Back to Journals » Nature and Science of Sleep » Volume 8
Periodic limb movements of sleep: empirical and theoretical evidence supporting objective at-home monitoring
Authors Moro M, Goparaju B, Castillo J, Alameddine Y, Bianchi M
Received 3 December 2015
Accepted for publication 26 February 2016
Published 8 August 2016 Volume 2016:8 Pages 277—289
DOI https://doi.org/10.2147/NSS.S101753
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Steven A Shea
Marilyn Moro,1 Balaji Goparaju,1 Jelina Castillo,1 Yvonne Alameddine,1 Matt T Bianchi1,2
1Neurology Department, Massachusetts General Hospital, 2Division of Sleep Medicine, Harvard Medical School, Boston, MA, USA
Introduction: Periodic limb movements of sleep (PLMS) may increase cardiovascular and cerebrovascular morbidity. However, most people with PLMS are either asymptomatic or have nonspecific symptoms. Therefore, predicting elevated PLMS in the absence of restless legs syndrome remains an important clinical challenge.
Methods: We undertook a retrospective analysis of demographic data, subjective symptoms, and objective polysomnography (PSG) findings in a clinical cohort with or without obstructive sleep apnea (OSA) from our laboratory (n=443 with OSA, n=209 without OSA). Correlation analysis and regression modeling were performed to determine predictors of periodic limb movement index (PLMI). Markov decision analysis with TreeAge software compared strategies to detect PLMS: in-laboratory PSG, at-home testing, and a clinical prediction tool based on the regression analysis.
Results: Elevated PLMI values (>15 per hour) were observed in >25% of patients. PLMI values in No-OSA patients correlated with age, sex, self-reported nocturnal leg jerks, restless legs syndrome symptoms, and hypertension. In OSA patients, PLMI correlated only with age and self-reported psychiatric medications. Regression models indicated only a modest predictive value of demographics, symptoms, and clinical history. Decision modeling suggests that at-home testing is favored as the pretest probability of PLMS increases, given plausible assumptions regarding PLMS morbidity, costs, and assumed benefits of pharmacological therapy.
Conclusion: Although elevated PLMI values were commonly observed, routinely acquired clinical information had only weak predictive utility. As the clinical importance of elevated PLMI continues to evolve, it is likely that objective measures such as PSG or at-home PLMS monitors will prove increasingly important for clinical and research endeavors.
Keywords: periodic limb movements, polysomnography, predictors, sleep, decision analysis, cost-effectiveness, diagnostic
Introduction
Periodic limb movements of sleep (PLMS), brief muscle activations occurring at regular intervals, currently require laboratory polysomnography (PSG) for quantification.1 Periodic limb movement disorder is the combination of periodic limb movement index (PLMI) of ≥15 events per hour and symptoms attributable to the PLMS. Elevated PLMI is commonly observed in patients with restless legs syndrome (RLS),2,3 which itself has been linked to obesity,4 cardiovascular disease,5,6 cerebrovascular disease,7,8 and anxiety and depression.9–11 PLMS have been associated with a variety of health problems as well, including posttraumatic stress disorder,12 narcolepsy,13 renal disease,14 congestive heart failure,15 alcoholism,16 mood disorders,10,17–20 Parkinson’s disease,21–25 and attentional problems.26,27 Most importantly, PLMS has been associated with higher risk of cardiovascular and/or cerebrovascular events.7,28,29 Despite this growing list of associations, aggressive investigation for PLMS is not commonly undertaken (compared, eg, to sleep apnea), perhaps in part since causality remains uncertain particularly regarding whether PLMS treatment can mitigate these risks.
The range of reported PLMS prevalence is highly variable, from 4% to 47% in adults depending on the population and definitions used,3 and PLMS are commonly comorbid with obstructive sleep apnea (OSA),20 insomnia,3 and advanced age.30 Given the potential connection between PLMS and cardiovascular and cerebrovascular morbidity, the question arises as to how to best identify patients with PLMS, the majority of which do not have RLS symptoms. Such information could improve screening decisions and transform PLMS from mainly an incidental PSG finding to an actively sought aspect of clinical sleep evaluations. Clinical suspicion of PLMS in non-RLS patients centers mainly around bed-partner report of the limb movements, the reliability of which is uncertain. We approached this problem by empirical data analysis from our center and by theoretical decision modeling to place the empirical findings into broader clinical context. Specifically, we tested the hypothesis that PLMS indices can be predicted by a combination of demographic and self-reported symptoms in a retrospective database study from our sleep laboratory. We subsequently performed cost-effectiveness modeling to compare clinical screening, in-laboratory PSG, and at-home device monitoring, for diagnosis and management of PLMS.
Methods
We performed a retrospective analysis of clinical PSGs performed in our center. The Partners Healthcare Institutional Review Board approved the use of this clinical database without additional informed consent. Prespecified exclusions included age <18 years, missing presleep clinical questionnaires, or <3 hours of total PSG sleep time.
We considered two groups in this retrospective analysis based on OSA metrics (n=209 without OSA and n=443 with OSA; the OSA cases were previously reported in an unrelated study of sleep perceptions)31. We prespecified that OSA was defined by the apnea–hypopnea index (AHI) as AHI≥5, while No-OSA was defined as AHI <5 and a respiratory disturbance index (RDI) of <10. The RDI cutoff for No-OSA cases was prespecified to exclude patients with frequent nonhypoxic events in the No-OSA group.
PSGs were scored according to American Academy of Sleep Medicine standard definitions for stages, breathing, and movements. We used the 4% rule for hypopneas and nasal pressure flattening with arousal/recovery for respiratory event-related electroencephalogram (EEG) arousals. The RDI includes apneas, hypopneas, and respiratory event-related EEG arousals, per hour of sleep. PLMS were scored according to standard American Academy of Sleep Medicine criteria; scoring conventions include censoring limb movements associated with scored respiratory events. Each patient undergoing PSG in our center completes a self-report inventory of symptoms and clinical history. We prespecified that the presence of RLS symptom means that one or more of the following queries were positive: 1) “When awake, my legs have a funny or uncomfortable sensation”, 2) “This problem is worse in the evening”, and 3) “When I move around, this sensation gets better”. PLMI and limb movement arousal index (LMAI) were nonnormally distributed and thus analyzed by nonparametric methods. Self-reported medications were manually categorized: antihypertensive, psychiatric, sleeping aid, medication used to treat RLS (dopaminergic agents, gabapentin, pregabalin), and stimulant.
We used SAS version 9.3 (SAS Institute, Inc., Cary, NC, USA) for statistical analyses. To determine significant predictors for PLMS, covariates collected during the diagnostic night were all included in logistic regression (LR) analyses for PLMI. Spearman’s correlation analyses were performed to determine possible interaction terms to be included in LR analyses for PLMI. For LR analyses, PLMI values were transformed to a categorical variable (PLMI ≥15 threshold). Diagnostic PSG data were also analyzed under Wilcoxon rank-sum and chi-square (χ2) tests for determining differences in PMLI and LMAI distributions by OSA status.
We performed decision analysis with TreeAge software (TreeAge Pro, 2015; Williamstown, MA, USA). Markov modeling was used to evaluate the cost-effectiveness of different strategies to diagnose PLMS in adults and initiate treatment to prevent a composite outcome of cardiovascular and/or cerebrovascular events. The time horizon was 5 years. The population was assumed to be adults free of RLS (which could independently indicate treatment) and free of OSA (an independent reason and benefit of laboratory PSG testing). Elevated PLMI is linked to increased cardiovascular or cerebrovascular event risk in the model, and this risk is assumed to decrease with pharmacological therapy. Such risk mitigation has not yet been demonstrated in clinical trials, yet is plausible based on cardiac physiology data32–35 and that dopaminergic medications decrease PLMS indices.36
Patients testing positive for PLMS (whether true or false positive) may initiate dopaminergic treatment. Health outcomes were expressed in quality-adjusted life-years gained. We instituted a utility reduction for surviving a cardiovascular or cerebrovascular event of 0.84,37 but no change in utility was related to PLMS itself (ie, it is assumed to be asymptomatic) or its treatment (ie, medication adverse effects were not modeled but are indirectly captured in the probability of accepting treatment). Costs were considered from a third-party payer perspective. Costs included treatment of PLMS (cRx) and clinical visits (cCL), as well as for different PLMS testing methods: home-based PLMS device (cHD), cost for administering a clinical tool for predicting PLMS diagnosis (cCT), and cost of PSG testing (cPSG). The cost of having a cardiovascular or cerebrovascular event (cCV) was assumed to be a one-time cost for the acute event. For simplicity, we did not model costs of chronic cardiovascular or cerebrovascular care, which would further increase the expected cost of untreated PLMS and is thus a conservative assumption. The clinical tool sensitivity and specificity were assumed to be 65% each, in keeping with the small odds ratio values found in the correlation analysis of this work; the cost was assumed to be $10 (USD) for time to administer.
Table 1 shows the costs, utilities, and probabilities used in the model. Costs and utilities were collected and compared using a willingness to pay threshold of $50,000 (USD) per quality-adjusted life-year. The base case values are shown in Table 1. The annual cost for PLMS treatment (cRx) was $485 (USD), and the cost for home device (cHD) usage for diagnosis was estimated at $100 (USD). The sensitivity (sensHD) and specificity (specHD) of HD were defined as 82.4% and 70.8%, respectively, for detecting binary presence/absence of elevated PLMS (>15/h) based on a recent study,38 which is within the ranges reported in the literature for leg actigraphy.39 Sensitivity and specificity of PSG for this binary determination were defined as 90% and 95%, respectively. The cost of PSG was estimated at $800 (USD). Modifying factors for cardiovascular or cerebrovascular risk included increased cardiovascular or cerebrovascular risk given PLMS (a), increased cardiovascular or cerebrovascular risk in those who have had a cardiovascular or cerebrovascular event (g), and decreased cerebrovascular risk in true positives who accept treatment.
Results
We investigated subjective and objective PSG findings in patients undergoing clinical PSG in our laboratory: one group had normal breathing metrics defined by AHI <5 and RDI <10 (“No-OSA” group) and one group had AHI ≥5 on their diagnostic PSG (Dx-OSA) and returned for continuous positive airway pressure titration on a subsequent night (Rx-OSA). Baseline PSG characteristics were similar, aside from the expected OSA-defining metrics (Table 2). OSA patients were older and had a higher body mass index (BMI) compared to No-OSA patients, and were more likely to be male, consistent with known OSA risk factors (Table 3).
No-OSA and OSA groups had similar PLMI and LMAI values during diagnostic PSG (Table 2). Small but significantly higher values of median PLMI and LMAI were observed during Rx-OSA compared to Dx-OSA nights. However, cumulative density function plots show that the distributions separate more clearly after the 50th percentile range, leading us in subsequent analyses to utilize the clinically relevant cutoff of PLMI ≥15 as the target predictor for logistic models (later) (Figure 1), which is the ~65th–75th percentile for Dx-OSA and Rx-OSA.
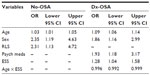
Before performing multiple LR models, we investigated PLMI and LMAI values across clinical and demographic categories for each group of PSGs (No-OSA, Dx-OSA, and Rx-OSA), which showed distinct clinical associations in the OSA versus the No-OSA groups (Table S1). Next, correlations between PLMI values and clinical variables (Tables S2–S5) were undertaken to inform regression modeling for predictors of elevated PLMI (≥15), adjusting for age, sex, BMI, and self-reported medications (Table 4). Age (P=0.02), male sex (P=0.01), and self-reported RLS (P=0.02) predicted PLMI ≥15 in No-OSA patients. For the Dx-OSA group, predictors were age (P<0.0001), male sex (P=0.01), and self-reported intake of psychiatric medications (P=0.01). The Epworth Sleepiness score and its interaction with age were also significant (P=0.02), although the latter was quite small (β=−0.004) and is of uncertain clinical importance. The odds ratios are given in Table 3. The portion of the variance in PLMI status explained by the models for No-OSA and Dx-OSA groups was modest: ~9% and ~14%, respectively.
Given the challenges of predicting elevated PLMS according to the earlier analysis, we undertook a decision modeling approach to compare strategies for diagnosing and subsequently treating patients with elevated PLMS. As there is much uncertainty with regard to clinical and device-based screening for elevated PLMS, as well as the impact of treatment on associated morbidities, we made several simplifying assumptions that could be addressed in future prospective studies. We assumed a cardiovascular risk associated with untreated PLMS that is mitigated by pharmacological treatment. We compared four strategies to approach the problem of elevated PLMS in a presumed asymptomatic adult population (Figure 2). The “do-nothing” strategy and obtaining PSG on all patients represent boundary conditions of baseline health burden of PLMS (do-nothing) versus better accuracy of diagnosis as well as higher cost of testing (PSG for all). These were compared with two more realistic strategies: 1) assessing PLMS risk using a clinical tool based on the aforementioned LR model, followed by confirmatory PSG testing in those who screen positive, and 2) actigraphic HD testing for PLMS. Several reports of lower limb actigraphy have been published;39 we utilized values from the PAM-RL device.38
We performed sensitivity analysis across three key parameters (Figure 3). Prevalence is uncertain and varies across certain populations, so we considered this across a tenfold range from 3.5% to 35%. The sensitivity, specificity, and cost of a HD were also investigated, as device-based PLMS diagnostics are not common clinically and lack extensive study. The resulting plots provide an overview of the main considerations that would drive decision making for the question of medical benefit from testing and treating PLMS. First, the cost of home testing is not predicted to play a major role, as the difference between decision boundaries was small when testing was free versus costing $100 per person. This is perhaps not unexpected, as the cost of home testing is small in general compared to the cost of medical morbidity associated with untreated PLMS and that of pharmacotherapy for PLMS. Second, the pretest probability of PLMS played a major role in the optimal decision pathway, with higher values driving the decision to pursue some form of objective diagnostic testing. Interestingly, because the accuracy of the clinical tool was modest, it is never favored in the decision space. Instead, the two dominant pathways are the do-nothing path and the home-test path. PSG is also not preferred across this range of values, when considered from the sole perspective of identifying PLMS, suggesting that the increased cost does not justify the improved accuracy.
Discussion
Elevated PLMI was not uncommon among adult patients undergoing PSG testing, with ~26% having PLMI ≥15/h. Among the 13% of the study population who self-reported leg jerking during sleep, approximately one-third of them (4.5%) showed PLMI ≥15/h during their diagnostic PSG night, indicating that this self-reported symptom is not strongly predictive of elevated PLMI (ie, low positive predictive value of symptoms). Self-reported RLS symptoms and the Epworth Sleepiness scores, along with age, male sex, and self-reported psychiatric medications, were each associated with PLMI ≥15, although the odds ratios were fairly small. Nevertheless, the correlations are generally consistent with prior work.3,8,34,40–46 Other work identified a female predominance of PLMS,47 but this was not seen in this study. Previous work has linked antidepressants to PLMS,40,45 and we observed an association of psychiatric medications with elevated PLMI, albeit only in those with comorbid OSA.
The limited predictive power of simple symptom inventories suggests that PLMS may go undetected in practice, except for incidental findings on PSG obtained for other reasons (usually OSA). Various studies have reported that symptoms of sleepiness and PLMS are not well correlated,48–52 except when RLS is present.3 Given that current clinical practice requires PSG to quantify PLMS,53 yet PSG utilization is increasingly restricted, understanding the extent to which clinical information alone will predict elevated PLMI on PSG remains an important goal. This work supports prior literature in the suggestion that, in the absence of RLS, it can be quite challenging to predict the presence of elevated PLMS by clinical history alone.
Clinical implications of PLMS
The clinical importance of PLMS is suggested by associations with a variety of medical and psychiatric comorbidities discussed earlier. Genetics and epidemiology studies suggest that the objective sleep disturbance and the genetic correlates with RLS are linked to the underlying PLMS.54 It is possible that the cardiovascular risk associated with RLS is mediated by objective sleep disturbance from PLMS via changes in heart rate, sympathetic activity, cortisol secretion, and cardiac vagal modulation.35,44,55 Increased heart rate is associated with PLMS more frequently than EEG arousals,35 even when LMAs cannot be detected.55,56 Another possible link with daytime hypertension could be through interference with normal nocturnal blood pressure dipping:42,57 The absence of dipping contributes to a greater cardiovascular risk58–60 and hypertension61,62 and cerebrovascular disease.63 PLMS are also associated with elevated plasma C-reactive protein and fibrinogen, markers of cardiovascular risk, in patients with OSA.64
Dopaminergic therapy decreases the number of PLMS and normalizes the increased PLMS-related heart rate variability response in patients with RLS.33 Whether such pharmacological therapy for PLMS improves cardiovascular outcomes is unknown. Future prospective trials are needed, ideally with objective monitoring to track clinical response.
Objective measurement of PLMS
In the absence of accurate clinical predictors, PLMS may remain underdiagnosed in the adult population. As home testing kits for diagnosing OSA increasingly replace in-laboratory PSG, even incidental findings of PLMS may decrease, exacerbating underdiagnosis of PLMS because no type 3 or type 4 kits measure PLMS.65 Likewise, as home autotitrations replace in-laboratory titrations, the relationship of PLMS to OSA and its therapy may not be realized. The current data and prior literature suggest that PAP treatment is associated with increased PLMS,66,67 although this may be related to conventions that restrict scoring PLMS occurring near breathing events. In our cohort, there was no relation of PLMI with OSA severity category during diagnostic PSG assessments: for AHI <5, AHI 5–15, and AHI >15, the median and IQR of PLMI in each group was 4.1 (0.7–14.5), 5.0 (0.9–15.5), and 3.6 (0.6–19.8), respectively. The group PLMI values were not statistically different by Mann–Whitney nonparametric test. Furthermore, there was no correlation between PLMI and AHI across the categories (Spearman’s R value 0.03; P>0.5). Nevertheless, recent work highlights the potential clinical utility of quantifying limb movements in sleep associated with respiratory events,68 an area that deserves further investigation.
The potential for at-home testing of leg movements could refine diagnostic phenotyping through multiple-night recordings and provide an objective marker for treatment response over time. In addition, home PLMS monitoring could provide an alternative to formal PSG for objective screening purposes. Leg actigraphy has been used in several studies to quantify PLMS, with a range of sensitivity and specificity generally in the 60%–90% range.39 Specifically, ankle-placed devices such as the PAM-RL have better sensitivity and thus better “rule-out” value, compared to devices placed on the dorsum of the foot (eg, Actiwatch), which has higher specificity and thus better “rule-in” value. Given observed night-to-night variability in PLMS,38,69,70 the capacity to record multiple nights will further inform diagnostic phenotyping by improving upon the single-night snapshot of laboratory PSG. Obtaining improved phenotype information via home tracking is important for clinical care and for outcomes-based research studies in this area by improving power of clinical trials and for individualized management plans in clinical practice. We speculate that at-home monitoring for PLMS will gain momentum because of the combination of poor clinical predictors of PLMS, potential morbidity risk of untreated PLMS, and the necessity to utilize alternatives to PSG for objective testing.
Decision modeling
We undertook a cost-effectiveness model to compare different diagnostic approaches for detecting (and treating) occult PLMS based on the assumption that elevated PLMI has health consequences and that these consequences are reduced with dopaminergic medication treatment. Although HD screening for occult PLMS is not currently considered standard of care, the model provides a framework for identifying key issues that can inform prospective studies in this regard. Across a 5-year time horizon during which cardiovascular events can occur, the HD strategy was cost-effective under plausible estimates of device accuracy, device cost, and PLMS prevalence. Importantly, the expected low cost per person of HD for PLMS did not impact the preferred strategy as much as prevalence of elevated PLMI. Even when the cost of home testing is free, we observe a relatively stronger impact of prevalence than on sensitivity and specificity of a HD. This finding suggests that accurate pretest probability estimation, whether by symptoms or by medical history, will be useful in future studies of the impact of therapy in populations with elevated PLMI. Including quality-of-life benefits of treating PLMS, which were omitted here because we assumed the PLMS to be occult, would be predicted to further favor testing from a cost-effectiveness perspective.
Limitations
Our study has several important limitations that could be addressed in future studies. We used data obtained from single nights of PSG, while it is likely that night variability occurs in PLMS. This could have reduced our power to find correlations with clinical or demographic factors, due to single-night sampling for assigning PLMS status. A related issue to clinical prediction is that symptoms and medicines were self-reported via simple questionnaire, rather than obtained in structured interview settings or with additional validation steps. Medication lists also do not capture reasons for therapy or compliance with therapy, which might also contribute to heterogeneity. Additionally, our clinical questionnaire only asks about a subset of medical and psychiatric disorders, and the responses are self-reported. Future work based on validation by electronic records can validate these items and explore more deeply the potential relationships. Our clinical laboratory is part of a tertiary referral center, and thus, the results may not generalize. Finally, with respect to the modeling, several simplifying assumptions were implemented and require further experimental investigation, most importantly, the risk reduction conferred by dopaminergic agents.
Disclosure
Dr Bianchi received funding from the Department of Neurology, Massachusetts General Hospital; the Center for Integration of Medicine and Innovative Technology; the Department of Defense; the Milton Family Foundation; MC10, Inc.; Insomnisolv, Inc.; and the American Sleep Medicine Foundation. He has a patent pending on a home sleep monitoring device. He has consulting agreements with Grand Rounds and International Flavors & Fragrances, has received travel funding from Servier, serves on the advisory board of Foramis, and has provided expert testimony in sleep medicine. The other authors report no conflicts of interest in this work.
References
ICSD-2. International Classification of Sleep Disorders: Diagnostic and Coding Manual. Second ed. Westchester: American Academy of Sleep Medicine; 2005. | |
Innes KE, Selfe TK, Agarwal P. Prevalence of restless legs syndrome in North American and Western European populations: a systematic review. Sleep Med. 2011;12(7):623–634. | |
Hornyak M, Feige B, Riemann D, Voderholzer U. Periodic leg movements in sleep and periodic limb movement disorder: prevalence, clinical significance and treatment. Sleep Med Rev. 2006;10(3):169–177. | |
Gao X, Schwarzschild MA, Wang H, Ascherio A. Obesity and restless legs syndrome in men and women. Neurology. 2009;72(14):1255–1261. | |
Li Y, Walters AS, Chiuve SE, Rimm EB, Winkelman JW, Gao X. Prospective study of restless legs syndrome and coronary heart disease among women. Circulation. 2012;126(14):1689–1694. | |
Winkelman JW, Shahar E, Sharief I, Gottlieb DJ. Association of restless legs syndrome and cardiovascular disease in the Sleep Heart Health Study. Neurology. 2008;70(1):35–42. | |
Walters AS, Rye DB. Review of the relationship of restless legs syndrome and periodic limb movements in sleep to hypertension, heart disease, and stroke. Sleep. 2009;32(5):589–597. | |
Ferini-Strambi L, Walters AS, Sica D. The relationship among restless legs syndrome (Willis-Ekbom Disease), hypertension, cardiovascular disease, and cerebrovascular disease. J Neurol. 2014;261(6):1051–1068. | |
Winkelman JW, Finn L, Young T. Prevalence and correlates of restless legs syndrome symptoms in the Wisconsin Sleep Cohort. Sleep Med. 2006;7(7):545–552. | |
Gupta R, Lahan V, Goel D. A study examining depression in restless legs syndrome. Asian J Psychiatr. 2013;6(4):308–312. | |
Kim KW, Yoon IY, Chung S, et al. Prevalence, comorbidities and risk factors of restless legs syndrome in the Korean elderly population – results from the Korean Longitudinal Study on Health and Aging. J Sleep Res. 2010;19(1 pt 1):87–92. | |
Breslau N, Roth T, Burduvali E, Kapke A, Schultz L, Roehrs T. Sleep in lifetime posttraumatic stress disorder: a community-based polysomnographic study. Arch Gen Psychiatry. 2004;61(5):508–516. | |
Baker TL, Guilleminault C, Nino-Murcia G, Dement WC. Comparative polysomnographic study of narcolepsy and idiopathic central nervous system hypersomnia. Sleep. 1986;9(1 pt 2):232–242. | |
Benz RL, Pressman MR, Hovick ET, Peterson DD. Potential novel predictors of mortality in end-stage renal disease patients with sleep disorders. Am J Kidney Dis. 2000;35(6):1052–1060. | |
Hanly PJ, Zuberi-Khokhar N. Periodic limb movements during sleep in patients with congestive heart failure. Chest. 1996;109(6):1497–1502. | |
Gann H, Feige B, Fasihi S, van Calker D, Voderholzer U, Riemann D. Periodic limb movements during sleep in alcohol dependent patients. Eur Arch Psychiatry Clin Neurosci. 2002;252(3):124–129. | |
Winkelmann J, Prager M, Lieb R, et al. “Anxietas tibiarum”. Depression and anxiety disorders in patients with restless legs syndrome. J Neurol. 2005;252(1):67–71. | |
Brand S, Beck J, Hatzinger M, Holsboer-Trachsler E. Patients suffering from restless legs syndrome have low internal locus of control and poor psychological functioning compared to healthy controls. Neuropsychobiology. 2013;68(1):51–58. | |
Lee HB, Ramsey CM, Spira AP, Vachon J, Allen R, Munro CA. Comparison of cognitive functioning among individuals with treated restless legs syndrome (RLS), untreated RLS, and no RLS. J Neuropsychiatry Clin Neurosci. 2014;26(1):87–91. | |
Al-Alawi A, Mulgrew A, Tench E, Ryan CF. Prevalence, risk factors and impact on daytime sleepiness and hypertension of periodic leg movements with arousals in patients with obstructive sleep apnea. J Clin Sleep Med. 2006;2(3):281–287. | |
Raggi A, Bella R, Pennisi G, Neri W, Ferri R. Sleep disorders in Parkinson’s disease: a narrative review of the literature. Rev Neurosci. 2013;24(3):279–291. | |
Schulte EC, Winkelmann J. When Parkinson’s disease patients go to sleep: specific sleep disturbances related to Parkinson’s disease. J Neurol. 2011;258(Suppl 2):S328–S335. | |
Young A, Home M, Churchward T, Freezer N, Holmes P, Ho M. Comparison of sleep disturbance in mild versus severe Parkinson’s disease. Sleep. 2002;25(5):573–577. | |
Dhawan V, Dhoat S, Williams AJ, et al. The range and nature of sleep dysfunction in untreated Parkinson’s disease (PD). A comparative controlled clinical study using the Parkinson’s disease sleep scale and selective polysomnography. J Neurol Sci. 2006;248(1–2):158–162. | |
Wetter TC, Collado-Seidel V, Pollmacher T, Yassouridis A, Trenkwalder C. Sleep and periodic leg movement patterns in drug-free patients with Parkinson’s disease and multiple system atrophy. Sleep. 2000;23(3):361–367. | |
Miano S, Parisi P, Villa MP. The sleep phenotypes of attention deficit hyperactivity disorder: the role of arousal during sleep and implications for treatment. Med Hypotheses. 2012;79(2):147–153. | |
Cortese S, Brown TE, Corkum P, et al. Assessment and management of sleep problems in youths with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2013;52(8):784–796. | |
Koo BB, Blackwell T, Ancoli-Israel S, et al; Osteoporotic Fractures in Men(MrOS) Study Group. Association of incident cardiovascular disease with periodic limb movements during sleep in older men: outcomes of sleep disorders in older men (MrOS) study. Circulation. 2011;124(11):1223–1231. | |
Lindner A, Fornadi K, Lazar AS, et al. Periodic limb movements in sleep are associated with stroke and cardiovascular risk factors in patients with renal failure. J Sleep Res. 2012;21(3):297–307. | |
Martin J, Shochat T, Ancoli-Israel S. Assessment and treatment of sleep disturbances in older adults. Clin Psychol Rev. 2000;20(6):783–805. | |
Castillo J, Goparaju B, Bianchi MT. Sleep-wake misperception in sleep apnea patients undergoing diagnostic versus titration polysomnography. J Psychosom Res. 2014;76(5):361–367. | |
Allena M, Campus C, Morrone E, et al. Periodic limb movements both in non-REM and REM sleep: relationships between cerebral and autonomic activities. Clin Neurophysiol. 2009;120(7):1282–1290. | |
Manconi M, Ferri R, Zucconi M, et al. Effects of acute dopamine-agonist treatment in restless legs syndrome on heart rate variability during sleep. Sleep Med. 2011;12(1):47–55. | |
Pennestri MH, Montplaisir J, Fradette L, Lavigne G, Colombo R, Lanfranchi PA. Blood pressure changes associated with periodic leg movements during sleep in healthy subjects. Sleep Med. 2013; 14(6):555–561. | |
Sforza E, Nicolas A, Lavigne G, Gosselin A, Petit D, Montplaisir J. EEG and cardiac activation during periodic leg movements in sleep: support for a hierarchy of arousal responses. Neurology. 1999;52(4):786–791. | |
Montplaisir J, Nicolas A, Denesle R, Gomez-Mancilla B. Restless legs syndrome improved by pramipexole: a double-blind randomized trial. Neurology. 1999;52(5):938–943. | |
Sullivan PW, Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Med Decis Making. 2006;26(4):410–420. | |
Kobayashi M, Namba K, Ito E, et al. The validity of the PAM-RL device for evaluating periodic limb movements in sleep and an investigation on night-to-night variability of periodic limb movements during sleep in patients with restless legs syndrome or periodic limb movement disorder using this system. Sleep Med. 2014;15(1):138–143. | |
Plante DT. Leg actigraphy to quantify periodic limb movements of sleep: a systematic review and meta-analysis. Sleep Med Rev. 2014;18(5):425–434. | |
Yang C, White DP, Winkelman JW. Antidepressants and periodic leg movements of sleep. Biol Psychiatry. 2005;58(6):510–514. | |
Hoque R, Chesson AL Jr. Pharmacologically induced/exacerbated restless legs syndrome, periodic limb movements of sleep, and REM behavior disorder/REM sleep without atonia: literature review, qualitative scoring, and comparative analysis. J Clin Sleep Med. 2010;6(1):79–83. | |
Calhoun DA, Harding SM. Sleep and hypertension. Chest. 2010; 138(2):434–443. | |
Boehm G, Wetter TC, Trenkwalder C. Periodic leg movements in RLS patients as compared to controls: are there differences beyond the PLM index? Sleep Med. 2009;10(5):566–571. | |
Winkelman JW. The evoked heart rate response to periodic leg movements of sleep. Sleep. 1999;22(5):575–580. | |
Zhang B, Hao Y, Jia F, et al. Sertraline and periodic limb movements during sleep: an 8-week open-label study in depressed patients with insomnia. Sleep Med. 2013;14(12):1405–1412. | |
Carrier J, Frenette S, Montplaisir J, Paquet J, Drapeau C, Morettini J. Effects of periodic leg movements during sleep in middle-aged subjects without sleep complaints. Mov Disord. 2005;20(9):1127–1132. | |
Ohayon MM, Roth T. Prevalence of restless legs syndrome and periodic limb movement disorder in the general population. J Psychosom Res. 2002;53(1):547–554. | |
Mendelson WB. Are periodic leg movements associated with clinical sleep disturbance? Sleep. 1996;19(3):219–223. | |
Youngstedt SD, Kripke DF, Klauber MR, Sepulveda RS, Mason WJ. Periodic leg movements during sleep and sleep disturbances in elders. J Gerontol A Biol Sci Med Sci. 1998;53(5):M391–M394. | |
Nicolas A, Lesperance P, Montplaisir J. Is excessive daytime sleepiness with periodic leg movements during sleep a specific diagnostic category? Eur Neurol. 1998;40(1):22–26. | |
Bastuji H, Garcia-Larrea L. Sleep/wake abnormalities in patients with periodic leg movements during sleep: factor analysis on data from 24-h ambulatory polygraphy. J Sleep Res. 1999;8(3):217–223. | |
Hilbert J, Mohsenin V. Can periodic limb movement disorder be diagnosed without polysomnography? A case-control study. Sleep Med. 2003;4(1):35–41. | |
Kushida CA, Littner MR, Morgenthaler T, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28(4):499–521. | |
Stefansson H, Rye DB, Hicks A, et al. A genetic risk factor for periodic limb movements in sleep. N Engl J Med. 2007;357(7):639–647. | |
Sforza E, Juony C, Ibanez V. Time-dependent variation in cerebral and autonomic activity during periodic leg movements in sleep: implications for arousal mechanisms. Clin Neurophysiol. 2002;113(6):883–891. | |
Gosselin N, Lanfranchi P, Michaud M, et al. Age and gender effects on heart rate activation associated with periodic leg movements in patients with restless legs syndrome. Clin Neurophysiol. 2003;114(11):2188–2195. | |
Sayk F, Teckentrup C, Becker C, et al. Effects of selective slow-wave sleep deprivation on nocturnal blood pressure dipping and daytime blood pressure regulation. Am J Physiol Regul Integr Comp Physiol. 2010;298(1):R191–R197. | |
Tsioufis C, Andrikou I, Thomopoulos C, Syrseloudis D, Stergiou G, Stefanadis C. Increased nighttime blood pressure or nondipping profile for prediction of cardiovascular outcomes. J Hum Hypertens. 2011;25(5):281–293. | |
Ben-Dov IZ, Kark JD, Ben-Ishay D, Mekler J, Ben-Arie L, Bursztyn M. Predictors of all-cause mortality in clinical ambulatory monitoring: unique aspects of blood pressure during sleep. Hypertension. 2007; 49(6):1235–1241. | |
Carney RM, Steinmeyer B, Freedland KE, et al. Nocturnal patterns of heart rate and the risk of mortality after acute myocardial infarction. Am Heart J. 2014;168(1):117–125. | |
Espinar-Sierra J, Vela-Bueno A, Luque-Otero M. Periodic leg movements in sleep in essential hypertension. Psychiatry Clin Neurosci. 1997;51(3):103–107. | |
Ali NJ, Davies RJ, Fleetham JA, Stradling JR. Periodic movements of the legs during sleep associated with rises in systemic blood pressure. Sleep. 1991;14(2):163–165. | |
Otzenberger H, Simon C, Gronfier C, Brandenberger G. Temporal relationship between dynamic heart rate variability and electroencephalographic activity during sleep in man. Neurosci Lett. 1997;229(3):173–176. | |
Murase K, Hitomi T, Hamada S, et al. The additive impact of periodic limb movements during sleep on inflammation in patients with obstructive sleep apnea. Ann Am Thorac Soc. 2014;11(3):375–382. | |
Collop NA, Tracy SL, Kapur V, et al. Obstructive sleep apnea devices for out-of-center (OOC) testing: technology evaluation. J Clin Sleep Med. 2011;7(5):531–548. | |
Baran AS, Richert AC, Douglass AB, May W, Ansarin K. Change in periodic limb movement index during treatment of obstructive sleep apnea with continuous positive airway pressure. Sleep. 2003;26(6):717–720. | |
Seo WH, Guilleminault C. Periodic leg movement, nasal CPAP, and expiratory muscles. Chest. 2012;142(1):111–118. | |
Aritake S, Blackwell T, Peters KW, et al; Osteoporotic Fractures in Men (MrOS) Study Research Group. Prevalence and associations of respiratory-related leg movements: the MrOS sleep study. Sleep Med. 2015;16(10):1236–1244. | |
Mosko SS, Dickel MJ, Ashurst J. Night-to-night variability in sleep apnea and sleep-related periodic leg movements in the elderly. Sleep. 1988;11(4):340–348. | |
Sforza E, Haba-Rubio J. Night-to-night variability in periodic leg movements in patients with restless legs syndrome. Sleep Med. 2005;6(3):259–267. | |
Pietzsch JB, Garner A, Cipriano LE, Linehan JH. An integrated health-economic analysis of diagnostic and therapeutic strategies in the treatment of moderate-to-severe obstructive sleep apnea. Sleep. 2011;34(6):695–709. | |
Meyers J, Candrilli S, Allen R, Manjunath R, Calloway M. Health care resource utilization and costs associated with restless legs syndrome among managed care enrollees treated with dopamine agonists. Managed care. 2012;21(10):44–51. |
Supplementary materials
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.