Back to Journals » International Journal of Women's Health » Volume 15
Perinatal Outcome of Pregnant Women with RhD Sensitization: A Five-Year Cross-Sectional Study at a Tertiary Care Hospital in Ethiopia
Authors Kureba AA , Gudu W , Mersha A, Jemal E, Abdosh AA
Received 26 December 2022
Accepted for publication 6 April 2023
Published 12 April 2023 Volume 2023:15 Pages 571—578
DOI https://doi.org/10.2147/IJWH.S402373
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Elie Al-Chaer
Abdulhakim Abdurahman Kureba,1 Wondimu Gudu,1 Anteneh Mersha,1 Elias Jemal,2 Abdulfetah Abdulkadir Abdosh1
1Department of Obstetrics and Gynecology, St. Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia; 2Department of Obstetrics and Gynecology, Haramaya University Hiwot Fana Comprehensive Specialized Hospital, Harar, Ethiopia
Correspondence: Abdulhakim Abdurahman Kureba, Department of Obstetrics and Gynecology, St. Paul’s Hospital Millennium Medical College, 1271, Swaziland St, Addis Ababa, Ethiopia, Tel +251911937561, Email [email protected]
Background: Isoimmunization is a process of immunizing an antigen-negative pregnant individual with a paternally derived fetal antigen. Although the Rh systems contain many antigen subtypes (D, C, c, E, e), the RhD antigen is highly immunogenic. This research aimed to investigate the perinatal Outcome of pregnant women with RhD sensitization at St. Paul’s Hospital Millennium Medical College (SPHMMC), Ethiopia.
Methodology: A facility-based retrospective cross-sectional study was conducted on 98 pregnant women with RhD alloimmunization at SPHMMC from September 11, 2016, to September 10, 2021. SPSS 26 was used for data analysis. Descriptive statistics were utilized to assess the perinatal outcome of pregnant women with RhD alloimmunization. Fisher’s exact test was used to determine which association, and a P value < 0.05 was considered statistically significant.
Results: From the 98 pregnancies (06 - hydropic, 92 - non-hydropic) at high risk for fetal anemia, 45.9% of cases had MCA-PSV above 1.5MoM. Among these, 21.42% of all fetuses received an intrauterine transfusion. Forty-three IUTs were performed in 21 fetuses. The median number of transfusions per fetus was two. About 52.4% of the transfused fetuses had severe anemia, and 28.6% had moderate anemia. Prediction of MCA PSV ≥ 1.5MOM in diagnosing moderate-severe anemia in pregnant women with RhD sensitization 81%. General neonatal survival of alloimmunizations was 93.8%, 90.5% with IUT, 50% with hydrops fetalis, and 96.7% without hydrops.
Conclusion: This research provides evidence that MCA PSV ≥ 1.5MoM is modest predictor of moderate-severe anemia in untransfused fetuses. This study was a step toward the development of more extensive and multicenter studies on the Perinatal Outcome of pregnant women with RhD sensitization in Ethiopia. Extra studies are needed to evaluate strategies for estimates of fetal anemia after blood transfusion as a result of the absence of information on the IUT database.
Keywords: intrauterine transfusion, RhD alloimmunization, fetal hydrops, fetal anemia
Introduction
Isoimmunization is the process of immunizing an antigen-negative pregnant individual with a paternally derived fetal antigen if antigen was initially absent. Although the Rh systems consist of many antigen subtypes (eg, D, C, c, E, e), the D antigen is highly immunogenic; therefore, it is most involved in Rh incompatibility abnormalities.1 The volume necessary to cause alloimmunization varies from patient to patient. It is probably related to the Rh-positive RBC’s immune capacity and the mother’s immune responsiveness.2 RhD alloimmunization is a severe preventable disease that develops in Rh-negative pregnant women.3
Maternal RhD alloimmunization are the main cause of fetal anemia and are responsible for fetal and newborn morbidity and mortality. Hydrops fetalis and intrauterine or perinatal death are its most severe consequences.4 Once produced, maternal RhD antibodies continue to exist for life, resulting in fetal alloimmune-induced hemolytic anemia.5 Therefore, early disease detection with raised antibody titer is of utmost importance.6
MCA PSV above 1.5 Multiple of a Median (MoM) identified moderate and severe fetal anemia with sensitivity of 100% and a false-positive rate (FPR) of 12%.7 Currently, MCA PSV is used commonly and has replaced amniocentesis in managing Rh alloimmunization.7
Intrauterine transfusions (IUT) have been the backbone of treatment of fetal isoimmunization since the early 1980s. Perinatal survival rates are more excellent than 90% if anemia is diagnosed and treated early in the experienced center.8 Therefore, postnatal management of newborns is primarily centered on treating jaundice with phototherapy and exchanging transfusions to prevent complications.9
Over the years, the severe cases of Rh alloimmunization have decreased in developed countries. However, the disease burden is worse in developing countries. It continues to endanger women’s obstetric care in sub-Saharan Africa, where universal anti-D prevention for Rh-negative mothers is suboptimal.8,10
In Ethiopia, multicenter trials are scarce. Obstetrics centers have published no study in the country regarding the perinatal outcomes of pregnant mothers with Rh alloimmunization. Our study aims to identify perinatal outcome of pregnant women with RhD sensitization in fetuses/neonates with and without hydrops.
Materials and Methods
Study Setting
The study was conducted at St. Paul’s Hospital Millennium Medical College (SPHMMC) in Addis Ababa, Ethiopia. The hospital has a tertiary healthcare level serving the population of Ethiopia. In the hospital, a delivery and high-risk pregnancy follow-up clinic operates within the Department of Obstetrics – Gynecology, where patients are diagnosed with RhD sensitization. Patients were referred to a high-risk clinic where they were registered, followed then gave birth in the labor ward.
Study Design and Participants
A facility-based cross-sectional study was conducted in SPHMMC by reviewing medical charts of all pregnant women with RhD alloimmunization who gave birth at SPHMMC from September 11, 2016, to September 10, 2021GC. The exclusion criteria were patients who came on referral after giving birth at other health Institutions and pregnant women with fetal congenital Anomalies. Chart numbers of all RhD-sensitized pregnant and their neonate born from RhD-sensitized pregnant women were collected from logbooks of the ALS Team at the labor ward, NICU, Maternity ward, and high-risk clinic. Then, each patient chart was retrieved from the archive rooms of the hospital. Accordingly, 98 patients were diagnosed with RhD alloimmunization.
Data Collection Tool and Procedure
A data-gathering checklist was prepared to extract the necessary information from patient charts. Four trained OBGYN residents participated in the data gathering process. The acceptability of each patient card was checked before the data-gathering process was started. Each data-gathering checklist was coded and had three parts: 1. Socio-demography, 2. Perinatal Outcome and 3. Neonatal Outcome. Data-gathering checklist is listed as Appendix.
Data-Processing and Analysis
First, data was checked for errors, coded, edited, and entered SPSS version 26 (SPSS Inc, Chicago, 2019) for statistical analysis. Then, a descriptive statistical analysis, such as frequencies, percentages, and mean, was performed. Finally, Fisher’s exact test has been used to determine association, and the value of P < 0.05 has been considered statistically significant.
Ethical Consideration
To maintain confidentiality, patient identifiers like names and phone numbers were removed from the checklist. Ethical clearance letters with reference numbers PM/23/537 were received from the respective institutional review boards (IRBs) of SPHMMC. Study was performed in compliance with the standards of declaration On The Helsinki and informed consent had not been taken from patients as the study used secondary data from medical charts and maintained confidentiality.
Results
Ninety-eight pregnant with anti-D antibodies during the study were identified and included.
Demographic Data
Reviewing the past medical records of the 98 antibodies positive cases, we found that average age of women has been 32 years (25–38 years), and most patients were multiparous women (98%) with a minimum of two, and maximum of 11 pregnancies and only 2% were primiparous. It is listed in Table 1.
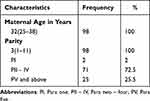 |
Table 1 Demographic Data Relating to Pregnant Women with RhD Sensitization |
Perinatal Outcomes
Of the 98 pregnancies at high risk for fetal anemia, 45 cases had MCA PSV above 1.5 MoM. Among 45 patients with MCA PSV above 1.5Multiple of a Median (MoM), 21 fetuses (21.42% of all fetuses) received an RBC intrauterine transfusion.
Out of 45 cases with MCA PSV above 1.5 MoM, three instances of intrauterine deaths and three neonatal deaths occurred after delivery. Overall, live birth after IUT was 90.5% for these intrauterine transfusions. It is listed in Table 2.
 |
Table 2 Perinatal Outcomes After Intrauterine Transfusion of Pregnant Women with RhD Sensitization |
In our study, out of 66 (67.3%) cases, with a critical titer of more than 1:16, 21 (21.4%) cases had MCA PSV less than 1.5 MoM and no fetal or neonatal death was reported in this group.
Intrauterine Transfusion (IUT)
Forty-three intrauterine RBC transfusions were achieved in 21 fetuses. Average number of blood transfusions per fetus was two: the maximum was six, and the minimum was one transfusion. Nine (42.85%) cases received one transfusion, six (28.6%) instances required three transfusions, four (19%) patients required two transfusions, one (4.8%) case required five transfusions, and one (4.8%) case required six transfusions.
Four (19%) of the 21 transfusion fetuses had fetal hydrops during follow-up. Of these, three (75%) court cases resulted in live births, one (25%) case resulted in posttransfusion deaths, and of the 17 non-hydropic transfused fetuses, 16 (94.1%) led to live births.
Perinatal Outcome with Hydrops
Perinatal outcomes in six hydropic fetuses demonstrated that there was one (16.7%), intrauterine fetal death (IUFD), one IUFD after intrauterine transfusion (16.7%), and one neonatal death (16.7%), with three living babies at discharge with a survival rate in hydropic babies of 50%. In the 92 non-hydropic fetuses, only one intrauterine fetal death and two neonatal deaths were noted, with survival rates of 96.7%. It is listed in Table 3.
 |
Table 3 Perinatal Outcomes According to a Fetus with/Without Hydrops of Pregnant Women with Rh Sensitization |
Performance of MCA-PSV ≥1.5MOM in the Diagnosis of Moderate-Severe and Severe Fetal Anemia
Eleven (52.4%) of the 21 transfused fetuses had pretransfusion Hb >7g/dl below normal for GA, which showed severe anemia in the fetus, and six (28.6%) cases had moderate anemia. Prediction of MCA PSV ≥1.5MOM in diagnosing moderate-severe and serious fetal anemia caused by pregnancy with RhD sensitization 81%. It is listed in Table 4.
 |
Table 4 Performance of MCA PSV ≥1.5MOM in the Diagnosis of Moderate-Severe and Severe Fetal Anemia in Pregnant Women with RhD Sensitization |
Neonatal Outcome
The most typical complication of the neonate was related to its RhD-sensitized status. Neonatal jaundice occurs in most neonates. Depending upon the jaundice severity, treatment was given. About 20 (95.23%) neonates received phototherapy and supportive measures. Out of this, 15 (71.4%) did require exchange transfusion. However, in the following line, it is explained otherwise that 4 (19%) neonates intervention needed in the form of Simple transfusion. It is listed in Table 5.
 |
Table 5 Neonatal Outcomes After Intrauterine Transfusion of Pregnant Women with RhD Sensitization |
Finally, overall neonatal survival of alloimmunizations was 93.8%, 50% with hydrops fetalis, and 96.7% without hydrops. The mean GA at delivery was 37 (28–42).
Discussion
All pregnant patients should undergo determination of blood type and antibody screen by Indirect Coombs Test (If Rh-negative) at the first prenatal visit. The recommended antenatal dose of anti-D is a single 300 microgram intramuscular (IM) dose given to all Rh-negative non-alloimmunized pregnant women once at 28–32 weeks which takes care of small fetomaternal bleeding (FMH) happening during pregnancy. The same dose is again repeated shortly after birth within 72 h (if missed, within 28 days) for combating the FMH going on during parturition.11,12
Despite the prevalence of the Rh-negative is significantly lesser among Africans (8%) than Caucasians (15%), isoimmunization remains a significant factor in perinatal morbidity and continues to compromise women’s obstetric care in Sub-Saharan Africa due to the poor antenatal practices to identify Rh-negative women, absence of a universal access program for all Rh-negative women, failure to recognize sensitizing events in pregnancy and to treat them appropriately, failure and absence of facilities to determine the degree of fetomaternal bleeding and the unaffordability of anti-D immunoglobulin.13,14
This study evaluated 98 cases with RhD sensitization at SPHMMC from September 11, 2016, to September 10, 2021. According to data collected over five years, we found that average age of women had been 32 years (range=25–38 years), and most cases were multiparous. Similar results were reported by the authors such as Omkar Potdar and Lauren Andersson. They noted that patients whose mean age of 30 years, except for one woman, were all Multigravida (MG) with a minimum of two and maximum of seven pregnancies, respectively.8,15
Once a mother has been identified as having a clinically significant RBC alloantibody, further monitoring and evaluation are required. As the pregnancy progresses, serial RBC antibody titers may be used. Once a critical titer threshold (1:16) is reached, and the fetus is found to be at risk of carrying the antigen by paternal zygosity testing or more direct measurements, or the fetal result is unknown, the fetus must be assessed for clinical anemia.16 Out of 98 cases, 66 (67.3%) had a critical titer of more than 1:16. In the Study by Shradha et al, out of 24 indirect antiglobulin test (ICT) positive cases, only one had a titer of 1:16 and followed every week, but a rising trend was not seen. The titer of 6 patients out of 24 was >1:32.17 In a study by Sánchez-Durán et al, of the 194 high-risk pregnancies, 38 had titers <1:16 (resulting in 38 livebirths), and 156 had titers ≥1:16.18
Correct pregnancy monitoring allows optimum planning in terms of the time and place of delivery, even when it comes to pregnant women with an MCA PSV under 1.5 MoM throughout gestation. In our series, from the 98 pregnancies with high-risk fetal anemia, 45 cases had MCA PSV above 1.5 MoM. Among these three cases of fetal deaths, three neonatal deaths occurred after delivery. Overall neonatal survival of alloimmunizations was 93.8%.
Among 45 cases with MCA PSV above 1.5 MoM, 21 (21.42% of all fetuses) received an RBC intrauterine transfusion. In a study by Sánchez-Durán et al, of the 194 high-risk pregnancies, 57 had MCA-PSV >1.5 MoM, and 45 fetuses (23.2% of all fetuses) received IUT.18
Forty-three IUT were performed in 21 fetuses. The median figure of a blood transfusion per fetus was two: the maximum was six, and the minimum was one transfusion. The earliest gestational week at first IUT was 24 + 4/7, and the latest was 35 + 0/7. For most of them, 61.9% of their 1st Intrauterine transfusions were at GA of 29–32+6 weeks. Overall, live birth after IUT was 90.5%. In the study by Ayse et al, 110 intrauterine transfusions were done for 42 fetuses, and average number of blood transfusions per fetus was two: the maximum was seven transfusions, and the minimum was one transfusion. The mean gestational week at first blood transfusion was 27.07 ± 4.13. The earliest gestational week at IUT was 20 + 2/7, and the latest was 33 1/7. The overall survival is 80.95% which was lower than our study.19 In a survey by Chatziantoniou et al, In six pregnancies, a total of 21 IUTs were needed (mean 3.5 transfusions per pregnancy, range 3–5); the Mean gestation at first IUT was 25.5 weeks (range 21–28).20
The current method for fetal anemia monitoring and intrauterine transfusion therapy referral is the MCA PSV measurement found by Martinez‐Portilla et al. This method identifies a fetus at risk of moderate and severe anemia when levels reach a value above 1.5 MoM for a particular gestational age. The method has a sensitivity of 100% and a 12% rate of false positives.21 In our study, 11 (52.4%) of the 21 transfused fetuses had pretransfusion Hb >7g/dl below normal for GA, which showed severe anemia in the fetus, and 6 (28.6%) cases had moderate anemia. Prediction of MCA PSV ≥1.5MOM in diagnosing moderate-serious and serious fetal anemia in pregnant women with RhD sensitization 81%. In a study by David A., the median Hb at presentation (at first in-utero transfusion) was 7.3 g% (IQR 4.6–8.8 g%). Of these, 30% (n = 20) had a Hb ≤2 SD for gestation (GA) (moderate anemia) and 70% (n = 47) had a Hb ≤5 SD for GA (severe anemia) at initial fetal blood sampling.19
In our study, we did not evaluate strategies for estimation of fetal anemia after blood transfusion as a result of an absence of information on the IUT database. In a meta-analysis published by Martinez – Portilla et al, the performance of MCA PSV ≥1.5MoM for predicting moderate-severe fetal anemia regardless of the number of preceding blood transfusions. The constructed heroic curve had an area below the curve (AUC) of 83%. When considering only not transfused fetuses, predictions improved, achieving AUC of 87%, susceptibility of 86% and a specificity of 71%. However, decrease sensitivity to predictions of moderate-severe anemia by MCA PSV ≥1.5MoM have been observed (estimate, –5.5% (95% CI, –10.7 to 0.3%), P = 0.039) as number of preceding transfusions increased.21
The most typical complication of the neonate was related to its RhD isoimmunized status. Neonatal jaundice occurs in many neonates. Depending upon the jaundice severity, treatment was given. In this study, out of 21 fetuses of IUT, about 20 (95.23%) neonates received phototherapy and supportive measures. Out of this, (n-15) 71.4% did require exchange transfusion. However, in the following line, it is declared otherwise than four (19%) neonates’ interventions are needed in the form of Simple transfusion. Our results were comparable to a study done by Omkar et al.8
In six hydropic fetuses, there were one intrauterine fetal death (IUFD), one intrauterine transfusion fetal death, and one neonatal death, with three living babies at discharge with a survival rate in hydropic babies of 50%. In the 92 non-hydropic fetuses, only one intrauterine fetal death and two neonatal deaths were noted, with survival rates of 96.7%. According to Şavkli et al, the survival rate was 78% and 92% in hydropic and non-hydropic cases, respectively.22
Finally, our study’s overall neonatal survival of alloimmunizations was 93.8%. In a study done by Nabeel et al, out of 424 Rh antibodies positive women, 91 patients (21.5%) had a significant level of antibody titer (>1:16) and were managed as high-risk patients. The perinatal deaths in the 424 alloimmunized women were as follows: 16 cases of intrauterine fetal deaths and five early neonatal deaths, making neonatal survival 95.5%.3
Strengths and Limitations of This Study
One of the strengths of the research was that it was the first study addressing RhD alloimmunization in Ethiopia. Another strength is that it has been carried out in a center with a relatively extensive experience in the isoimmunization, blood transfusion, and neonatal managing, yielding many cases and better outcomes.
The study limitations were a retrospective study and lack of available information on the IUT database. Another restriction is that data on neonatal outcomes were incomplete since some charts were missed. Also, the sample size of cases with hydrops and IUT were small and made it difficult to generalize the results.
Conclusion
This research provides evidence that MCA PSV ≥1.5MoM is modest predictor of moderate-severe anemia in untransfused fetuses. This study was a step toward the development of more extensive and multicenter studies on the perinatal Outcome of pregnant women with RhD sensitization in Ethiopia. Extra studies are needed to evaluate strategies for estimates of fetal anemia after blood transfusion as result of absence of information on the IUT database.
Abbreviations
RBC, Red blood cell; RhD, Rhesus D; IgG, Immunoglobulin G; IgM, Immunoglobulin M; HDFN, Haemolytic Disease of the Fetus and Newborn; MCA PSV, Middle Cerebral Arteries Peak Systolic Velocity; IUT, Intrauterine Blood transfusion; MoM, Multiples of the Median; NICU, Neonatal Intensive Care Unit; ICT, Indirect Coombs Test; ALS, Advanced life support; SPHMMC, St. Poul Hospital Millennium Medical College; AA, Addis Ababa; MG, Multigravida; PI, Para one; PII – IV, Para two – four; PV, Para five; GA, Gestational age.
Ethical Approval
Ethical clearance letters with PM/23/537 reference numbers were obtained from the institutional review boards (IRBs) of St. Paul’s Hospital Millennium Medical College after evaluating the research proposal. And consent for publication was waived as the study used secondary data from medical charts and all identifiers on charts such as record numbers, phone numbers, names, and addresses were not disclosed to maintain the confidentiality of patient data.
Acknowledgments
We would like to thank the OBGYN Department of SPHMMC for giving us a chance to do this research and help in a time of need on the contents of a manuscript and for agreeing to be responsible for all aspects of this work.
Funding
St. Paul’s Hospital Millennium Medical College funded this project.
Disclosure
The authors have no competing interests in this work.
References
1. George Uchenna Eleje CPI COE, Joseph Chinedu Umeobika, Charlotte Blanche Oguejiofor. Fetomaternal-outcomes-of-women-with-rhesus-isoimmunization-in-A-Nigerian-teinstitution. Preg Neonatal Med. 2017;1(1):21–27.
2. American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 192: management of alloimmunization during pregnancy. Obstet Gynecol. 2018;131(3):e82–e90. doi:10.1097/aog.0000000000002528
3. Bondagji NS. Rhesus alloimmunization in pregnancy. Saudi Med. 2011;32(10):1040. doi:10.35841/Pregnancy-Neonatal.1000102
4. Phung TV, Houfflin‐Debarge V, Ramdane N, et al. Maternal red blood cell alloimmunization requiring intrauterine transfusion: a comparative study on management and outcome depending on the type of antibody. Transfusion. 2018;58:1199–1205. doi:10.1111/trf.14542
5. Seriki SA. Factors that determine fatality of rhesus incompatibility. Reprod Med. 2020;7(4). doi:10.19080/GJORM.2020.07.5556720
6. Rashmi Tripathi NS, Singh N. Maternal and perinatal outcome in Rh negative mothers. Int J Reprod Contracept Obstet Gynecol. 2018;7(8):3141–3146. doi:10.18203/2320-1770.ijrcog20183306
7. Dodd JM, Andersen C, Dickinson JE, et al. Fetal middle cerebral artery Doppler to time intrauterine transfusion in red-cell alloimmunization: a randomized trial. Ultrasound Obstet Gynecol. 2018;51(3):306–312. doi:10.1002/uog.18807
8. Omkar PH, Purnima S. Perinatal outcome after intrauterine transfusion in Rh Isoimmunized mothers. J Obstet Gynaecol India. 2018. doi:10.1007/s13224-018-1108-6
9. Giancarlo MMEN, Stone J, Berghella V, Sciscione AC, Tate D, Mauro H; Society for Maternal-Fetal Medicine. Society for Maternal-Fetal Medicine (SMFM) clinical guideline #8: the fetus at risk for anemiae diagnosis and management. Am J Obstet Gynecol. 2015;14. doi:10.1016/j.ajog.2015.01.059
10. Giancarlo Mari ME, Vincenzo Berghella AC, Sciscione DT, Mauro H. Society for Maternal-Fetal Medicine (SMFM) clinical guideline #8: the fetus at risk for anemiae diagnosis and management. Am J Obstet Gynecol. 2015;14. doi:10.1016/j.ajog.2015.01.059
11. Tanushree S, Madhushree S. Rh alloimmunisation: current updates in antenatal and postnatal management. Indian J Pediatr. 2020. doi:10.1007/s12098-020-03366-0
12. Ghesquie` Re L, Garabedian C, Coulon C, et al. Management of red blood cell alloimmunization in pregnancy. J Gynecol Obstet Hum Reprod. 2018;47:197–204. doi:10.1016/j.jogoh.2018.02.00
13. Dean L. Blood Groups and Red Cell Antigens. Bethesda (MD): National Center for Biotechnology Information (US); 2005.
14. Osaro E, Charles AT. Rh isoimmunization in SubSaharan Africa indicates need for universal access to anti-RhD immunoglobulin and effective management of D-negative pregnancies. Int J Womens Health. 2010;1:429–437. doi:10.2147/IJWH.S15165
15. Andersson L, Szabo F. The incidence, and outcome of clinically significant antibodies detected in Rhesus-D positive pregnant women of the Northern Territory. Aust N Z J Obstet Gynaecol. 2017;1–4. doi:10.1111/ajo.12750
16. JenniferWebb MD, Delaney M. Red blood cell alloimmunization in the pregnant patient. Transfus Med. 2018;32:213–219. doi:10.1016/j.tmrv.2018.07.002
17. Shradha BM, Sahay PB. Obstetrical and perinatal outcome in rhesus antigen negative pregnancy. Int J Sci Study. 2016;3(11):124–129. doi:10.17354/ijss/2016/70
18. Sánchez-Durán MÁ, Higueras MT, Halajdian-Madrid C, et al. Management and outcome of pregnancies in women with red cell isoimmunization: a 15-year observational study from a tertiary care university hospital. BMC Pregnancy Childbirth. 2019;19(356). doi:10.1186/s12884-019-2525-y
19. Somerset DA, Martin J, William M, Mark D, Kilby MD. An audit of outcome in intravascular transfusions using the intrahepatic portion of the fetal umbilical vein compared to cordocentesis. Fetal Diagn Ther. 2006;21:272–276. doi:10.1159/000091355
20. Chatziantoniou V, Maggs T, Rozette C, Fountain C, Watts T. A descriptive single-centre experience of the management and outcome of maternal alloantibodies in pregnancy. Transfus Med. 2017;27(4):275–285. doi:10.1111/tme.12430
21. Martinez‐Portilla RJ, Lopez‐Felix J, Hawkins‐Villareal A, et al. Performance of fetal middle cerebral artery peak systolic velocity for prediction of anemia in untransfused and transfused fetuses: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2019;54:722–731. doi:10.1002/uog.20273
22. Şavkli AÖ, Çetin BA, Acar Z, et al. Perinatal outcomes of intrauterine transfusion for foetal anaemia due to red blood cell alloimmunisation. J Obstet Gynaecol. 2020;40(5):649–653. doi:10.1080/01443615.2019.1647521
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
