015-0494-z
8. Kosuga T, Ichikawa D, Komatsu S, et al. Clinical and surgical factors associated with organ/space surgical site infection after laparoscopic gastrectomy for gastric cancer. Surg Endosc. 2017;31(4):1667–1674. doi:10.1007/s00464-016-5156-7
9. Özmen T, Javadov M, Yeğen CS. Factors affecting surgical site infection rate after elective gastric cancer surgery. Ulus Cerrahi Derg. 2016;32(3):178–184.
10. Ornek D, Metin S, Deren S, et al. The influence of various anesthesia techniques on postoperative recovery and discharge criteria among geriatric patients. Clinics. 2010;65(10):941–946. doi:10.1590/S1807-59322010001000003
11. Tsuchiya M, Shiomoto K, Mizutani K, et al. Reduction of oxidative stress a key for enhanced postoperative recovery with fewer complications in esophageal surgery patients: randomized control trial to investigate therapeutic impact of anesthesia management and usefulness of simple blood test for prediction of high-risk patients. Medicine. 2018;97(47):e12845.
12. Chang CC, Lin HC, Lin HW, Lin HC. Anesthetic management and surgical site infections in total hip or knee replacement: a population-based study. Anesthesiology. 2010;113(2):279–284. doi:10.1097/ALN.0b013e3181e2c1c3
13. Sandini M, Ruscic KJ, Ferrone CR, et al. Intraoperative dexamethasone decreases infectious complications after pancreaticoduodenectomy and is associated with long-term survival in pancreatic cancer. Ann Surg Oncol. 2018;25(13):4020–4026. doi:10.1245/s10434-018-6827-5
14. Adamina M, Warschkow R, Näf F, et al. Monitoring c-reactive protein after laparoscopic colorectal surgery excludes infectious complications and allows for safe and early discharge. Surg Endosc. 2014;28(10):2939–2948. doi:10.1007/s00464-014-3556-0
15. Yamamoto S, Fujita S, Akasu T, Ishiguro S, Kobayashi Y, Moriya Y. Wound infection after elective laparoscopic surgery for colorectal carcinoma. Surg Endosc. 2007;21(12):2248–2252. doi:10.1007/s00464-007-9358-x
16. Adcock IM, Mumby S. Glucocorticoids. Handb Exper Pharmacol. 2017;237:171–196.
17. Weren M, Demeere JL. Methylprednisolone vs. dexamethasone in the prevention of postoperative nausea and vomiting: a prospective, randomised, double-blind, placebo-controlled trial. Acta Anaesthesiol Belg. 2008;59(1):1–5.
18. Chen P, Li X, Sang L, Huang J. Perioperative intravenous glucocorticoids can decrease postoperative nausea and vomiting and pain in total joint arthroplasty: a meta-analysis and trial sequence analysis. Medicine. 2017;96(13):e6382. doi:10.1097/MD.0000000000006382
19. Gan TJ, Diemunsch P, Habib AS, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2014;118(1):85–113.
20. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20(1):1–19. doi:10.1007/s10120-016-0622-4
21. Zhu M, Zhou C, Huang B, Ruan L, Liang R. Granisetron plus dexamethasone for prevention of postoperative nausea and vomiting in patients undergoing laparoscopic surgery: a meta-analysis. J Int Med Res. 2017;45(3):904–911. doi:10.1177/0300060517703276
22. Meikle AW, Tyler FH. Potency and duration of action of glucocorticoids. Effects of hydrocortisone, prednisone and dexamethasone on human pituitary-adrenal function. Am J Med. 1977;63(2):200–207. doi:10.1016/0002-9343(77)90233-9
23. Treillet E, Laurent S, Hadjiat Y. Practical management of opioid rotation and equianalgesia. J Pain Res. 2018;11:2587–2601. doi:10.2147/JPR.S170269
24. Clotz MA, Nahata MC. Clinical uses of fentanyl, sufentanil, and alfentanil. Clin Pharm. 1991;10(8):581–593.
25. Silvasti M, Rosenberg P, Seppälä T, Svartling N, Pitkänen M. Comparison of analgesic efficacy of oxycodone and morphine in postoperative intravenous patient-controlled analgesia. Acta Anaesthesiol Scand. 1998;42(5):576–580. doi:10.1111/j.1399-6576.1998.tb05169.x
26. Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) hospital infection control practices advisory committee. Am J Infect Control. 1999;27(2):
27. Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol. 1992;13(10):606–608. doi:10.2307/30148464
28. Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16(3):128–140. doi:10.1016/0196-6553(88)90053-3
29. Yangco B. CDC definitions for nosocomial infections. Am J Infect Control. 1989;17(1):42–43. doi:10.1016/S0196-6553(89)80013-6
30. Türe M, Kurt I, Kürüm T. Analysis of intervariable relationships between major risk factors in the development of coronary artery disease: a classification tree approach. Anatol J Cardiol. 2007;7(2):140–145.
31. Xin Z, Yuan J, Hua L, et al. A simple tool detected diabetes and prediabetes in rural Chinese. J Clin Epidemiol. 2010;63(9):1030–1035. doi:10.1016/j.jclinepi.2009.11.012
32. Hirao M, Tsujinaka T, Imamura H, et al. Overweight is a risk factor for surgical site infection following distal gastrectomy for gastric cancer. Gastric Cancer. 2013;16(2):239–244. doi:10.1007/s10120-012-0174-1
33. Hennessey DB, Burke JP, Ni-Dhonochu T, Shields C, Winter DC, Mealy K. Preoperative hypoalbuminemia is an independent risk factor for the development of surgical site infection following gastrointestinal surgery: a multi-institutional study. Ann Surg. 2010;252(2):325–329. doi:10.1097/SLA.0b013e3181e9819a
34. Toner AJ, Ganeshanathan V, Chan MT, Ho KM, Corcoran TB. Safety of perioperative glucocorticoids in elective noncardiac surgery: a systematic review and meta-analysis. Anesthesiology. 2017;126(2):234–248. doi:10.1097/ALN.0000000000001466
35. Slieker JC, Komen N, Mannaerts GH, et al. Long-term and perioperative corticosteroids in anastomotic leakage: a prospective study of 259 left-sided colorectal anastomoses. Arch Surg. 2012;147(5):447–452. doi:10.1001/archsurg.2011.1690
36. Eriksen TF, Lassen CB, Gögenur I. Treatment with corticosteroids and the risk of anastomotic leakage following lower gastrointestinal surgery: a literature survey. Colorectal Dis. 2014;16(5):O154–160. doi:10.1111/codi.12490
37. Laaninen M, Sand J, Nordback I, Vasama K, Laukkarinen J. Perioperative hydrocortisone reduces major complications after pancreaticoduodenectomy: a randomized controlled trial. Ann Surg. 2016;264(5):696–702. doi:10.1097/SLA.0000000000001883
38. Bressan AK, Isherwood S, Bathe OF, Dixon E, Sutherland FR, Ball CG. Preoperative single-dose methylprednisolone prevents surgical site infections after major liver resection: a Randomized controlled trial. Ann Surg. 2020. doi:10.1097/SLA.0000000000004720
39. McSorley ST, Roxburgh CSD, Horgan PG, McMillan DC. The Impact of preoperative dexamethasone on the magnitude of the postoperative systemic inflammatory response and complications following surgery for colorectal cancer. Ann Surg Oncol. 2017;24(8):2104–2112. doi:10.1245/s10434-017-5817-3
40. Oberhofer D, Juras J, Pavicić AM, Rancić Zurić I, Rumenjak V. Comparison of C-reactive protein and procalcitonin as predictors of postoperative infectious complications after elective colorectal surgery. Croat Med J. 2012;53(6):612–619. doi:10.3325/cmj.2012.53.612
41. Tassoudis V, Vretzakis G, Petsiti A, et al. Impact of intraoperative hypotension on hospital stay in major abdominal surgery. J Anesth. 2011;25(4):492–499. doi:10.1007/s00540-011-1152-1
42. Ishikawa K, Kusumi T, Hosokawa M, Nishida Y, Sumikawa S, Furukawa H. Incisional surgical site infection after elective open surgery for colorectal cancer. Int J Surg Oncol. 2014;2014:419712. doi:10.1155/2014/419712
43. Anannamcharoen S, Vachirasrisirikul S, Boonya-Assadorn C. Incisional surgical site infection in colorectal surgery patients. J Med Assoc Thai. 2012;95(1):42–47.
44. Abdelmalak BB, Cata JP, Bonilla A, et al. Intraoperative tissue oxygenation and postoperative outcomes after major non-cardiac surgery: an observational study. Br J Anaesth. 2013;110(2):241–249. doi:10.1093/bja/aes378
45. Devereaux PJ, Yang H, Yusuf S, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371(9627):1839–1847.
46. Walsh M, Devereaux PJ, Garg AX, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology. 2013;119(3):507–515. doi:10.1097/ALN.0b013e3182a10e26
47. Yilmaz HO, Babazade R, Leung S, et al. Postoperative hypotension and surgical site infections after colorectal surgery: a retrospective cohort study. Anesth Analg. 2018;127(5):1129–1136. doi:10.1213/ANE.0000000000003666
48. Wesselink EM, Kappen TH, Torn HM, Slooter AJC, van Klei WA. Intraoperative hypotension and the risk of postoperative adverse outcomes: a systematic review. Br J Anaesth. 2018;121(4):706–721. doi:10.1016/j.bja.2018.04.036
49. Bijker JB, van Klei WA, Kappen TH, van Wolfswinkel L, Moons KG, Kalkman CJ. Incidence of intraoperative hypotension as a function of the chosen definition: literature definitions applied to a retrospective cohort using automated data collection. Anesthesiology. 2007;107(2):213–220. doi:10.1097/01.anes.0000270724.40897.8e
50. Salmasi V, Maheshwari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology. 2017;126(1):47–65. doi:10.1097/ALN.0000000000001432
51. Zhou J, Hiki N, Mine S, et al. Role of prealbumin as a powerful and simple index for predicting postoperative complications after gastric cancer surgery. Ann Surg Oncol. 2017;24(2):510–517. doi:10.1245/s10434-016-5548-x
52. Papazian L, Klompas M, Luyt CE. Ventilator-associated pneumonia in adults: a narrative review. Intensive Care Med. 2020;46(5):888–906. doi:10.1007/s00134-020-05980-0
53. Staehr AK, Meyhoff CS, Rasmussen LS. Inspiratory oxygen fraction and postoperative complications in obese patients: a subgroup analysis of the PROXI trial. Anesthesiology. 2011;114(6):1313–1319. doi:10.1097/ALN.0b013e31821bdb82
54. Beal MW, Brown DC, Shofer FS. The effects of perioperative hypothermia and the duration of anesthesia on postoperative wound infection rate in clean wounds: a retrospective study. Vet Surg. 2000;29(2):123–127. doi:10.1111/j.1532-950X.2000.00123.x
55. Teng Z, Zhu Y, Liu Y, et al. Restrictive blood transfusion strategies and associated infection in orthopedic patients: a meta-analysis of 8 randomized controlled trials. Sci Rep. 2015;5(1):13421. doi:10.1038/srep13421
56. García-Delgado M, Navarrete-Sánchez I, Colmenero M. Preventing and managing perioperative pulmonary complications following cardiac surgery. Curr Opin Anaesthesiol. 2014;27(2):146–152. doi:10.1097/ACO.0000000000000059
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.