Back to Journals » International Medical Case Reports Journal » Volume 10
Perioperative care in an adolescent patient with heparin-induced thrombocytopenia for placement of a cardiac assist device and heart transplantation: case report and literature review
Authors Kamata M, Sebastian R, McConnell PI, Gomez D, Naguib A, Tobias JD
Received 27 July 2016
Accepted for publication 5 November 2016
Published 14 February 2017 Volume 2017:10 Pages 55—63
DOI https://doi.org/10.2147/IMCRJ.S118250
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Mineto Kamata,1 Roby Sebastian,1,2 Patrick I McConnell,3 Daniel Gomez,4 Aymen Naguib,1,2 Joseph D Tobias1,2,5
1Department of Anesthesiology and Pain Medicine, Nationwide Children’s Hospital, 2Department of Anesthesiology and Pain Medicine, The Ohio State University College of Medicine, 3Department of Cardiothoracic Surgery, Nationwide Children’s Hospital, 4Cardiovascular Perfusion Services and Heart Center, Nationwide Children’s Hospital and The Ohio State University, 5Department of Pediatrics, The Ohio State University College of Medicine, Columbus, OH, USA
Abstract: Heparin-induced thrombocytopenia (HIT) can cause life-threatening complications following the administration of heparin. Discontinuation of all sources of heparin exposure and the use of alternative agents for anticoagulation are necessary when HIT is suspected or diagnosed. We present the successful use of bivalirudin anticoagulation in an adolescent patient during cardiopulmonary bypass who underwent both placement of a left ventricular assist device and subsequent heart transplantation within a 36-hour period. The pathophysiology and diagnosis of HIT are reviewed, previous reports of the use of direct thrombin inhibitors for cardiac surgery are presented, and potential dosing regimens for bivalirudin are discussed.
Keywords: bivalirudin, anticoagulation, cardiopulmonary bypass, heart transplant
Introduction
Heparin-induced thrombocytopenia (HIT) is a potentially life-threatening condition that may follow the administration of heparin. Based on the clinical picture and mechanism, HIT is classified as HIT type 1 (HIT-1) and HIT type 2 (HIT-2). HIT-1 is characterized by an early, transient, nonimmune-mediated decrease in platelet count following exposure to heparin.1 While HIT-1 does not result in adverse clinical effects even without cessation of heparin therapy, significant morbidity and even mortality can occur with HIT-2. This immune-mediated complication occurs in ~1–5% of patients receiving unfractionated heparin (UFH) and <1% of patients receiving low-molecular-weight heparin (LMWH).2–4 In children, the frequency of HIT-2 is reported to range from 2.3 to 3.7% with a 1–3% incidence in those receiving UFH during cardiac surgery.5 There is a bimodal age distribution with a peak during the neonatal period and a second peak during adolescence, which may be reflective of the peak ages for the administration of heparin during surgical procedures.
HIT-2 results from the formation of a complex consisting of heparin, platelet factor 4 (PF-4), and immunoglobulin against them (usually IgG but also occasionally IgA or IgM).1 The complex binds to and activates platelets via the Fc receptor (RcγRII) with the subsequent release of prothrombotic platelet-derived microparticles, resulting in platelet consumption and thrombocytopenia. These microparticles also promote excessive thrombin generation, frequently resulting in thrombosis. The immune complexes also interact with monocytes, leading to tissue factor production, which results in antibody-mediated endothelial injury. Both of these latter processes contribute further to the activation of the coagulation cascade, thrombin generation, and thrombotic complications.
HIT-2 can cause limb- and life-threatening venous and arterial thrombosis (38–76% incidence) with a mortality rate of 20–30% if alternative anticoagulation therapy is not instituted.6,7 The clinical presentation of patients with HIT may vary with clinical signs and symptoms related to either thrombocytopenia or thrombosis. Clinical symptomatology typically occurs 5–10 days after heparin exposure with a ≥50% decrease in platelet count and resistance to heparin anticoagulation or unexplained thrombotic events.8 HIT is clinically judged using the following “4 T’s” scoring system: thrombocytopenia, timing of platelet count fall, thrombosis, and other causes for thrombocytopenia. The diagnosis of HIT is also based on laboratory findings, including an enzyme-linked immunosorbent assay (ELISA) and serotonin release assay.
We present the successful use of bivalirudin anticoagulation in an adolescent during cardiopulmonary bypass (CPB) who underwent both placement of a left ventricular assist device (LVAD) and subsequent heart transplantation within a 36-hour period. In both cases, anticoagulation was provided using bivalirudin. Options for anticoagulation during cardiac surgery in patients with HIT-2 are discussed. Previous reports of the use of direct thrombin inhibitors (DTIs) for cardiac surgery are reviewed with special attention to those involving assist device placement or cardiac transplantation.
Case report
The requirement for patient consent to publish this case report was waived by the Institutional Review Board of the Nationwide Children’s Hospital (Columbus, OH, USA) in accordance with their policy. A 15-year-old 84 kg adolescent who was previously healthy other than attention-deficit hyperactivity disorder (ADHD) presented with a history of fever, fatigue, abdominal pain, malaise, and orthopnea for 2 weeks. Echocardiography showed severely depressed left ventricular systolic function with an ejection fraction (EF) of 7%. He was admitted to the cardiothoracic intensive care unit (CTICU) with a diagnosis of dilated cardiomyopathy and inotropic support instituted with a milrinone infusion. Magnetic resonance imaging (MRI) demonstrated a right ventricular thrombus, which necessitated anticoagulation with heparin. Heparin was started at 10 U/kg/hour and maintained at an infusion rate of 10–20 U/kg/hour to maintain a therapeutic antifactor Xa level of 0.5–1.0 IU/mL and an activated partial thromboplastin time (APTT) within the range of 60–85 seconds. The platelet count at admission was 140,000/mm3 and reached a nadir of 82,000/mm3 after 12 days of heparin therapy. The diagnosis of HIT was based on clinical and laboratory findings, including an ELISA and serotonin release assay. Anticoagulation was changed to argatroban and then transitioned to oral warfarin. After the prothrombin time international normalized ratio (PT-INR) stabilized with warfarin, an implantable cardioverter-defibrillator (ICD) was placed for nonsustained ventricular tachycardia on the 29th day of the hospitalization. Given the concerns of thrombosis, anticoagulation with warfarin was neither interrupted nor reversed with vitamin K. Although his myocardial function temporarily improved allowing for discharge home, he was subsequently readmitted to the CTICU with an acute deterioration of cardiac function and listed for heart transplantation. His cardiac function continued to deteriorate, and he underwent placement of an LVAD while waiting for a heart to be available.
Perioperative management for LVAD
In the night before the surgery, anticoagulation with warfarin (PT-INR 2.12) was reversed with 10 mg of intravenous vitamin K. Other preoperative laboratory evaluations revealed a hemoglobin of 10.1 g/dL, a hematocrit of 30.6%, and a platelet count of 188,000/mm3. The patient was transported to the operating room for CPB and LVAD implantation with inotropic support, including milrinone (1.0 µg/kg/minute) and dobutamine (7.5 µg/kg/minute). After applying the American Society of Anesthesiologists’ (ASA) monitors, anesthesia was induced with fentanyl (6 µg/kg), etomidate (0.1 mg/kg), and midazolam (0.05 mg/kg). Endotracheal intubation was facilitated with rocuronium (1 mg/kg). Following anesthetic induction, a radial arterial cannula and a right internal jugular central venous cannula were placed. Anesthesia was maintained with isoflurane, a dexmedetomidine infusion (0.5 µg/kg/min), fentanyl (total intraoperative dose of 20 µg/kg), and rocuronium. Given the presence of HIT, a DTI (bivalirudin) was used for anticoagulation during CPB. A bolus dose of bivalirudin (1.5 mg/kg) was administered prior to CPB followed by a continuous infusion starting at 2.0 mg/kg/hour. Additionally, the CPB circuit was primed with bivalirudin (50 mg). The activated clotting time (ACT) was maintained at >400 seconds, and the APTT was also monitored at various points throughout the case. Bivalirudin dosing and the ACT values are listed in Table 1. Due to his projected wait time for transplantation, a durable implantable LVAD was selected. Following implantation of a HeartMate II (St. Jude Medical, Pleasanton, CA, USA) continuous-flow LVAD and weaning from CPB, hemostasis was achieved by the administration of fresh frozen plasma (FFP) (25 mL/kg), platelet concentrates (7.3 mL/kg), cryoprecipitate (2.5 mL/kg), and recombinant factor VIIa (90 µg/kg). Anesthesia, surgery, and CPB times were 593, 495, and 124 minutes, respectively. Inotropic support with milrinone and dobutamine was continued throughout the procedure. Following the procedure, the patient remained intubated, and he was transported to the CTICU. Postoperative laboratory evaluation at arrival to the CTICU revealed a prothrombin time (PT) of 18.1 seconds, a PT-INR of 1.46, an APTT of 54 seconds, and a fibrinogen of 251 mg/dL. During the initial 24 postoperative hours, there was a total drainage of 17 mL/kg from the chest tubes. The following blood products were transfused during this time: packed red blood cells (pRBCs) (12 mL/kg), FFP 3.6 (mL/kg), and platelets (3 mL/kg). He was extubated on postoperative day (POD) 1. The plan was to initiate anticoagulation therapy with argatroban and then transition back to oral coumadin; however, on POD 2 while on no anticoagulation, a heart donor became available and the patient was proceeded to heart transplantation.
Perioperative management for heart transplantation
Preoperative laboratory evaluation revealed a hemoglobin of 8.8 g/dL, a hematocrit of 26.7%, and a platelet count of 135,000/mm3. Inotropic support included milrinone (0.5 µg/kg/minute) and sodium nitroprusside (0.5 µg/kg/minute). Anesthesia was induced with fentanyl (4 µg/kg), midazolam (0.05 mg/kg), and propofol (1.5 mg/kg), followed by rocuronium for neuromuscular blockade. Anesthesia was maintained with isoflurane, a dexmedetomidine infusion (0.5 µg/kg/hour), fentanyl (total intraoperative dose of 12 µg/kg), and rocuronium. CPB management was similar to that used during LVAD placement. Bivalirudin was again used for anticoagulation with a continuous infusion that was started at 2.5 mg/kg/hour and proceeded by a bolus dose of 1.5 mg/kg. Additionally, the CPB circuit was primed with bivalirudin (50 mg). The bivalirudin dosing regimen and the ACT values are listed in Table 2. Post-CPB hemostasis and reversal of anticoagulation was performed with the administration of FFP (38 mL/kg), platelets (6.9 mL/kg), cryoprecipitate (1.1 mL/kg), and recombinant factor VIIa (90 µg/kg). Anesthesia, surgery, CPB, and aortic cross clamp times were 565, 455, 175, and 162 minutes, respectively. The patient was transported to the CTICU with his trachea intubated and mechanical ventilation provided. Milrinone and sodium nitroprusside were infusing at 0.5 µg/kg/minute. Postoperative laboratory evaluation at arrival to the CTICU revealed a PT of 23.3 seconds, a PT-INR of 2.04, an APTT of 64 seconds, and a fibrinogen of 243 mg/dL. Postoperative chest tube output for the first 24 postoperative hours was 19 mL/kg. During this time, the following blood products and colloids were administered: pRBCs (18 mL/kg), FFP (7.5 mL/kg), platelets (6.3 mL/kg), and albumin (2.4 mL/kg). The bleeding decreased and no transfusion was needed after POD 2. He was again extubated on POD 1. His hospital course was unremarkable. He was discharged home on POD 18 after heart transplantation.
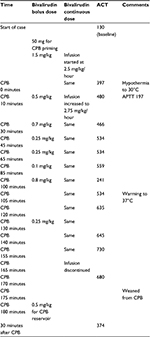  | Table 2 Bivalirudin dosing and ACT values during CPB for heart transplant Abbreviations: ACT, activated clotting time; APTT, activated partial thromboplastin time; CPB, cardiopulmonary bypass. |
Discussion
Following the suspicion or diagnosis of HIT, all sources of heparin exposure should be discontinued immediately. As needed, alternative nonheparin anticoagulation agents should be initiated (Table 3). The choice of the alternative anticoagulant agent depends on several factors including patient-related factors (renal and hepatic function), local and regional availability of the specific agents, metabolic considerations, half-life, institutional experience, and available laboratory monitoring. Although it has a lower chance of eliciting HIT, LMWH is contraindicated because of its high cross-reactivity with heparin–PF4 antibodies. The orally administered vitamin K antagonists (warfarin) are not effective as the initial, sole anticoagulation therapy in the acute phase of HIT because of a slow onset and the risk of causing limb or skin necrosis. However, warfarin can be administered in the event that long-term anticoagulation therapy is needed after the platelet count recovers. Although current guidelines recommend heparin bridging with discontinuation of warfarin in patients with significant thromboembolic risk undergoing surgery,9 preliminary evidence demonstrates that pacemaker and ICD placement can be safely performed in selected patients without a perioperative pause in warfarin anticoagulation.10–12 Given these data, we chose not to interrupt or reverse warfarin therapy prior to placement of the ICD in our patient.
When a patient with HIT requires elective cardiac surgery, surgery should be postponed until the heparin–PF4 antibody levels have become negative if this is clinically feasible. Heparin–PF4 antibodies are transient and are usually nondetectable after 100 days.13 Heparin can be safely used for patients with previous HIT who require cardiac surgery if they no longer have circulating HIT antibodies.14,15 However, it is generally recommended that both UFH and LMWH be avoided for pre- and postoperative anticoagulation. If a patient with a history of HIT requires cardiac surgery with CPB while the heparin–PF4 antibody is still present, alternative means of anticoagulation are required, generally the DTIs (Table 3).14–16 DTIs act on the thrombin molecule, inhibiting the conversion of fibrinogen to fibrin.
Introduced in 1909, hirudin was the first parental anticoagulant available for clinical use.16,17 It is a natural thrombin inhibitor, which is produced by the salivary gland of the leech.16 Although hirudin was used for anticoagulation during the first clinical applications of hemodialysis, heparin subsequently became available in the early 1950s and, since then, has become the favored agent for parenteral anticoagulation. Hirudin is no longer available for routine clinical use. Lepirudin is a recombinant form of hirudin (r-hirudin), consisting of a single polypeptide chain of 65 amino acids. It forms an irreversible (1:1) complex with thrombin. The amino-terminal domain binds to the active site of the thrombin, and the carboxyl-terminal domain interacts with the fibrinogen-binding site, completely inhibiting the procoagulant actions of thrombin. Unlike heparin, lepirudin also inhibits clot-bound thrombin, including thrombin on fibrin that may line the CPB circuit. Lepirudin is primarily eliminated by the kidneys with a plasma half-life of 80 minutes. Given its renal excretion, its half-life is prolonged in renal insufficiency or failure and dose adjustments are needed based on the degree of renal dysfunction. In the absence of a known antidote or drug to reverse its effect, hemofiltration has been used to enhance the elimination of lepirudin at the end of CPB.18,19 Although clinical trials have demonstrated its efficacy to provide anticoagulation, allergic reactions ranging from urticaria to hemodynamic instability have been reported.20,21 These anaphylactoid reactions may occur regardless of previous exposure, although reexposure to lepirudin is associated with a greater risk when compared with first exposure.22 Therefore, lepirudin administration should be limited to a single exposure whenever clinically feasible. Lepirudin is no longer available in the USA.
Argatroban is a DTI that was first described in Japan in 1981 for clinical use.23 It is a small, synthetic, nonantigenic molecule derived from L-arginine. Argatroban binds directly and reversibly to the catalytic site of thrombin, thereby preventing the conversion of fibrinogen to fibrin. It undergoes hepatic metabolism with a half-life that is shorter than lepirudin (40–60 vs 80 minutes).24 The half-life is significantly increased, and clearance decreased in patients with hepatic impairment, mandating dose reduction.25 There is no antidote to reverse its anticoagulant effects. Anecdotal experience has demonstrated the successful use of argatroban for anticoagulation in pediatric patients during ECMO, CPB, and surgery for congenital heart disease.26,27
Bivalirudin, which was used for anticoagulation during CPB for both LVAD placement and heart transplantation in our patient, is also a parenteral DTI. It is a 20-amino acid synthetic peptide consisting of two hirudin peptide fragments connected by a tetraglycine spacer. Anticoagulation is achieved through specific and reversible interaction with the catalytic site of thrombin. The amino-terminal segment electively binds to the catalytic site of thrombin and the carboxyl-terminal portion binds to the fibrinogen site on thrombin. The half-life of bivalirudin is only 25 minutes, and it is predominantly (80%) eliminated through proteolytic cleavage within the plasma. A minority (20%) is excreted by the kidneys.28 Despite limited dependence on renal excretion, its clearance is reduced by ~80% in patients with renal failure. As such, argatroban that undergoes hepatic metabolism is generally preferred in patients with renal insufficiency or failure.14 As with the other DTIs, no antidote is available to reverse its anticoagulant effects. Although argatroban and bivalirudin are approved by the US Food and Drug Administration (FDA) for anticoagulation in adult patients with HIT, neither is approved for administration in the pediatric population.
Unlike heparin therapy where the ACT or heparin assays are readily available to monitor anticoagulation, there are no readily available laboratory monitor devices or tests to accurately and rapidly measure the therapeutic effect of the DTIs. Although the PT, INR, APTT, thrombin time, and ACT increase after the administration of bivalirudin, the best method for monitoring anticoagulation produced by this agent remains to be established. Although there is a dose–response relationship between the concentration of bivalirudin and prolongation of the ACT or APTT at lower concentrations of bivalirudin (eg, for cardiac catheter procedures), neither correlate well at the higher concentrations required for CPB.29 The ecarin clotting time (ECT) has been suggested to be superior to the ACT for bivalirudin anticoagulation monitoring during CPB. Anecdotal reports and case series suggest that the ACT can be used during CPB with the goal being an ACT of ≥400 seconds.30,31 Given the lack of ready availability to ECT monitoring in most institutions, we chose to use a combination of ACT and APTT to monitor anticoagulation. As noted in our case, local clot formation can occur in areas of stagnant flow (eg, CPB reservoir and pericardial cavity) and may reflect local bivalirudin metabolism.
Given the shorter half-life and normal renal function in our patient, we chose to use bivalirudin during both LVAD placement and CPB in our patient. Anecdotal reports have demonstrated the efficacy of bivalirudin for providing anticoagulation in various clinical scenarios in the pediatric population during CPB as well as during heart transplantation (Table 4).29,32–47 The first report of bivalirudin use in a pediatric patient involved its use for CPB during orthotropic heart transplantation in a 5-year-old child with HIT.32 Prior to the initiation of CPB, the circuit was primed with a 50 mg bolus of bivalirudin. During CPB, a bivalirudin infusion was started at ~10% of the recommended adult dose. This included an intravenous bolus dose of 0.15 mg/kg followed by an infusion at 0.25 mg/kg/hour. The ACT was used for monitoring anticoagulation, and repeated bolus doses of bivalirudin (total 0.9 mg/kg) were required during the first 30 minutes of CPB to achieve the target range of ACT >400 seconds. Although significant bleeding occurred after separation from CPB, hemostasis was achieved with administration of blood products, including recombinant factor VII and ultrafiltration.
We report the successful use of bivalirudin to provide anticoagulation during LVAD placement and then CPB for heart transplantation in an adolescent with dilated cardiomyopathy and HIT. Bivalirudin is approved by the FDA for anticoagulation in adult patients undergoing percutaneous coronary intervention (PCI) with or at risk of HIT or HIT and thrombosis syndrome (HITTS). However, bivalirudin is not approved for administration in the pediatric population. As with the other DTIs, no antidote is available to reverse its anticoagulant effects. The half-life of bivalirudin is shorter than the other DTIs and it is predominantly (80%) eliminated through proteolytic cleavage within the plasma. As other anecdotal reports have demonstrated, bivalirudin was an effective method of anticoagulation during CPB for our patient with HIT during both procedures. Our dosing regimen included an initial bolus of 1.5 mg/kg to patient with 50 mg added to the priming volume of the CPB circuit. This was followed by a continuous infusion starting at 2.5 mg/kg/hour. This dosing regimen is similar to those reported for adults.48 Given the decreased enzymatic metabolism with changes in body temperature, special precautions are needed when bivalirudin is administered for anticoagulation during CPB in the presence of hypothermia. In the absence of accurate monitoring for bivalirudin, the duration of hypothermia should be as short as feasible, and if possible, deep hypothermia should be avoided. The patient’s temperature should be maintained normothermia at the end of CPB and into the postoperative period to ensure rapid metabolism. Following weaning from CPB, after the needed pump volume is reinfused to patient, a bolus dose of bivalirudin should be administered into the CPB circuit to prevent clot formation in reservoir in the event that a return to bypass support is needed. Additionally, continued circulation of the blood in the CPB machine is needed to prevent clot formation in stagnant areas. The remaining pump volume should be treated using a cell saver to clear any remaining bivalirudin. Although there is no specific monitoring device for the anticoagulation effect of bivalirudin, ACT prolongation to >400 seconds or >2.5 times baseline has been suggested.48 In the absence of a known antidote to reverse its effect, modified ultrafiltration can be used to enhance the elimination of bivalirudin. The reader is referred to Warkentin and Koster48 for additional useful information regarding bivalirudin use during CPB.
Conclusion
We present the successful use of bivalirudin anticoagulation in an adolescent with HIT during CPB who underwent both placement of a LVAD and subsequent heart transplantation. Although bivalirudin is not currently approved for the administration in the pediatric population, anticoagulation for both cases was safely achieved using bivalirudin as has been reported in previous case reports. Advantages of bivalirudin when compared with other DTIs include a shorter half-life of bivalirudin and limited dependence on renal and hepatic clearance. Although reversal is not feasible, modified ultrafiltration can be used to enhance elimination.
Disclosure
The authors report no conflicts of interest in this work.
References
Mureebe L, Silver D. Heparin-induced thrombocytopenia: pathophysiology and management. Vasc Endo Surg. 2002;36:163–170. | ||
Warkentin TE, Sheppard JA, Horsewood P, Simpson PJ, Moore JC, Kelton JG. Impact of the patient population on the risk for heparin-induced thrombocytopenia. Blood. 2000;96(5):1703–1708. | ||
Martel N, Lee J, Wells PS. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood. 2005;106(8):2710–2715. | ||
Jang IK, Hursting MJ. When heparins promote thrombosis: review of heparin-induced thrombocytopenia. Circulation. 2005;111:2671–2683. | ||
Vakil NH, Kanaan AO, Donovan JL. Heparin-induced thrombocytopenia in the pediatric population: a review of current literature. J Pediatr Pharmacol Ther. 2012;17(1):12–30. | ||
Hirsh J, Heddle N, Kelton JG. Treatment of heparin-induced thrombocytopenia: a critical review. Arch Intern Med. 2004;164(4):361–369. | ||
Lewis BE, Wallis DE, Berkowitz SD, et al; ARG-911 Study Investigators. Argatroban anticoagulant therapy in patients with heparin-induced thrombocytopenia. Circulation. 2001;103(14):1838–1843. | ||
Frost J, Mureebe L, Russo P, Russo J, Tobias JD. Heparin-induced thrombocytopenia in the pediatric intensive care unit population. Pediatr Crit Care Med. 2005;6(2):216–219. | ||
Douketis JD, Berger PB, Dunn AS, et al. The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:299S–339S. | ||
Yokoshiki H, Mitsuyama H, Watanabe M, Mizukami K, Matsui Y, Tsutsui H. Anticoagulation management in the perioperative phase of implantable cardioverter defibrillator implantation. Circ J. 2013;77(8):2003–2008. | ||
Crosato M, Calzolari V, Franceschini Grisolia E, et al. Implanting cardiac rhythm devices during uninterrupted warfarin therapy: a prospective, single center experience. J Cardiovasc Med (Hagerstown). 2015;16(7):503–506. | ||
Airaksinen KE, Korkeila P, Lund J, et al. Safety of pacemaker and implantable cardioverter-defibrillator implantation during uninterrupted warfarin treatment – the FinPAC study. Int J Cardiol. 2013;168(4):3679–3682. | ||
Warkentin TE, Kelton JG. Temporal aspects of heparin-induced thrombocytopenia. N Engl J Med. 2001;344(17):1286–1292. | ||
Linkins LA, Dans AL, Moores LK, et al. Treatment and prevention of heparin-induced thrombocytopenia: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e495–e530. | ||
Watson H, Davidson S, Keeling D. Haemostasis and Thrombosis Task Force of the British Committee for standards in haematology. Guidelines on the diagnosis and management of heparin-induced thrombocytopenia: second edition. Br J Haematol. 2012;159:528–540. | ||
Greinacher A, Lubenow N. Recombinant hirudin in clinical practice: focus on lepirudin. Circulation. 2001;103(10):1479–1484. | ||
Engelmann F, Stade C. Über die Bedeutung des Blutegelextraktes für die Therapie der Eklampsie [The importance of the leech extract for the therapy of eclampsia]. Münchner Medizinische Wochenschrift. 1909;43:2203–2207. | ||
Petros S. Lepirudin in the management of patients with heparin-induced thrombocytopenia. Biologics. 2008;2(3):481–490. | ||
Willey ML, de Denus S, Spinler SA. Removal of lepirudin, a recombinant hirudin, by hemodialysis, hemofiltration, or plasmapheresis. Pharmacotherapy. 2002;22(4):492–499. | ||
Bircher AJ, Czendlik CH, Messmer SL, Müller P, Howald H. Acute urticaria caused by subcutaneous recombinant hirudin: evidence for an IgG-mediated hypersensitivity reaction. J Allergy Clin Immunol. 1996;98(5 pt 1):994–996. | ||
Badger NO, Butler K, Hallman LC. Excessive anticoagulation and anaphylactic reaction after rechallenge with lepirudin in a patient with heparin-induced thrombocytopenia. Pharmacotherapy. 2004;24(12):1800–1803. | ||
Greinacher A, Lubenow N, Eichler P. Anaphylactic and anaphylactoid reactions associated with lepirudin in patients with heparin-induced thrombocytopenia. Circulation. 2003;108(17):2062–2065. | ||
Okamoto S, Hijikata A, Kikumoto R, et al. Potent inhibition of thrombin by the newly synthesized arginine derivative No. 805. The importance of stereo-structure of its hydrophobic carboxamide portion. Biochem Biophys Res Commun. 1981;101:440–446. | ||
Moledina M, Chakir M, Gandhi PJ. A synopsis of the clinical uses of argatroban. J Thromb Thrombolysis. 2001;12(2):141–149. | ||
Swan SK, Hursting MJ. The pharmacokinetics and pharmacodynamics of argatroban: effects of age, gender, and hepatic or renal dysfunction. Pharmacotherapy. 2000;20(3):318–329. | ||
Latham GJ, Jefferis Kirk C, Falconer A, Dickey R, Albers EL, McMullan DM. Challenging argatroban management of a child on extracorporeal support and subsequent heart transplant. Semin Cardiothorac Vasc Anesth. 2016;20(2):168–174. | ||
Dyke PC 2nd, Russo P, Mureebe L, Russo J, Tobias JD. Argatroban for anticoagulation during cardiopulmonary bypass in an infant. Paediatr Anaesth. 2005;15(4):328–333. | ||
Kentin TE, Koster A. Bivalirudin: a review. Expert Opin Pharmacother. 2005;6(8):1349–1371. | ||
Rayapudi S, Torres A Jr, Deshpande GG, et al. Bivalirudin for anticoagulation in children. Pediatr Blood Cancer. 2008;51(6):798–801. | ||
Cho L, Kottke-Marchant K, Lincoff AM. Correlation of point-of-care ecarin clotting time versus activated clotting time with bivalirudin concentrations. Am J Cardiol. 2003;91:1110–1113. | ||
Koster A, Chew D, Gründel M, et al. Bivalirudin monitored with the ecarin clotting time for anticoagulation during cardiopulmonary bypass. Anesth Analg. 2003;96:383–386. | ||
Almond CS, Harrington J, Thiagarajan R, et al. Successful use of bivalirudin for cardiac transplantation in a child with heparin-induced thrombocytopenia. J Heart Lung Transplant. 2006;25(11):1376–1379. | ||
Gates R, Yost P, Parker B. The use of bivalirudin for cardiopulmonary bypass anticoagulation in pediatric heparin-induced thrombocytopenia patients. Artif Organs. 2010;34(8):667–669. | ||
Dragomer D, Chalfant A, Biniwale R, Reemtsen B, Federman M. Novel techniques in the use of bivalirudin for cardiopulmonary bypass anticoagulation in a child with heparin-induced thrombocytopenia. Perfusion. 2011;26(6):516–518. | ||
Argueta-Morales IR, Olsen MC, DeCampli WM, Munro HM, Felix DE. Alternative anticoagulation during cardiovascular procedures in pediatric patients with heparin-induced thrombocytopenia. J Extra Corpor Technol. 2012;44(2):69–74. | ||
Faella KH, Whiting D, Fynn-Thompson F, Matte GS. Bivalirudin anticoagulation for a pediatric patient with heparin-induced thrombocytopenia and thrombosis requiring cardiopulmonary bypass for ventricular assist device placement. J Extra Corpor Technol. 2016;48(1):39–42. | ||
Pollak U, Yacobobich J, Tamary H, Dagan O, Manor-Shulman O. Heparin-induced thrombocytopenia and extracorporeal membrane oxygenation: a case report and review of the literature. J Extra Corpor Technol. 2011;43(1):5–12. | ||
Ranucci M, Ballotta A, Kandil H, et al; Surgical and Clinical Outcome Research Group. Bivalirudin-based versus conventional heparin anticoagulation for postcardiotomy extracorporeal membrane oxygenation. Crit Care. 2011;15(6):R275. | ||
Nagle EL, Dager WE, Duby JJ, et al. Bivalirudin in pediatric patients maintained on extracorporeal life support. Pediatr Crit Care Med. 2013;14(4):e182–e188. | ||
Preston TJ, Dalton HJ, Nicol KK, Ferrall BR, Miller JC, Hayes D Jr. Plasma exchange on venovenous extracorporeal membrane oxygenation with bivalirudin anticoagulation. World J Pediatr Congenit Heart Surg. 2015;6(1):119–122. | ||
Rutledge JM, Chakravarti S, Massicotte MP, Buchholz H, Ross DB, Joashi U. Antithrombotic strategies in children receiving long-term Berlin Heart EXCOR ventricular assist device therapy. J Heart Lung Transplant. 2013;32(5):569–573. | ||
Zamora R. Successful anticoagulation with bivalirudin in antithrombin-deficient pediatric patient undergoing stent placement. Catheter Cardiovasc Interv. 2006;68(2):292–295. | ||
Breinholt JP, Moffett BS, Texter KM, Ing FF. Successful use of bivalirudin for superior vena cava recanalization and stent placement in a child with heparin-induced thrombocytopenia. Pediatr Cardiol. 2008;29(4):804–807. | ||
Forbes TJ, Hijazi ZM, Young G, et al. Pediatric catheterization laboratory anticoagulation with bivalirudin. Catheter Cardiovasc Interv. 2011;77(5):671–679. | ||
Young G, Tarantino MD, Wohrley J, Weber LC, Belvedere M, Nugent DJ. Pilot dose-finding and safety study of bivalirudin in infants <6 months of age with thrombosis. J Thromb Haemost. 2007;5:1654–1659. | ||
Malloy KM, McCabe TA, Kuhn RJ. Bivalirudin use in an infant with persistent clotting on unfractionated heparin. J Pediatr Pharmacol Ther. 2011;16(2):108–112. | ||
O’Brien SH, Yee DL, Lira J, Goldenberg NA, Young G. UNBLOCK: an open-label, dose-finding, pharmacokinetic and safety study of bivalirudin in children with deep vein thrombosis. J Thromb Haemost. 2015;13(9):1615–1622. | ||
Warkentin TE, Koster A. Bivalirudin: a review. Expert Opin Pharmacother. 2005;6(8):1349–1371. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



