Back to Journals » Research and Reports in Urology » Volume 11
Perioperative outcomes in elderly patients undergoing nephrectomy for renal cell carcinoma
Authors Sirithanaphol W, Pachirat K, Rompsaithong U , Kiatsopit P, Ungareevittaya P, Chindaprasirt J
Received 20 June 2019
Accepted for publication 8 July 2019
Published 23 July 2019 Volume 2019:11 Pages 195—199
DOI https://doi.org/10.2147/RRU.S220221
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jan Colli
Wichien Sirithanaphol,1 Kachit Pachirat,1 Ukrit Rompsaithong,1 Pakorn Kiatsopit,1 Piti Ungareevittaya,2 Jarin Chindaprasirt3
1Division of Urologic Surgery, Department of Surgery, Khon Kaen University, Khon Kaen, Thailand; 2Department of Pathology, Khon Kaen University, Khon Kaen, Thailand; 3Medical Oncology Division, Department of Internal Medicine, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand
Purpose: To determine if age should be considered a relative contraindication to surgery for safety reasons.
Methods: Renal cell carcinoma (RCC) patients who underwent nephrectomy from January 2007 to December 2017 were analyzed retrospectively. Patients were grouped into age<65 and age≥65 years. The demographic data, surgical outcomes, complication, hospital stay, blood loss, and survival were compared between the two groups.
Results: A total of 101 patients were included; 74 in the younger group, and 27 in the older group. Compared to the young group, lower BMI, higher anemia, higher ASA grade, and comorbidities were frequent in the elderly. The operative time, blood loss, and renal function decline were comparable between two age groups. The complication rates in the older and younger group were 22% and 12%, respectively. The survival time was shorter in older patients compared to the younger ones; hazard ratio 2.25; 95%CI 1.08–4.69, p-value=0.031.
Conclusion: Nephrectomy in elderly patients is safe and feasible and preoperative assessment along with diligent postoperative care may further increase survival. Age alone cannot be regarded as a contraindication for nephrectomy in RCC.
Keywords: geriatric, kidney cancer, kidney surgery, aged population
Introduction
In recent years, many countries are facing an aging society as the proportion of people aged over 65 years is growing tremendously.1 The number of elderly cancer patients has also been growing simultaneously.2
Renal cell carcinoma (RCC), a primary tumor of the kidney, predominantly occurs in the elderly (>65 years), according to the Surveillance Epidemiology and End Results (SEER) Cancer Statistics Review.3 Nephrectomy is the standard of care for RCC with a proven survival benefit in localized disease.4 For advanced disease, the surgery helps control the symptoms with also the added value for survival.5,6 However, it is a major surgery which could result in multiple complications including blood loss, kidney injury, and even death.7,8
Elderly patients usually have multiple comorbidities which further compromise the surgical outcome. In recent years, there are many studies on the short-term safety and long-term survival benefit of nephrectomy for RCC in the elderly. Some studies have suggested the higher risk of complications9 in the elderly than in the young, while others reported that the operation could be done safely in the elderly with low morbidity and mortality rate.7,10,11 Thus, the safety of nephrectomy in elderly patients remains controversial.
The aim of our research was to determine if age should be considered a relative contraindication to surgery for safety reasons.
Materials and methods
Study population
This was a retrospective cohort study which included RCC patients who underwent nephrectomy at Srinagarind Hospital, Khon Kaen University between 1 January 2007 and 31 December 2017. The inclusion criteria were age 18 years old or over, a diagnosis of RCC, and underwent partial or radical nephrectomy. The pathological review was done and patients with a diagnosis other than RCC were excluded.
Data collection and definition
Baseline clinical data, operative details, and perioperative complications were collected. Overall survival (OS) was defined as survival time from the date of RCC diagnosis to death from any cause. Preoperative functional status was assessed globally according to the American Society of Anesthesiologists (ASA) classification.12 Acute kidney injury was defined by the KDIGO guidelines as follows:13 increase in serum creatinine by ≥0.3 mg/dL within 48 hrs, or increase in serum creatinine to ≥1.5 times baseline, or urine volume <0.5 mL/kg/hour for six hours.
Surgical technique
The operation was either radical or partial nephrectomy. Radical nephrectomy was done with early control of the renal artery and vein. Lymphadenectomy was not a routine practice; it was done only if the surgeon suspected the involvement by either enlarged size in preoperative imaging or during surgery.
Statistical analysis
Baseline and clinical characteristics were analyzed using descriptive statistics and presented as the median and interquartile range (IQR). Wilcoxon rank-sum test, Chi-square and Fisher’s exact test were used. Survival analysis was performed using the Kaplan-Meier method and compared among groups using the log-rank test. For all statistical comparisons, a p-value of less than 0.05 was considered statistically significant. All data analysis was performed using STATA software (StataCorp LP, College Station, TX, USA).
Ethical consideration
Ethical approval was provided by the Khon Kaen University Faculty of Medicine Ethics Committee as instituted by the Declaration of Helsinki (Number HE621159). The patient consent to review the medical record was not required by the committee due to the retrospective nature of the study. All the data was anonymized and maintained with confidentiality.
Results
Patient characteristics
Of all 101 patients included, 27 patients were older than 65 years. The distribution of age at diagnosis is shown in Figure 1. Most of the patients presented at age 50–69 years old. The tumor size, metastasis, and histology did not differ significantly among the two groups.
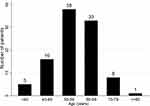 |
Figure 1 Age at diagnosis. |
The elderly group had a higher percentage of anemia and a lower body mass index compared to younger patients, with no significant difference, at 75% vs 53% and 22.0 vs 23.9 kg/m2, respectively (Table 1). Only 4% of patients in the younger group were classified as ASA class III, while there was 18.5% in the older cohort. The comorbidities were significantly higher in older groups (chronic kidney and heart disease) and older patients had significantly lower baseline glomerular filtration rate (GFR) by Cockcroft-Gault formula14 at a median of 47.3 mL/min compared to 79.8 mL/min in the younger group.
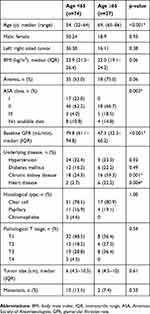 |
Table 1 Baseline characteristics of 101 patients underwent nephrectomy for RCC |
Perioperative outcomes
Ninety percent of patients in both groups underwent open surgery and almost all underwent radical nephrectomy. The median operative time was comparable between younger and older patients (Table 2); 130 vs 140 mins, respectively and there was no significant difference of the median blood loss between the two groups. Older patients required significantly longer hospitalization than the younger group.
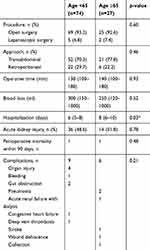 |
Table 2 Surgical outcomes after nephrectomy |
The overall complication rate in the older cohort was 22% compared to 12% in younger patients, with the details in Table 2. Organ injury was the most common complication including colon, spleen, and pancreatic injury resulting in colon repair, splenectomy, and endoscopic retrograde cholangiopancreatography and stent, respectively. Postoperative major bleeding occurred in one case which required reoperation. There was no intraoperative mortality and no patient died within 30 days after surgery.
Follow up and survival time
The median follow-up time was 6.6 years and at the time of analysis, 39 (40.2%) patients had died. For M0 disease, older patients (≥65 years) were at higher risk for death compared to younger ones significantly; median survival of 5.0 years vs not reached, the hazard ratio (HR) 2.25; 95%CI 1.08–4.69, p-value=0.031 as shown in Figure 2. The survival rate is shown in Table 3.
 |
Table 3 The survival rate of patients with M0 disease |
 |
Figure 2 Kaplan-Meier survival curve (age≥65vs <65) for non-metastatic RCC. (HR 2.25; 95% CI 1.08–4.69, p-value=0.031). |
Twelve patients (10:2, young:old) underwent cytoreductive nephrectomy; the median survival was 1.8 (1.0–3.7) years. For the two patients who were older than 65 years old, the first patient died at 1 year while another one died at 1.9 years.
Discussion
In the present study, we verified the perioperative complications and survival outcome after nephrectomy in elderly patients. The morbidity and mortality rate was low, and there was no 30-day postoperative death and a 90-day mortality rate was 3.7%.
The elderly had higher ASA grades, and more comorbidities especially heart and chronic kidney disease, which is consistent with other studies involving surgery in the elderly.11,15,16 However, there were no significant differences in perioperative outcomes apart from a longer hospital stay. Operative time, blood loss, and rate of acute kidney injury in elderly patients were comparable with those younger patients, consistent with earlier reports.7,10 Although the baseline GFR was worse in the older cohort, the rate of postoperative acute kidney injury was comparable. Nevertheless, for those older-old patients (≥80 years), Bensalah et al9 reported a higher risk for impaired renal function after surgery, which was consistent with the finding in our cohort.
As reported in other series, common complications were bleeding, organ injury, and infections. Operative time and blood loss were comparable to other studies8,10,11 and the morbidity rate was acceptable. Risk of overall complications increased with age and the hospital stay was longer in the elderly cohort, similar to other reported major surgeries cohort.17 It should be noted that not only the complication rate was higher in the elderly group, those who did have the complications tend to be more severe and complex; 6 events in 3 patients. The respiratory complications were found only in elderly patients, similar to other studies involving major surgery such as hepatectomy and cardiac surgery.15,17 This could be explained by the lower BMI in the elderly group and a higher risk for frailty and sarcopenia which led to reduced respiratory muscle activity, worse nutritional state, and less functional reserves.18,19
Regarding the oncological outcome, the overall survival was significantly lower in elderly patients both in the localized and metastatic setting, in concordance with earlier studies.3,20 No significant difference in cancer-specific survival (CSS) in RCC between older and younger patients was observed in many studies,10,11 however, the CSS could be confounded by many factors especially in the retrospective setting and may not represent the real data, hence we did not compare the CSS between two groups.
Patients in the present series, even with small lesions, preferred to have the tumors removed, as a result, no patient was treated with active surveillance. Moreover, tumor ablation, another treatment option for small RCC,21 was not available in the center at that time, therefore almost all patients were treated with surgery if feasible. The difference between radical and partial nephrectomy was not addressed here because more than 90% of the operations were radical in nature. However, if the patients are candidates for nephron-sparing surgery, preoperative aspects and dimensions used for an anatomical (PADUA) classification of renal tumors would benefit in predicting surgical outcomes and should be incorporated in the assessment.22
Conclusion
In summary, nephrectomy in elderly patients is a safe and feasible procedure. Preoperative assessment along with diligent postoperative care may further increase OS in the elderly, although it is apparent from the results of this study that age alone cannot be regarded as a contraindication for nephrectomy in renal cell carcinoma.
Disclosure
The authors report no conflicts of interest in this work.
References
1. United Nations Department of Economic and Social Affairs Population Division. World Population Ageing 2017 – Highlights (ST/ESA/SER.A/397); 2017. Available from: http://www.un.org/en/development/desa/population/publications/pdf/ageing/WPA2017_Highlights.pdf. Accessed 1 May, 2019.
2. Wirasorn K, Suwanrungruang K, Sookprasert A, Limpawattana P, Sirithanaphol W, Chindaprasirt J. Hospital-based population of elderly cancer cases in Northeastern Thailand. Asian Pac J Cancer Prev. 2016;17(2):767–770. doi:10.7314/apjcp.2016.17.2.767
3. Saad AM, Gad MM, Al-Husseini MJ, Ruhban IA, Sonbol MB, Ho TH. Trends in renal-cell carcinoma incidence and mortality in the United States in the last 2 decades: a SEER-based study. Clin Genitourin Cancer. 2019;17(1):46–57 e45. doi:10.1016/j.clgc.2018.10.002
4. Colombo JR
5. Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345(23):1655–1659. doi:10.1056/NEJMoa003013
6. Mickisch GH, Garin A, van Poppel H, et al. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet. 2001;358(9286):966–970. doi:10.1016/s0140-6736(01)06103-7
7. Lowrance WT, Yee DS, Savage C, et al. Complications after radical and partial nephrectomy as a function of age. J Urol. 2010;183(5):1725–1730. doi:10.1016/j.juro.2009.12.101
8. Stephenson AJ, Hakimi AA, Snyder ME, Russo P. Complications of radical and partial nephrectomy in a large contemporary cohort. J Urol. 2004;171(1):130–134. doi:10.1097/01.ju.0000101281.04634.13
9. Bensalah K, Sadiq A, Guille F, Lobel B, Patard JJ. [Risks and benefits of total nephrectomy in elderly patients over the age of 80]. Prog Urol. 2005;15(4):632–635.
10. Gao X, Hu L, Pan Y, Zheng L. Surgical outcomes of nephrectomy for elderly patients with renal cell carcinoma. Pak J Med Sci. 2018;34(2):288–293. doi:10.12669/pjms.342.14062
11. Staehler M, Haseke N, Stadler T, et al. Renal surgery in the elderly: morbidity in patients aged >75 years in a contemporary series. BJU Int. 2008;102(6):684–687. doi:10.1111/j.1464-410X.2008.07794.x
12. American Society of Anesthesiologists. New classification of physical status. Anesthesiology. 1963;24:111.
13. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–c184. doi:10.1159/000339789
14. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi:10.1159/000180580
15. Scandroglio AM, Finco G, Pieri M, et al. Cardiac surgery in 260 octogenarians: a case series. BMC Anesthesiol. 2015;15:15. doi:10.1186/s12871-015-0044-6
16. Tzeng CW, Cooper AB, Vauthey JN, Curley SA, Aloia TA. Predictors of morbidity and mortality after hepatectomy in elderly patients: analysis of 7621 NSQIP patients. HPB (Oxford). 2014;16(5):459–468. doi:10.1111/hpb.12155
17. Yu X, Yan YC, Chen G, Yu H. The efficacy and safety of totally laparoscopic hepatectomy for non-cirrhotic hepatocellular carcinoma in the elderly. BMC Surg. 2018;18(1):118. doi:10.1186/s12893-018-0444-x
18. Angulo J, El Assar M, Rodriguez-Manas L. Frailty and sarcopenia as the basis for the phenotypic manifestation of chronic diseases in older adults. Mol Aspects Med. 2016;50:1–32. doi:10.1016/j.mam.2016.06.001
19. Chindapasirt J. Sarcopenia in cancer patients. Asian Pac J Cancer Prev. 2015;16(18):8075–8077. doi:10.7314/apjcp.2015.16.18.8075
20. Karakiewicz PI, Jeldres C, Suardi N, et al. Age at diagnosis is a determinant factor of renal cell carcinoma-specific survival in patients treated with nephrectomy. Can Urol Assoc J. 2008;2(6):610–617. doi:10.5489/cuaj.978
21. Palumbo C, Cyr SJ, Mazzone E, et al. Impact of tumor size on cancer-specific mortality rate after local tumor ablation in T1a renal-cell carcinoma. J Endourol. 2019. doi:10.1089/end.2019.0179
22. Ficarra V, Novara G, Secco S, et al. Preoperative aspects and dimensions used for an anatomical (PADUA) classification of renal tumours in patients who are candidates for nephron-sparing surgery. Eur Urol. 2009;56(5):786–793. doi:10.1016/j.eururo.2009.07.040
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
