Back to Journals » Clinical Ophthalmology » Volume 13
Periocular injection of candesartan-PLGA microparticles inhibits laser-induced experimental choroidal neovascularization
Authors Okuda Y, Fukumoto M, Horie T, Oku H , Takai S, Nakanishi T, Matsuzaki K , Tsujimoto H , Ikeda T
Received 23 July 2018
Accepted for publication 20 November 2018
Published 31 December 2018 Volume 2019:13 Pages 87—93
DOI https://doi.org/10.2147/OPTH.S181110
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Yoshitaka Okuda,1 Masanori Fukumoto,1 Taeko Horie,1 Hidehiro Oku,1 Shinji Takai,2 Toyofumi Nakanishi,3 Kaori Matsuzaki,4 Hiroyuki Tsujimoto,4 Tsunehiko Ikeda1
1Department of Ophthalmology, Osaka Medical College, Osaka, Japan; 2Department of Innovative Medicine, Graduate School of Medicine, Osaka Medical College, Osaka, Japan; 3Department of Clinical and Laboratory Medicine, Osaka Medical College, Osaka, Japan; 4Research and Development Division, Hosokawa Micron Corporation, Osaka, Japan
Purpose: Microparticle technology enables local administration of medication. The purpose of this study was to examine the inhibitory effect of locally administered candesartan (CAN)-encapsulated microparticles on experimental choroidal neovascularization (CNV).
Methods: Laser photocoagulation was used to induce CNV in Brown Norway rats. The rats were pretreated with subconjunctival injections of CAN (5.0 mg/eye) or phosphate buffer saline for 3 days before photocoagulation. The volume of CNV was evaluated 7 days after laser injury using the lectin staining technique. The infiltration of macrophages within the CNV lesion was determined using immunofluorescent staining with an anti-CD68 antibody. mRNA levels of MCP-1, IL1-β and VEGF in the retinal pigment epithelium/choroid complex were determined using quantitative PCR (q-PCR).
Results: CNV volume was significantly suppressed by the treatment with CAN compared with that in vehicle-treated eyes (P<0.05, two-tailed Student’s t-test). Subconjunctival injections of CAN decreased the numbers of CD68+ cells in the CNV lesion. The increased mRNA levels of MCP-1, IL1-β, and VEGF induced by photocoagulation was significantly suppressed following the local administration of CAN (P<0.05, two-tailed Student’s t-test).
Conclusion: Local administration of CAN inhibited experimentally induced CNV possibly through anti-inflammatory effects.
Keywords: age-related macular degeneration, choroidal neovascularization, renin-angiotensin system, candesartan, macrophage, monocyte chemotactic protein 1, poly(lactic-co-glycolic acid)
Introduction
Age-related macular degeneration (AMD) is the leading cause of blindness in the USA in people over 50 years of age.1
AMD is classified into two types, dry (atrophic) and wet (exudative).2 The wet type affects approximately 10%–15% of individuals with AMD, where choroidal neovascularization (CNV) is associated with the vision loss.3,4 Although the molecular and cellular mechanisms of CNV formation are not fully understood, recent experimental and clinical studies have shown that vascular endothelial growth factors (VEGFs) play a central role in CNV pathogenesis. Inflammatory cells, such as macrophages, are also involved in the pathogenesis of AMD as they are known to release VEGFs to promote healing via inflammation.5 Pharmacologic depletion of macrophages in murine CNV tissues results in significant reduction of CNV volume.6,7 Moreover, genetic ablation of monocyte chemotactic protein (MCP-1) inhibits CNV formation in the rat model.8
The renin-angiotensin system (RAS) is a well-recognized circulating hormone system that plays a critical role in controlling blood pressure. The principle effector of this system is angiotensin II (ANG II), which works through two main receptors, angiotensin receptor type 1 (AT1R) and angiotensin receptor type 2 (AT2R).9 AT1R serves as a major mediator for controlling the ultimate effects of ANG II. In clinical settings, AT1R blockers (ARBs) have been commonly used for the therapy of hypertension. There is increasing evidence that the RAS is involved in the regulation of angiogenesis, inflammation, and tissue remodeling.10,11 Previously, we showed that treatment with candesartan (CAN), an ARB, suppressed the increased expression of VEGF in the retina of a type 2 diabetic rat model.12 Recently, orally administered losartan, another ARB, was shown to inhibit experimental CNV; however, large dose systemic administration of ARBs does result in general adverse effects, such as hypotension.13
Poly(lactic-co-glycolic acid) (PLGA) polymers are widely used as a biodegradable material for encapsulating a variety of drugs, DNA, and proteins.14 The complete release of encapsulated drugs is accomplished through degradation and erosion of the surface of the polymer matrix.15 The PLGA degradation products of lactic and glycolic acids are then metabolized via the Krebs cycle and ultimately converted to water and carbon dioxide, which are excreted through the respiratory system.16 Because PLGA is biocompatible, PLGA microspheres and nanoparticles are now widely accepted. Most importantly, the degradation rates of the polymer and the release of the encapsulated drugs can be regulated by the crystallinity, hydrophilicity, and molecular weight of the encapsulated drugs.17,18
We hypothesized that locally delivered ARBs can inhibit the RAS pathway and attenuate CNV formation through the inhibition of macrophage-induced inflammatory reactions. To test this hypothesis, experimental CNV was generated by laser photocoagulation in rats; CAN was encapsulated into PLGA microparticles; and CAN microparticles were locally delivered into the periocular space via sub-Tenon’s injection.
Materials and methods
Animals
For this study, we used 7–9-week-old male BN/SsNSlc rats (body weight, 160–200 g; Japan SLC, Inc., Shizuoka, Japan). The animals had free access to food and water, and they were handled in accordance with the ARVO Resolution for the Use of Animals in Ophthalmic and Vision Research. The experimental protocol, which conformed to the Animal Research: Reporting In Vivo Experiments (ARRIVE) guidelines, was approved by the Committee of Animal Use and Care of the Osaka Medical College (approval number: 30016). For the experiments described here, a total of 28 adult rats were used.
Anesthesia and euthanasia
All surgeries were performed under general anesthesia, which was induced by an intraperitoneal injection of a mixture of medetomidine (0.75 mg), midazolam hydrochloride (4.0 mg), and butorphanol tartrate (5.0 mg/kg body wt), and all efforts were made to minimize suffering. Rats were euthanized by exposure to CO2 at a rate of 6 L/min in a 13.8 L cage with wood-shaving bedding.
CAN-PLGA particle formulation
PLGA was generous gift from Hosokawa Micron Corporation (Osaka, Japan). CAN (0.1 g) and PLGA (1 g) were dissolved in dichloromethane (3.7 mL) and ethanol (2.2 mL). This solution was added to 100 mL of an aqueous PVA (0.25% wt/vol.) solution and homogenized at 50,000× g for 3 minutes. The contents were stirred for 3 hours at 40°C under a safety hood in order to completely remove the chloroform and ethanol by evaporation. The PLGA microparticles were separated by centrifugation at 41,000× g for 10 minutes at 4°C. The resulting pellets were washed twice with distilled water and freeze-dried to obtain lyophilized particles.
Particle size and drug-loading measurement of CAN-PLGA microparticle size
The particle size distribution of the CAN-PLGA microparticles dispersed in the distilled water was measured by laser diffraction (Microtrac MT 3300, Nikkiso, Japan).
Drug loading
The encapsulation ratios of CAN in the microparticles were analyzed by using high-speed liquid chromatography at 254 nm (UV detector SPD-20A, Shimadzu, Japan) according to the following procedures. After dissolving 10 mg of the microparticles in 8 mL of acetonitrile, 2 mL of 1% acetic acid was added to separate the PLGA. The suspension was then centrifuged at 41,000× g for 20 minutes to remove the PLGA. Thus, the dissolved CAN in the supernatant could be quantified with a 15 cm long C18 column (Inertsil ODS-3, GL Science, Tokyo, Japan, acetonitrile/1% acetic acid =80/20 mobile phase) at 40°C.
Induction of CNV
Laser photocoagulation was performed under general anesthesia as described before. Prior to laser pulse application, the pupils of the rats were dilated with focal administration of a 0.5% phenylephrine hydrochloride-0.5% tropicamide solution (Santen Pharmaceutical, Osaka, Japan). Lubricating eyedrops (Genteal; Alcon Laboratories, Fort Worth, TX, USA) placed on a glass cover slip were applied to the cornea, and the retina was viewed through a slit lamp microscope (NOVUS Spectra™; Lumenis, Tokyo, Japan).
While viewing the ocular fundus, five laser spots were created per eye around the optic disc. The laser parameters were set to the following: intensity, 200 mW; duration, 0.1 second; and spot diameter, 75 μm. The presence of a bubble at the time of the laser application was taken as an indication that Bruch’s membrane had been sufficiently ruptured.
Treatment with CAN
PLGA particles carrying CAN (5.0 mg/eye) were administered by periocular administration into the posterior subconjunctival space using a 27G needle in one eye 2 days before laser irradiation (CAN-PLGA group). Similarly, the contralateral eye of the same animal was administered phosphate buffer saline (PBS) and served as the control. Locally applied CAN corresponded to approximately 50 μg/kg body wt.
Quantification of laser-induced CNV
Five rats were euthanatized 7 days after laser photocoagulation. Eyes were immediately enucleated and fixed with 4% paraformaldehyde (PFA) in PBS for 1 hour at room temperature. After removal of the retinas, posterior eye cups consisting of the retinal pigment epithelium (RPE)/choroid/sclera were dissected and permeabilized with 0.1% Triton X-100 in PBS for 1 hour at room temperature. The CNV lesions were stained with Alexa Fluor 488 conjugated isolectin B4 (IB4, 10 μg/mL, Life Technologies, Carlsbad, CA, USA) at room temperature overnight. After washing with PBS, the posterior eye cups were flat mounted onto slides with the scleral side down. Z-stack images were taken using a TCS SP8 confocal microscope (Leica, Wetzlar, Germany). ImageJ software was used to analyze Z-stack images. The summation of the whole stained area in each section, multiplied by the distance between the sections was calculated and estimated as each CNV volume. CNV volume for each eye was determined by calculating the average of 5 laser-induced CNVs. Then, the average CNV volume of the control group (n=5) was compared with the CAN-PLGA group (n=5).
Immunohistochemistry for infiltrating macrophages
Seven days after laser photocoagulation, another five rats were euthanized and posterior eye cups consisting of the RPE/choroid/sclera were created as described. After blocking with 5% normal goat serum plus 2% BSA and 0.1% Triton X-100 in PBS, the posterior eye cups were flat mounted onto slides with the scleral side down, and incubated with the primary antibody, mouse monoclonal anti-CD68 (1:500, Serotec, Oxford, UK) overnight at 4°C. Then, they were incubated for 2 hours at room temperature in Alexa 488-conjugated secondary antibodies (Life Technologies; diluted by 1:500) and cover slipped. In order to determine the number of CD68+ cells, posterior eye cups were photographed under a fluorescence microscope (BZ-X700, Keyence, Osaka, Japan). We counted CR68+ cells in the area of 100×100 μm2 at the center of the CNV and expressed the level as their density. The level of CD68+ cells for each eye was calculated as the average density of CD68+ cells of five laser-induced CNVs. The outcomes were compared between the control (n=5) and the CAN-PLGA group (n=5).
Quantitative PCR analysis
We determined the changes in the expression of several genes in the RPE/choroid complex by quantitative PCR (qPCR) using the other 18 rats. Laser spots were created were created as mentioned earlier 7 days prior to the euthanasia. After removal of the retina from the RPE, the choroid and RPE were carefully dissected away from the sclera resulting in the isolation of RPE–choroid complexes. The RPE–choroid complexes were then homogenized in lysis buffer and total RNA was extracted using a NucleoSpin RNA kit (TakaRa, Ohtsu, Shiga, Japan). The RNA quality and quantity were assessed with a BioSpectrometer (Eppendorf, Hamburg, Germany).
Total RNA was reverse transcribed with the PrimeScript reverse transcriptase reagent (TaKaRa). The cDNA was used for quantitative real-time PCR amplification with the TaqMan Gene Expression Assays for targeted genes (Applied Biosystems, Foster City, CA, USA).
Rat TaqMan Gene Assays for Mcp-1 (Rn00580555_m1), also known as Ccl2, Vegf (Rn01511601_m1), and Il-1β (Rn00580432_m1) were used. Amplicons were detected with the relevant probes tagged with an MGB quencher and a FAM dye. TaqMan rat 18 seconds rRNA control Expression Assays (Applied Biosystems) were used as the reference genes.
Real-time PCR was performed in Premix Ex Taq (Perfect Real Time; TakaRa). All reactions were run on a Thermal Cycler Dice Real time system TP870 (TakaRa) with the following cycling parameters: 30 seconds at 95°C followed by 40 cycles at 95°C for 5 seconds, and 60°C for 30 seconds. A standard curve of the cycle thresholds was established using serial dilutions of cDNA samples. Relative quantities of mRNAs were calculated with 18 seconds rRNA serving as an internal control.
Statistical analyses
The means and the standard error of the means (SEMs) were calculated, and the data were expressed as the mean ± SEM, unless otherwise noted. Two-tailed Student’s t-tests were used for statistical comparisons between the groups and the level of significance was set at P<0.05.
Results
All rats used in this study were in good health throughout the experimental procedures, and their eyes showed no obvious complications (eg, inflammation, corneal opacity, cataract, vitreous opacity, retinal hemorrhage) after subconjunctival injection.
The mean particle size of the CAN-PLGA particles and control PLGA particles obtained by laser diffraction was 5.4 μm and 4.9 μm, respectively. The CAN loading efficiency onto the microparticles was estimated to be 1.6%.
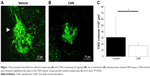
We first confirmed that CNVs were successfully created at all spots of photocoagulation. Five CNV lesions were clearly identified around the optic disc in each flat mounted retina, where laser spots had been created. The CNV volume was measured to evaluate the effects of the ARB on the development of CNV. CNV was significantly suppressed by administration of PLGA particles carrying CAN (Figure 1A and B). CAN-PLGA-treated eyes at the dose of 5 mg/eye resulted in a significant (P<0.05, two-tailed Student’s t-test) decrease in the CNV volume (17.5±2.1×104 μm3), compared with vehicle-treated rats (31.3±5.3×104 μm3) (Figure 1C).
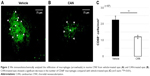
Because inflammatory cells, such as macrophages, play a critical role in the development of CNV, we immunohistochemically analyzed the infiltration of macrophages in our laser-induced murine CNV (Figure 2A).21 A significant decrease in the number of CD68+ macrophages was seen in the CNVs of CAN-PLGA-treated eyes, compared with vehicle-treated eyes. The mean ± SEM number of CD68+ macrophages was 88.6±17.4/mm2 in the CNVs of vehicle-treated eyes (n=5 eyes) and the mean number decreased significantly to 34.0±9.5/mm2 in the CNVs of CAN-PLGA-treated eyes (n=5 eyes, P<0.01, two-tailed Student’s t-test, Figure 2C).
To determine whether CAN-PLGA treatment affects angiogenic and inflammatory molecules related to the pathogenesis of CNV, mRNA levels of Mcp-1, Il1-β, and Vegf in the RPE–choroid complex were analyzed by qPCR using the 18 rats. These levels were significantly increased in the RPE–choroid complexes following CNV induction. Treatment with local administration of CAN-PLGA significantly suppressed mRNA levels of Mcp-1 (P<0.05, two-tailed Student’s t-test), Il-1β (P<0.01, two-tailed Student’s t-test), and Vegf (P<0.01, two-tailed Student’s t-test, Figure 3).
Discussion
First, injection of CAN-PLGA into the periocular tissue led to significant suppression of CNV (Figure 1). Second, the CAN-PLGA treatment resulted in decreased levels of macrophage infiltration into the CNV (Figure 2) and decreased levels of inflammation-related molecules in the RPE–choroid complexes (Figure 3).
In the present study, angiotensin receptor blockade with locally administered CAN significantly attenuated macrophage infiltration into the CNV.19,20 Macrophages are well-documented to localize at the site of CNV in patients with AMD.19,20 They are innate immune cells that play a key role in regulating angiogenesis, a key component that contributes significantly to the development of new vessels in CNV.20–22 Attenuation of macrophage infiltration or pharmacological deletion of macrophages has been shown to inhibit CNV formation, demonstrating that infiltrated macrophages contribute to CNV development.22–24 Evidence suggests that recruited macrophages at the site of CNV initiates the release of angiogenic cytokines, such as VEGF, which leads to the promotion of CNV.24
To elucidate the molecular mechanism by which locally administered CAN suppresses CNV, we explored the relationship of inflammatory cytokines, such as MCP-1, IL-1β, and VEGF, with the inhibition of CNV.25,26 The mRNA levels of Mcp-1, Il-1β, and Vegf were upregulated in the RPE–choroid complexes that underwent laser-induced CNVs, and CAN-encapsulated PLGA particles significantly depressed these increases.
MCP-1 is a main chemokine that causes a recruitment of macrophages. Thus, the CAN-induced decrease of Mcp-1 depressed the accumulation of macrophages at the CNV lesions, which were created by laser photocoagulation. Activated macrophages at these CNV lesions release VEGF and IL-1β.25,26 IL-1β is a proinflammatory cytokine that exerts pleiotropic effects on various cells and is reported to upregulate VEGF and VEGF receptor-2 (VEGFR-2) in inflammatory cells.27,28 VEGF induces MCP-1 secretion by vascular endothelial cells and blocking VEGF receptors reduces macrophage infiltration into the CNV tissue.29,30 Thus, in addition to the decrease of macrophage accumulation, CAN may suppress angiogenesis by attenuation of VEGF signaling pathways. Similar to other reports, our findings may add supporting evidence that inflammatory cytokines secreted by macrophages establish a vicious cycle that aggravates inflammatory changes and promotes CNV.31–33 Thus, it is reasonable to consider that the CAN-induced decrease of the inflammatory reaction may contribute to the smaller CNV volume.32–34
The use of polymeric encapsulation of drugs to produce long-lasting drug concentrations has received considerable attention over the past few years.35 Polylactic acid (PLA) and PLGA are approved for human use by the US Food and Drug Administration (FDA).35 They are biodegradable, biocompatible, and one of the most widely studied polymers for drug delivery purposes.16,35 By changing the copolymer composition and molecular weight, the release rate of the encapsulated drug or DNA fragment from the PLA or PLGA matrix can be extended from several days to several months.36 Several studies revealed that PLGA injected into periocular tissues has the capacity for sustained release of the encapsulated molecules.37,38
Although the results from this study are significant, there are also several limitations to this study. One limitation is a lack of information about drug release from CAN-PLGA microparticles.37,38 Although previous reports showed the ability of PLGA microparticles to release the encapsulated drug, additional research will be needed to optimize the release of the drug into the surrounding microenvironment. Another limitation of this study is the lack of confirmation that CAN underwent intraocular penetration. Periocular injection of triamcinolone acetonide is widely used in clinical practice for the treatment of posterior ocular diseases, such as diabetic macular edema and AMD.39,40 The transscleral permeability of substances is regulated by their molecular weight and lipid solubility.41 Triamcinolone acetonide and CAN cilexetil are both hydrophobic and are similar in molecular weights (434.5 g/mol and 440.45 g/mol, respectively). Thus, we suggest that the CAN released from the PLGA microparticles injected onto the episclera could penetrate the sclera and reach the RPE–choroid complex; however, further study is necessary to confirm drug penetration of this delivery system.42,43
Increased levels of VEGF are known to be present in surgically excised AMD-related CNVs leading to the development of VEGF antagonists as a therapeutic strategy for slowing AMD progression.24 However, intraocular injections of VEGF antagonists involve expensive medical procedures and can result in the formation of endophthalmitis, retinal detachment, and thromboembolic events. Our findings that local administration of CAN depressed the increase of mRNA levels of Vegf in laser-induced CNV may also be applicable for the AMD-associated CNV.
Conclusion
Subconjunctival administration of CAN-encapsulated PLGA microparticles depressed laser photocoagulation-induced CNV in rats. Future study is necessary to determine the efficacy to deliver CAN to the subretinal space, so that this microparticle technology can be used to develop an alternative treatment for the CNV.
Acknowledgments
The authors wish to thank Stephen Gee and Teruyo Kida, MD for editing the manuscript. Hosokawa Micron Corporation had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Bressler NM, Bressler SB, Fine SL. Age-related macular degeneration. Surv Ophthalmol. 1988;32(6):375–413. | ||
Ambati J, Fowler BJ. Mechanisms of age-related macular degeneration. Neuron. 2012;75(1):26–39. | ||
Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106–e116. | ||
Friedman DS, O’Colmain BJ, Muñoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564–572. | ||
Sarwar S, Clearfield E, Soliman MK. Aflibercept for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2016;8(2):CD011346. | ||
Espinosa-Heidmann DG, Suner IJ, Hernandez EP, Monroy D, Csaky KG, Cousins SW. Macrophage depletion diminishes lesion size and severity in experimental choroidal neovascularization. Investigative Opthalmology & Visual Science. 2003;44(8):3586–3592. | ||
Cao X, Shen D, Patel MM, et al. Macrophage polarization in the maculae of age-related macular degeneration: a pilot study. Pathol Int. 2011;61(9):528–535. | ||
Luhmann UF, Robbie S, Munro PM, et al. The drusenlike phenotype in aging Ccl2-knockout mice is caused by an accelerated accumulation of swollen autofluorescent subretinal macrophages. Invest Ophthalmol Vis Sci. 2009;50(12):5934–5943. | ||
de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52(3):415–472. | ||
Toko H, Zou Y, Minamino T, et al. Angiotensin II type 1a receptor is involved in cell infiltration, cytokine production, and neovascularization in infarcted myocardium. Arterioscler Thromb Vasc Biol. 2004;24(4):664–670. | ||
Takai S, Kirimura K, Jin D, et al. Significance of angiotensin II receptor blocker lipophilicities and their protective effect against vascular remodeling. Hypertens Res. 2005;28(7):593–600. | ||
Fukumoto M, Takai S, Ishizaki E, et al. Involvement of angiotensin II-dependent vascular endothelial growth factor gene expression via NADPH oxidase in the retina in a type 2 diabetic rat model. Curr Eye Res. 2008;33(10):885–891. | ||
Hikichi T, Mori F, Takamiya A, et al. Inhibitory effect of losartan on laser-induced choroidal neovascularization in rats. Am J Ophthalmol. 2001;132(4):587–589. | ||
Souza MC, Fialho SL, Souza PA, Fulgêncio GO, da Silva GR, Silva-Cunha A. Tacrolimus-loaded PLGA implants: in vivo release and ocular toxicity. Curr Eye Res. 2014;39(1):99–102. | ||
Fredenberg S, Wahlgren M, Reslow M, Axelsson A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems – a review. Int J Pharm. 2011;415(1–2):34–52. | ||
Jain RA. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials. 2000;21(23):2475–2490. | ||
Jain RA, Rhodes CT, Railkar AM, Malick AW, Shah NH. Controlled release of drugs from injectable in situ formed biodegradable PLGA microspheres: effect of various formulation variables. Eur J Pharm Biopharm. 2000;50(2):257–262. | ||
Duvvuri S, Janoria KG, Mitra AK. Development of a novel formulation containing poly(d,l-lactide-co-glycolide) microspheres dispersed in PLGA–PEG–PLGA gel for sustained delivery of ganciclovir. Journal of Controlled Release. 2005;108(2–3):282–293. | ||
Killingsworth MC, Sarks JP, Sarks SH. Macrophages related to Bruch’s membrane in age-related macular degeneration. Eye. 1990;4(Pt 4):613–621. | ||
Sarks JP, Sarks SH, Killingsworth MC. Morphology of early choroidal neovascularisation in age-related macular degeneration: correlation with activity. Eye. 1997;11(Pt 4):515–522. | ||
Combadière C, Feumi C, Raoul W, et al. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J Clin Invest. 2007;117(10):2920–2928. | ||
Ma W, Zhao L, Fontainhas AM, Fariss RN, Wong WT. Microglia in the mouse retina alter the structure and function of retinal pigmented epithelial cells: a potential cellular interaction relevant to AMD. PLoS One. 2009;4(11):e7945. | ||
Huang H, Parlier R, Shen JK, Lutty GA, Vinores SA. VEGF receptor blockade markedly reduces retinal microglia/macrophage infiltration into laser-induced CNV. PLoS One. 2013;8(8):e71808. | ||
Tsutsumi C, Sonoda KH, Egashira K, et al. The critical role of ocular-infiltrating macrophages in the development of choroidal neovascularization. J Leukoc Biol. 2003;74(1):25–32. | ||
Saijo Y, Tanaka M, Miki M, et al. Proinflammatory cytokine IL-1 beta promotes tumor growth of Lewis lung carcinoma by induction of angiogenic factors: in vivo analysis of tumor-stromal interaction. J Immunol. 2002;169(1):469–475. | ||
Oh H, Takagi H, Takagi C, et al. The potential angiogenic role of macrophages in the formation of choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1999;40(9):1891–1898. | ||
Marumo T, Schini-Kerth VB, Busse R. Vascular endothelial growth factor activates nuclear factor-kappaB and induces monocyte chemoattractant protein-1 in bovine retinal endothelial cells. Diabetes. 1999;48(5):1131–1137. | ||
Yamada M, Kim S, Egashira K, et al. Molecular mechanism and role of endothelial monocyte chemoattractant protein-1 induction by vascular endothelial growth factor. Arterioscler Thromb Vasc Biol. 2003;23(11):1996–2001. | ||
Low QE, Drugea IA, Duffner LA, et al. Wound healing in MIP-1alpha(−/−) and MCP-1(−/−) mice. Am J Pathol. 2001;159(2):457–463. | ||
Salcedo R, Ponce ML, Young HA, et al. Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood. 2000;96(1):34–40. | ||
Mclaren J, Prentice A, Charnock-Jones DS, et al. Vascular endothelial growth factor is produced by peritoneal fluid macrophages in endometriosis and is regulated by ovarian steroids. J Clin Invest. 1996;98(2):482–489. | ||
Beuscher HU, Günther C, Röllinghoff M. IL-1 beta is secreted by activated murine macrophages as biologically inactive precursor. J Immunol. 1990;144(6):2179–2183. | ||
Zhou Y, Yoshida S, Kubo Y, et al. Different distributions of M1 and M2 macrophages in a mouse model of laser-induced choroidal neovascularization. Mol Med Rep. 2017;15(6):3949–3956. | ||
Espinosa-Heidmann DG, Suner IJ, Hernandez EP, Monroy D, Csaky KG, Cousins SW. Macrophage depletion diminishes lesion size and severity in experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44(8):3586–3592. | ||
Shive MS, Anderson JM. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliv Rev. 1997;28(1):5–24. | ||
Lin SY, Chen KS, Teng HH, Li MJ, Mj L. In vitro degradation and dissolution behaviours of microspheres prepared by three low molecular weight polyesters. J Microencapsul. 2000;17(5):577–586. | ||
Ayalasomayajula SP, Kompella UB. Subconjunctivally administered celecoxib-PLGA microparticles sustain retinal drug levels and alleviate diabetes-induced oxidative stress in a rat model. Eur J Pharmacol. 2005;511(2–3):191–198. | ||
Amrite AC, Ayalasomayajula SP, Cheruvu NP, Kompella UB. Single periocular injection of celecoxib-PLGA microparticles inhibits diabetes-induced elevations in retinal PGE2, VEGF, and vascular leakage. Invest Ophthalmol Vis Sci. 2006;47(3):1149–1160. | ||
Yilmaz T, Weaver CD, Gallagher MJ, et al. Intravitreal triamcinolone acetonide injection for treatment of refractory diabetic macular edema: a systematic review. Ophthalmology. 2009;116(5):902–913. | ||
Katome T, Naito T, Nagasawa T, Shiota H. Efficacy of combined photodynamic therapy and sub-Tenon’s capsule injection of triamcinolone acetonide for age-related macular degeneration. J Med Invest. 2009;56(3–4):116–119. | ||
Cruysberg LP, Nuijts RM, Geroski DH, Koole LH, Hendrikse F, Edelhauser HF. In vitro human scleral permeability of fluorescein, dexamethasone-fluorescein, methotrexate-fluorescein and rhodamine 6G and the use of a coated coil as a new drug delivery system. J Ocul Pharmacol Ther. 2002;18(6):559–569. | ||
Sánchez-López E, Egea MA, Cano A, et al. PEGylated PLGA nanospheres optimized by design of experiments for ocular administration of dexibuprofen-in vitro, ex vivo and in vivo characterization. Colloids Surf B Biointerfaces. 2016;145:241–250. | ||
Pandit J, Sultana Y, Aqil M. Chitosan-coated PLGA nanoparticles of bevacizumab as novel drug delivery to target retina: optimization, characterization, and in vitro toxicity evaluation. Artif Cells Nanomed Biotechnol. 2017;45(7):1397–1407. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.