Back to Journals » Cancer Management and Research » Volume 13
Perioperative Safety and Effectiveness of Neoadjuvant Therapy with Fluorouracil, Leucovorin, Oxaliplatin, and Docetaxel Plus Apatinib in Locally Advanced Gastric Cancer
Authors Zhang Y, Zhang B, Yang J, Zhang J, Zhang W
Received 27 January 2021
Accepted for publication 26 February 2021
Published 10 March 2021 Volume 2021:13 Pages 2279—2286
DOI https://doi.org/10.2147/CMAR.S304093
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Seema Singh
Yonglei Zhang,1 Bin Zhang,1 Jinpo Yang,2 Jindai Zhang,1 Wei Zhang3
1Department of Gastrointestinal Surgery, The Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou, 450008, Henan Province, People’s Republic of China; 2Department of Medical Oncology, The Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou, 450008, Henan Province, People’s Republic of China; 3Department of Gastrointestinal Surgery, Henan Provincial People’s Hospital, Zhengzhou, 450008, Henan Province, People’s Republic of China
Correspondence: Bin Zhang Email [email protected]
Purpose: The trend in neoadjuvant therapy for locally advanced gastric cancer (LAGC) is to use more drugs or therapies in combination. This study aimed to assess the safety and effectiveness of neoadjuvant chemotherapy with fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) plus apatinib in the treatment of LAGC.
Patients and Methods: We collected clinical data from patients with LAGC who received neoadjuvant FLOT and apatinib therapy and underwent surgery from January 2017 to December 2020. Patients were divided into either the FLOT group (in which patients received FLOT neoadjuvant therapy and surgery) or the FLOTA group (in which patients received FLOT plus apatinib neoadjuvant therapy and surgery).
Results: The FLOT and FLOTA groups contained 44 and 31 patients, respectively. There were significant differences between the FLOT and FLOTA groups in the objective response rate (50.00% vs. 80.65%, respectively, p = 0.008) and average change from baseline in the target lesion size (− 26.16 ± 34.61 vs. − 54.32 ± 36.11, respectively, p < 0.001). There were also significant differences in the pretreatment clinical tumor-node-metastasis (cTNM) and post treatment cTNM stages for the FLOT group (p = 0.001) and for the FLOTA group (p < 0.001). There were no significant differences between the FLOT and FLOTA groups in post neoadjuvant therapy cTNM stages (p = 0.525), R0 rate (p = 0.397), tumor regression grade (p = 0.397), or post treatment pathological TNM stage (p = 0.180). Some neoadjuvant therapy-related adverse events occurred significantly more frequently in the FLOTA group, including diarrhea (all grades), pain (all grades), oral mucositis (all grades), and hand-foot syndrome (all grades).
Conclusion: The FLOTA regimen can achieve better perioperative efficacy and acceptable toxicity compared with that of the FLOT regimen in neoadjuvant treatment of LAGC. The FLOTA regimen for neoadjuvant therapy for LAGC merits further study.
Keywords: chemotherapy, FLOT, FLOTA, LAGC, toxicity
Introduction
Gastric cancer is the fifth most common cancer and the third most common cause of cancer death globally.1 Approximately 1 million new cases of stomach cancer are diagnosed globally each year.2 China is the country most affected by gastric cancer, accounting for 42.6% of the global incidence and 45% of all gastric cancer-related deaths.3 Surgery is currently required to cure gastric cancer. However, even after surgery, the prognosis for gastric cancer remains poor.3 The 5-year survival rate for gastric cancer patients undergoing resection alone is between 23% and 49% in Western countries and about 70% in Eastern countries.4 The 5-year survival rate is even lower for locally advanced gastric cancer (LAGC).5 Currently, more and more randomized clinical trials have shown that preoperative and postoperative therapy can improve the prognosis of patients with LAGC.2,3,6,7 Neoadjuvant therapy in particular is increasingly being accepted and performed by clinicians. An increasing number of neoadjuvant chemotherapy regimens for LAGC have been proven to have good efficacy.6,8,9
Among the numerous neoadjuvant chemotherapy regimens, the application of the fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) regimen in neoadjuvant therapy for gastric cancer began only recently.10 In the first large clinical trial, the FLOT protocol demonstrated better safety and efficiency than epirubicin, cisplatin, and fluorouracil or capecitabine regimens in patients with gastric cancer in 2016.11 The efficacy of FLOT was further demonstrated in subsequent clinical trials.12–14 In 2017, the AIO-FLOT3 trial showed that patients with LAGC who received FLOT neoadjuvant chemotherapy and proceeded to surgery had favorable survival.12 Results from another clinical trial in 2019 showed that perioperative FLOT improved overall survival compared with perioperative fluorouracil or capecitabine plus cisplatin and epirubicin in locally advanced, resectable gastric or gastroesophageal junction adenocarcinoma.13 In 2020, a clinical trial showed that a neoadjuvant FLOT regimen has similar safety and effectiveness for LAGC to that of a tegafur, gimeracil, and oteracil potassium capsule plus oxaliplatin regimen.14 With this evidence, the application of FLOT for neoadjuvant therapy in patients with gastric cancer will like become more popular.
As the first domestic multi-target tyrosine kinase inhibitor (TKI) in China, apatinib was approved for gastric cancer in 2014.15 Apatinib is in widespread use, and has been applied to various gastric cancer scenarios.16 Apatinib combined with chemotherapy can achieve a promising efficacy and acceptable safety in adjuvant therapy for advanced gastric cancer or neoadjuvant therapy for LAGC.17–19 As a major gastric cancer diagnosis and treatment center in central China, we has already treated many gastric cancer patients with FLOT or apatinib.20,21 Some of these patients received neoadjuvant chemotherapy with FLOT plus apatinib (FLOTA). In this study, we retrospectively collected and analyzed the clinical data for these patients, to provide a reference for clinical treatment decisions and the next stage of clinical trials.
Patients and Methods
Patients and Eligibility Criteria
We collected clinical data from patients with LAGC who received neoadjuvant FLOT and apatinib therapy and underwent surgery at The Affiliated Cancer Hospital of Zhengzhou University from January 2017 to December 2020. The inclusion criteria for these patients were as follows: 1) pathologically diagnosed gastric cancer; 2) multidisciplinary treatment for LAGC; 3) neoadjuvant FLOT or FLOTA therapy received; 4) surgically resected primary lesion; and 5) complete imaging, clinicopathological, and follow-up data.
The study followed the principles of the Declaration of Helsinki, and written informed consent was obtained from each patient. The study was approved by the Ethics Committee of the Cancer Hospital affiliated with Zhengzhou University.
Treatment Protocol
Patients were divided into the FLOT and FLOTA groups according to the treatment they received. In the FLOT group, on day 1, patients were administered intravenous 5-fluorouracil 2600 mg/m2 via a peripherally inserted central catheter for 24 hours. These patients also received intravenous leucovorin 200 mg/m2, oxaliplatin 85 mg/m2, and docetaxel 50 mg/m2. The treatment was repeated every 2 weeks, up to a maximum of four cycles.
In the FLOTA group, patients received apatinib 500 mg/m2 orally starting from day 1, in addition to receiving the same neoadjuvant chemotherapy regimen as in the FLOT group. The administration of apatinib was discontinued 7–14 days before surgery.
If severe toxicity occurred, apatinib and chemotherapy treatment was delayed until patient recovery, for a maximum of two weeks. All patients in both groups underwent surgical resection between 14 and 21 days after the completion of the last cycle of neoadjuvant therapy. Partial or total gastrectomy with D2 lymphadenectomy was performed.
Evaluation
Data were collected for medical history, physical examination, weight, Eastern Cooperative Oncology Group performance status (ECOG PS), complete blood count, and blood chemical tests at baseline and before the start of every neoadjuvant therapy cycle. Restaging by computed tomography (CT) or magnetic resonance imaging and endoscopy was done before surgery. Post neoadjuvant therapy tumor response evaluation was categorized as complete response (CR), partial response, stable disease, or progressive disease according to the response evaluation criteria in solid tumors. Differences in the objective response rate (ORR) and disease control rate (DCR) between the two groups were also assessed.
Tumor regression grading was performed according to the American Joint Committee on Cancer (AJCC) four-tiered grading system. Tumor staging (tumor-node-metastasis, TNM) was performed according to the seventh AJCC/Union for International Cancer Control tumor-node-metastasis classification for gastric cancer. All patients were assessed for toxicity according to the National Cancer Institute ’s common terminology criteria for adverse events version 4.0.
Statistical Analysis
All statistical analyses were performed using SPSS 21.0 software for Windows (IBM, Armonk, NY, USA). Quantitative variables are presented as medians (range) or number of patients (percentage). The Wilcoxon rank sum test with continuity correction was used for continuous variables. Fisher’s exact test was used for classification variables. All statistical analyses were two-sided, and p < 0.05 was considered statistically significant. The database was locked for statistical analysis in December 2020, and this was a descriptive analysis.
Results
Patient Characteristics
The basic characteristics of LAGC patients in this study are listed in Table 1. The FLOT and FLOTA groups contained 44 and 31 patients, respectively. These groups had average ages of 59.25 ± 7.76 and 60.19 ± 7.31 years, respectively. Almost all patients had a good performance status (ECOG PS 0/1). The histological subtypes included adenocarcinoma (not evaluated) (27.27% for FLOT vs. 32.26% for FLOTA), poorly differentiated adenocarcinoma (52.27% vs. 54.84%, respectively), and moderately and well-differentiated adenocarcinoma (20.45% vs. 16.13%, respectively). The pretreatment clinical TNM (cTNM) stages included IIB (9.10%), III (84.09%), IV (6.82%) for the FLOT group, and IIB (6.45%), III (80.65%), IV (12.90%) for the FLOTA group. There were no significant differences between the groups in terms of general patient characteristics (Table 1).
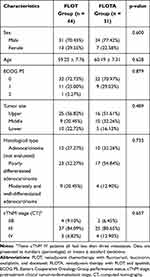 |
Table 1 Baseline Characteristics |
Clinical Effectiveness
The post neoadjuvant therapy CT evaluations are shown in Table 2. One of the 44 patients in the FLOT group and four of the 31 patients in the FLOTA group achieved CR. The FLOT group had an ORR of 50.00% and a DCR of 88.64%. The FLOTA group had an ORR of 80.65% and a DCR of 93.55%. The groups differed significantly with regard to ORR (50.00% vs. 80.65%, p = 0.008; Table 2) and average change from baseline in target lesion size (−26.16 ± 34.61 vs. −54.32 ± 36.11, p < 0.001, Table 2 and Figure 1). The post neoadjuvant therapy cTNM (ycTNM) stages were I (4.55%), IIA (20.45%), IIB (11.36%), and III (63.64%) for the FLOT group, and I (12.90%), IIA (25.81%), IIB (9.68%), and III (51.61%) for the FLOTA group. There were significant differences in cTNM stage and ycTNM stage for the FLOT group (p = 0.001) and for the FLOTA group (p < 0.001). There were no significant differences in ycTNM stages for the two groups (p = 0.525).
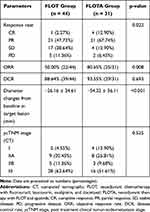 |
Table 2 Post Neoadjuvant Therapy CT Evaluation |
The clinicopathological results of both groups are shown in Table 3. The R0 rate for the FLOT group was 90.91%, and for the FLOTA group it was 96.77%. The percentages for tumor regression grades (TRGs) 0, 1, 2, and 3 for the FLOT group were 2.23%, 40.91%, 29.55%, and 27.27%, respectively; for the FLOTA group they were 9.68%, 54.84%, 22.58%, and 12.90%, respectively. The percentages for post treatment pathological TNM (ypTNM) stages I, II, and III for the FLOT group were 4.55%, 29.55%, and 65.91%, respectively; for the FLOTA group they were 12.90%, 41.94%, and 45.16%, respectively. There were no significant differences in R0 rate, TRG, or ypTNM stage for the two groups.
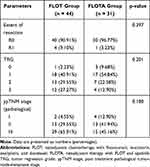 |
Table 3 Clinicopathological Results for the Two Groups |
Toxicity Evaluation
Neoadjuvant therapy-related adverse events (AEs) appeared to be more prevalent in the FLOTA group than in the FLOT group (Table 4). Most AEs were grade 1 or 2, although a few were grade 3 or 4; no drug-related deaths occurred. Some AEs occurred significantly more frequently in the FLOTA group, including diarrhea (all grades), pain (all grades), oral mucositis (all grades), and hand-foot syndrome (all grades) (Table 4).
 |
Table 4 Neoadjuvant Therapy-Related Adverse Effects in the Two Groups |
The postoperative complications of the two groups are shown in Table 5. There was no significant difference in postoperative stay at the hospital between the two groups, and the median length of stay in both cases was 10 days. There was no significant difference in overall postoperative morbidity between the two groups (Table 5). One patient underwent reoperation for intra-abdominal hemorrhage in the FLOT group. There were no deaths owing to postoperative complications in either group.
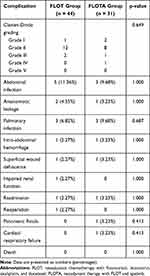 |
Table 5 Postoperative Complications in the Two Groups |
Discussion
Neoadjuvant therapy has been used for gastric cancer for more than 10 years.6,22 The purpose of neoadjuvant therapy includes further reducing the lesion size, improving the R0 resection rate, inhibiting micro-metastases, reducing the risk of tumor recurrence, and determining the sensitivity of patients to the corresponding treatment in advance.6 The current National Comprehensive Cancer Network recommendations for neoadjuvant chemotherapy include fluorouracil and cisplatin, fluorouracil and oxaliplatin, and FLOT.6 The neoadjuvant efficacy of FLOT was confirmed to be superior to that of the other two regimens in the latest clinical trial results.13 At present, FLOT is also the first recommendation for neoadjuvant chemotherapy in the diagnosis and treatment guidelines of the Chinese Society of Oncology.3 The trend in neoadjuvant therapy for LAGC is to use more drugs or therapies in combination, such as more chemotherapy drug combinations,13 a combination of chemotherapy and radiotherapy,23 or targeted combination chemotherapy.18 With the successful verification of targeted therapy combined with immunotherapy in advanced gastric cancer,24,25 this approach is also likely to be used in neoadjuvant therapy for LAGC. We believe this trend is occurring because the most important goals of neoadjuvant therapy are reduction and degradation, and because combining as many approaches as possible is more conducive to achieving this goal. Based on these factors, we have started to combine FLOT and apatinib, a targeted drug that is widely used for advanced gastric cancer in China, for neoadjuvant therapy in LAGC. Owing to the short follow-up period, we could not obtain sufficient overall survival data, so in this study we analyzed selected important data from the perioperative period of enrolled patients.
In this study, we retrospectively collected and analyzed clinical data for LAGC patients who received neoadjuvant therapy with FLOT or FLOTA and compared these two groups. The efficacy in the FLOT group in this study was similar to that observed in several other studies.11–14, 26 The FLOT plus apatinib combination therapy did not result in significantly higher DCR, nor did it significantly improve the TRG and ypTNM stages. We considered that this may be related to the small sample size. Nevertheless, the combination therapy results in significantly higher of ORR and significantly improve of the reduction degree of target lesion diameter. This suggests that apatinib can be added to the FLOT regimen for neoadjuvant therapy for LAGC to achieve a more desirable therapeutic effect. Notably, apatinib plus S-1 and oxaliplatin also showed predictable efficacy and good safety in neoadjuvant treatment of LAGC in a recent clinical trial.18 This suggests again that apatinib is a promising addition to neoadjuvant therapy in gastric cancer. However, we are unsure which chemotherapy regimen will be more effective in combination with apatinib. This requires further comparative studies. One study has shown that although neoadjuvant therapy is effective in perioperative evaluation of patients, long-term follow-up shows that neoadjuvant therapy does not improve long-term survival.27 This is one of the main points made by some studies casting doubt on neoadjuvant therapy.28 Therefore, to determine whether FLOTA neoadjuvant therapy improves long-term survival, it is necessary to continue with long-term follow-up of patients.
Clearly, the more drugs used in combination with neoadjuvant therapy, the more AEs.28 Studies have confirmed that either FLOT or apatinib is safe for the treatment of gastric cancer.11,29,30 However, when the two regimens are combined, patients receive three cytotoxic drugs and a multi-target TKI at the same time, which is likely more toxic. Other studies have shown that multi-target TKIs significantly enhance toxicity when used in combination with chemotherapy.31 The above reasons were the main concerns when we treated patients with the combination therapy in this study. We noted a significant increase in toxicity in the combined group, especially for diarrhea, pain, oral mucositis, and hand-foot syndrome. However, all patients were able to tolerate the toxicity of the combination therapy and successfully completed the treatment plan. This indicates that the addition of apatinib to FLOT is safe and tolerable, although it increases the incidence and intensity of toxicity. There was no significant difference in postoperative complications between the two groups. This suggests that preoperative treatment does not lead to an increase in the incidence of postoperative complications, as long as a sufficient time interval is maintained.
The main limitations of this study are its retrospective nature, small sample size, and short follow-up period. In addition, the inclusion of stage IV patients might have affected the results. Despite these deficiencies, we can confirm that FLOTA is safe and effective for neoadjuvant treatment of LAGC. To overcome the deficiencies of this study, prospective studies should be conducted, as well as comparative studies of apatinib in combination with other treatment regimens. In addition, efficacy markers for this treatment should also be studied simultaneously.
Conclusions
This study offers preliminary confirmation that the FLOTA regimen can achieve better perioperative efficacy and acceptable toxicity compared with that of the FLOT regimen in neoadjuvant treatment of LAGC. The FLOTA regimen for neoadjuvant therapy for LAGC merits further study.
Abbreviations
AJCC, American Joint Committee on Cancer; CR, Complete response; CT, Computed tomography; DCR, Disease control rate; FLOT, Fluorouracil, leucovorin, oxaliplatin, and docetaxel; FLOTA, Fluorouracil, leucovorin, oxaliplatin, and docetaxel plus apatinib; LAGC, Locally advanced gastric cancer; ORR, Objective response rate; TKI, Tyrosine kinase inhibitor; TNM, Tumor-node-metastasis.
Ethical Statement
The study was approved by the Ethics Committee of the Cancer Hospital affiliated with Zhengzhou University (Approval number: 20,201,110,003). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All patients provided written informed consent for data collection and research purposes.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Smyth EC, Nilsson M, Grabsch HI, et al. Gastric cancer. Lancet. 2020;396(10251):635–648. doi:10.1016/S0140-6736(20)31288-5
2. Venerito M, Ford AC, Rokkas T, et al. Review: prevention and management of gastric cancer. Helicobacter. 2020;25(Suppl S1):e12740. doi:10.1111/hel.12740
3. Wang FH, Shen L, Li J, et al. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer. Cancer Commun. 2019;39(1):10. doi:10.1186/s40880-019-0349-9
4. Rausei S, Lianos GD. Treatment of gastric cancer. Cancers. 2020;12(9):9. doi:10.3390/cancers12092627
5. Ilson DH. Advances in the treatment of gastric cancer. Curr Opin Gastroenterol. 2020;36(6):525–529. doi:10.1097/MOG.0000000000000679
6. Wang X-Z, Zeng Z-Y, Ye X, et al. Interpretation of the development of neoadjuvant therapy for gastric cancer based on the vicissitudes of the NCCN guidelines. World J Gastrointest Oncol. 2020;12(1):37–53. doi:10.4251/wjgo.v12.i1.37
7. Petrillo A, Smyth EC. Multimodality treatment for localized gastric cancer: state of the art and new insights. Curr Opin Oncol. 2020;32(4):347–355. doi:10.1097/CCO.0000000000000630
8. Zhao Q, Lian C, Huo Z, et al. The efficacy and safety of neoadjuvant chemotherapy on patients with advanced gastric cancer: a multicenter randomized clinical trial. Cancer Med. 2020;9(16):5731–5745. doi:10.1002/cam4.3224
9. Yan Y, Yang A, Lu L, et al. Impact of neoadjuvant therapy on minimally invasive surgical outcomes in advanced gastric cancer: an International Propensity Score-Matched Study. Ann Surg Oncol. 2020. doi:10.1245/s10434-020-09070-9
10. Kang Y-K, Cho H. Perioperative FLOT: new standard for gastric cancer? Lancet. 2019;393(10184):1914–1916. doi:10.1016/S0140-6736(18)33189-1
11. Al-Batran SE, Hofheinz RD, Pauligk C, et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the Phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. 2016;17(12):1697–1708. doi:10.1016/S1470-2045(16)30531-9
12. Al-Batran SE, Homann N, Pauligk C, et al. Effect of neoadjuvant chemotherapy followed by surgical resection on survival in patients with limited metastatic gastric or gastroesophageal junction cancer: the AIO-FLOT3 trial. JAMA Oncol. 2017;3(9):1237–1244. doi:10.1001/jamaoncol.2017.0515
13. Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393(10184):1948–1957. doi:10.1016/S0140-6736(18)32557-1
14. Sah BK, Zhang B, Zhang H, et al. Neoadjuvant FLOT versus SOX Phase II randomized clinical trial for patients with locally advanced gastric cancer. Nat Commun. 2020;11(1):6093. doi:10.1038/s41467-020-19965-6
15. Scott LJ. Apatinib: a review in advanced gastric cancer and other advanced cancers. Drugs. 2018;78(7):747–758. doi:10.1007/s40265-018-0903-9
16. Nie S, Yang G, Lu H. Current molecular targeted agents for advanced gastric cancer. Onco Targets Ther. 2020;13:4075–4088. doi:10.2147/OTT.S246412
17. Zhou N, Zhang C, Liu D, et al. Apatinib in combination with S-1 as first-line treatment in patients with advanced metastatic gastric cancer: results from an open, exploratory, single-arm, phase II trial. Oncologist. 2020. doi:10.1002/onco.13613
18. Zheng Y, Yang X, Yan C, et al. Effect of apatinib plus neoadjuvant chemotherapy followed by resection on pathologic response in patients with locally advanced gastric adenocarcinoma: a single-arm, open-label, phase II trial. Eur J Cancer. 2020;130:12–19. doi:10.1016/j.ejca.2020.02.013
19. Cheng H, Sun A, Guo Q, et al. Efficacy and safety of apatinib combined with chemotherapy for the treatment of advanced gastric cancer in the Chinese population: a systematic review and meta-analysis. Drug Des Devel Ther. 2018;12:2173–2183. doi:10.2147/DDDT.S170678
20. Zhang J, Zang X, Liu Y, et al. Efficacy and perioperative safety of FLOT regimen in neoadjuvant chemotherapy for gastric adenocarcinoma. Chin J Gen Surg. 2020;11(35):847–851. doi:10.3760/cma.j.cn113855-20200607-00460
21. Zhao Q, Guan L, Lv H, et al. Analysis of the efficacy prediction and prognostic factors for advanced gastric cancer treated with apatinib. China Oncol. 2018;3(28):203–209. doi:10.19401/j.cnki.1007-3639.2018.03.006
22. Byrd DR, Brierley JD, Baker TP, et al. Current and future cancer staging after neoadjuvant treatment for solid tumors. CA Cancer J Clin. 2020. doi:10.3322/caac.21640
23. Cats A, Jansen EPM, van Grieken NCT, et al. Chemotherapy versus chemoradiotherapy after surgery and preoperative chemotherapy for resectable gast ric cancer (CRITICS): an international, open-label, randomised Phase 3 trial. Lancet Oncol. 2018;19(5):616–628. doi:10.1016/S1470-2045(18)30132-3
24. Kawazoe A, Fukuoka S, Nakamura Y, et al. Lenvatinib plus pembrolizumab in patients with advanced gastric cancer in the first-line or second-line setting (EPOC1706): an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21(8):1057–1065. doi:10.1016/S1470-2045(20)30271-0
25. Fukuoka S, Hara H, Takahashi N, et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dos e-escalation, and dose-expansion phase ib trial (REGONIVO, EPOC1603). J Clin Oncol. 2020;38(18):2053–2061. doi:10.1200/JCO.19.03296
26. Wang K, Ren Y, Ma Z, et al. Docetaxel, oxaliplatin, leucovorin, and 5-fluorouracil (FLOT) as preoperative and postoperative chemotherapy compared with surgery followed by chemotherapy for patients with locally advanced gastric cancer: a propensity score-based analysis. Cancer Manag Res. 2019;11:3009–3020. doi:10.2147/CMAR.S200883
27. Schuhmacher C, Gretschel S, Lordick F, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for research and treatment of cancer randomized trial 40954. J Clin Oncol. 2010;28(35):5210–5218. doi:10.1200/JCO.2009.26.6114
28. Rausei S, Bali CD, Lianos GD. Neoadjuvant chemotherapy for gastric cancer. Has the time to decelerate the enthusiasm passed us by? Semin Oncol. 2020;47(6):355–360. doi:10.1053/j.seminoncol.2020.07.003
29. Anter AH, Abdel-Latif RM. The safety and efficacy of fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) combination in the front-line treatment for patients with advanced gastric or gastroesophageal adenocarcinoma: phase II trial. Med Oncol. 2013;30(1):451. doi:10.1007/s12032-012-0451-1
30. Geng R, Song L, Li J, et al. The safety of apatinib for the treatment of gastric cancer. Expert Opin Drug Saf. 2018;17(11):1145–1150. doi:10.1080/14740338.2018.1535592
31. Tian Z, Wang X, Liu Z, et al. Safety and efficacy of combination therapy with apatinib and doxorubicin in metastatic soft tissue sarcomas: an observational study from multiple institutions. Cancer Manag Res. 2019;11:5293–5300. doi:10.2147/CMAR.S207150
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

