Back to Journals » International Journal of Women's Health » Volume 14
Performance of the Human Papillomavirus E6/E7 mRNA Assay in the Primary Screening of Cervical Cancer: Opportunistic Screening in Fujian, China
Authors Zhuang L, Weng X, Wang L, Xie X, Zhong L , Liu D, Xiu Y
Received 22 July 2022
Accepted for publication 13 October 2022
Published 25 October 2022 Volume 2022:14 Pages 1519—1530
DOI https://doi.org/10.2147/IJWH.S383431
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Elie Al-Chaer
Lijuan Zhuang *, Xiulan Weng *, Lihua Wang, Xiaoyan Xie, Liying Zhong, Dabin Liu, Yingling Xiu
Department of Obstetrics and Gynecology, Fujian Maternity and Child Health Hospital, College of Clinical Medicine for Obstetrics & Gynecology and Pediatrics, Fujian Medical University, Fuzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yingling Xiu; Dabin Liu, Department of Obstetrics and Gynecology, Fujian Maternity and Child Health Hospital, College of Clinical Medicine for Obstetrics & Gynecology and Pediatrics, Fujian Medical University, 18 Daoshan Road, Fuzhou, People’s Republic of China, Tel +86-13860610354 ; +86-13489997701, Fax +86-591-87551247, Email [email protected]; [email protected]; [email protected]
Purpose: A high-risk human papillomavirus E6/E7 mRNA (HR-HPV mRNA) assay is widely used in cervical cancer screening in China. However, it is still unclear whether stand-alone HR-HPV mRNA testing is sufficient for primary screening. The purpose of this study was to investigate the feasibility of a stand-alone HR-HPV mRNA assay for primary screening of cervical cancer.
Methods: Women aged 21 and older were recruited in Fujian Province, China, from January 2020 to January 2022. Cervical exfoliated cells were collected for cervical cytology and HR-HPV mRNA assays, and women with positive results on either assay were referred for colposcopy. The screening effectiveness of the assay was calculated based on the cervical histology. When comparing the efficacy of the different screening strategies, only women aged 25 and older were included.
Results: A total of 9927 women were recruited. This study identified 217 cases of high-grade squamous intraepithelial disease or worse (HSIL+). The overall age-specific HR-HPV infection rate showed a U-shaped distribution. The sensitivity of the HR-HPV mRNA assay to identify CIN2+ and CIN3+ was 97.2% and 97.9%, respectively, which was significantly higher than that of cytology (82.9% and 88.6%, P< 0.001 and 0.002). The sensitivity of the HR-HPV mRNA primary screening strategy to identify CIN2+ and CIN3+ was 92.2% and 94.3%, respectively, which was similar to the co-testing strategy (P=0.336 and 0.394) and higher than the cytology primary screening (P=0.002 and 0.048). In addition, the HR-HPV primary screening strategy had a lower referral rate for colposcopy than cytology primary screening (5.4% vs 6.6%, P< 0.001), and the screening cost was lower than co-testing ($29,594.3 per 1000 screened women vs $55,140 per 1000 screened women, P< 0.001).
Conclusion: In conclusion, the detection of CIN2+/CIN3+ by HR-HPV mRNA is both specific and sensitive. It may be suitable for primary screening of cervical cancer in China.
Keywords: cervical cancer, HR-HPV, E6/E7 mRNA, primary screening, cervical intraepithelial neoplasia
Introduction
Cervical cancer is the fourth most common malignant tumor in women, and its incidence and mortality have been on the rise in China in recent years, with a trend of increasing impact on younger women.1–3 The incidence of cervical cancer in China and Fujian Province was 10.7/100,000 and 21.0/100,000, respectively, and the mortality rate was 4.4/100,000.4,5 Persistent infection with high-risk human papillomavirus (HPV) is a major causative factor for cervical cancer. Tertiary prevention of cervical cancer has been widely practiced worldwide.2 The prevalence of cervical HR-HPV varies among women of different ages. Previous evidence has found that in developing countries, women under 25 years of age and women over 50 years of age exhibit high rates of HR-HPV infection, whereas women between 25 and 50 years of age have lower rates.5–8
HPV vaccination is the most effective way to prevent cervical cancer, but China’s HPV vaccine was only first licensed in 2016,9 and the coverage rate is low. Therefore, it is very important to improve our current screening system. Pap smears have performed unsatisfactorily in developing countries and regions, with a sensitivity of only 30%-40%.10 The efficacy of fluid-based cytology has improved, but the number of cytopathologists in China remains inadequate, and the diagnostic techniques are immature, which leads to a hindrance in the popularization of this technique for routine screening. Screening methods using visual inspection with acetic acid and Lugol’s iodine (VIA/VILI) are not dependent on specific equipment and are simple and inexpensive to perform, but their sensitivity is low (40–60%).11,12
Given HPV’s major etiological role, HPV testing can be an accurate means of detecting women at risk for cervical cancer. In 2008, a high-risk HPV (HR-HPV) test was recommended in Europe for primary screening of cervical cancer in women over 25 years of age; in April 2014, the US Food and Drug Administration (FDA) approved the Cobas 4800 HPV-DNA test for primary cervical cancer screening of women over 25 years of age.13 The latest WHO guidelines published in 2021 similarly recommend HPV testing for primary screening (WHO, 2021). HPV testing is relatively accurate and consistent regardless of the test used, with HPV primary screening increasing the detection of cervical intraepithelial neoplasia (CIN) at grade 2 or worse (CIN2+) by 25%.14 The advantage of HPV primary screening is its high sensitivity, but it may lack specificity.
The four currently FDA-approved HPV tests include three DNA-based tests and one RNA-based test. The detection of HR-HPV E6/E7 mRNA has theoretically higher specificity.15 It is well known that E6/E7 oncogenes play a key role in the development of cervical cancer. Since E6/E7 overexpression occurs after the integration of HPV into the genome, it is important in the detection of high-grade cervical lesions. Previous evidence16,17 found that HR-HPV E6/E7 mRNA detection for cervical cancer screening can reduce the number of colposcopy referrals and reduce the number of HPV or cytology reviews, and thus reduce the cost of cervical cancer screening. Previous studies18,19 have compared the efficacy of HR-HPV E6/E7 mRNA testing with that of HC2 and cytology testing in cervical cancer screening, which also confirmed that the HR-HPV E6/E7 mRNA test was valid and feasible. However, that study was limited by its small sample size. Therefore, there is a need for a large prospective screening study to assess the performance of the HR-HPV E6/E7 mRNA assay in cervical cancer screening in China. Direct detection of HR-HPV E6/E7 in cervical samples may be more specific than the HR-HPV-DNA assay.20 The HR-HPV E6/E7 mRNA test met the cross-sectional clinical and reproducibility criteria for detecting CIN2+ in the International Guidelines for HR-HPV Testing Requirements in Cervical Cancer Screening in a noninferiority comparison with HR-HPV DNA testing.21
Therefore, through a large cohort study of cervical cancer screening in Fujian Province, China, this study evaluated the efficacy of different primary screening programs and various triage strategies to improve the level of cervical cancer prevention in China.
Materials and Methods
Study Population
Women who underwent cervical cancer screening at Fujian Maternal and Child Health Hospital from January 2020 to January 2022 were recruited for this study. The inclusion criteria were as follows: age ≥21 years; no serious organ dysfunction or mental illness; voluntary participation; and able to complete the questionnaire. The exclusion criteria were as follows: women with a history of hysterectomy; previous diagnosis of CIN or cervical cancer; pelvic radiation therapy; women who were pregnant or breastfeeding; and women being treated for other serious medical and surgical diseases. This study was approved by the Ethics Committee of Fujian Maternal and Child Health Hospital (approval number: 2020YJ239).
Study Design
One cervical specimen was collected from all participants at enrollment using a cell brush and preserved in suspension in PreservCyt collection media (Hologic Inc., MA, USA). Each specimen was used for liquid-based cytology (TCT) assays and Aptima HR-HPV mRNA assays (Hologic, CA, USA).
Participants with a cytologic diagnosis of atypical squamous cells of undetermined significance (ASCUS) or worse and/or positive on the HR-HPV mRNA assay were referred for colposcopy. Colposcopy was performed by a senior colposcopy physician with at least 10 years of experience. If abnormal epithelial cells were observed colposcopically, a colposcopy-guided biopsy was performed. If the colposcopic evaluation was inadequate, random biopsies were performed at the 3, 6, 9 and 12 o’clock positions of the cervix, and endocervical curettage (ECC) was performed.
Patients with ASCUS or low-grade squamous intraepithelial lesions (LSIL) on cytology and no obvious lesions on colposcopy at the first visit were not biopsied and were considered to have a histological status of “no high-grade squamous intraepithelial disease (HSIL)”. If cervical cancer is suspected at the time of sampling, a cervical biopsy is performed immediately.
Women with a negative combined screening result were considered to have a histological status of “normal/LSIL”. Biopsy results were classified into the following three broad groups: negative for intraepithelial lesion or malignancy (NILM, including no pathologic changes and benign or reactive lesions), LSIL (including CIN 1, HPV-affected), and high-grade cervical lesions or worse (HSIL+, including CIN2, CIN3 and cancer) (Figure 1).
The Process of the Different Referral Strategies
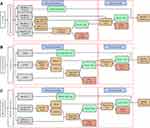
Three triage strategies were analyzed in this study: (1) Co-testing primary: Patients with both HR-HPV mRNA positive and abnormal cytology (ASCUS or worse) results were referred to colposcopy directly; patients with positive HPV-16/-18/-45 mRNA were directly referred for colposcopy, regardless of the cytology results (Figure 2A). (2) Primary cytology: Patients with cytology results of ASCUS were referred for colposcopy if they tested positive for HR-HPV mRNA. Patients with cytology results of LSIL or worse were referred to colposcopy directly regardless of the HR-HPV mRNA results (Figure 2B). (3) HR-HPV mRNA primary: For HR-HPV-positive patients, further genotyping should be performed. When HPV-16/-18/-45 is positive, they will be directly referred for colposcopy testing, or other genotype-positive patients with a cytology test result of ≥ASCUS will be referred for colposcopy testing (Figure 2C).
According to the ASCCP guidelines,22 HPV primary screening strategies are recommended for women aged 25 years and older. When evaluating the efficacy of the 3 different referral strategies, we excluded women 21–24 years of age and only analyzed women 25 years of age and older. For women diagnosed with CIN2+, according to the 2019 version of the ASCCP guidelines,23 women with CIN2 younger than 25 years old can be followed up for 12 months, and those aged 25–30 years who are concerned about the impact of cervical resection on subsequent pregnancy can be followed up for 12 months, but cervical excision should be performed if disease progression occurs. Cervical/hysterectomy was performed on CIN2 women ≥30 years of age and all CIN3+ women.
Liquid-Based Cytology (TCT) Assay
All samples were first subjected to TCT testing. The TCT results were assessed according to the 2014 Bethesda system by expert pathologists. For TCT detection, the cervical exfoliated cell suspension is first made into a single layer of cells on a glass slide and then fixed and Pap stained. The pathologist reads the slide under a microscope. The results are classified as follows: negative for intraepithelial lesion or malignancy (NILM); atypical squamous cells of undetermined significance (ASC-US); low-grade squamous intraepithelial lesion (LSIL); atypical squamous cells, not possible to exclude high-grade squamous intraepithelial lesion (ASC-H); high-grade squamous intraepithelial lesion (HSIL); squamous cervical cancer; atypical glandular cells (AGCs); and adenocarcinoma in situ (AIS).
HR-HPV mRNA Assay and Genotyping
TCT specimens were tested under blinded conditions using the Aptima® HR-HPV assay (Gen Probe; Hologic, CA, USA), an FDA-approved HR-HPV E6/E7 mRNA assay that detects 14 HR-HPV types (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68). All HR-HPV-positive samples were further genotyped for HPV-16, HPV-18, and HPV-45 using the Aptima® HPV 16 18/45 genotype test. Testing and reporting of the results was performed by a professional technician following the manufacturer’s instructions.
Data Analysis
Histologically confirmed HSIL+ was used as the endpoint for clinical observation. Sensitivity and specificity for the detection of HSIL+ were determined according to standard definitions. The 95% confidence intervals (CIs) were calculated according to the binomial method. The De Long test was used to determine statistical significance (P<0.05). The number of referrals for colposcopy performed to detect a single case of HSIL+ was calculated and used as a measure of the efficiency of referral for the screening method. There were two definitions of cost used: the first was the cost per 1000 women screened in the first round of screening, and the second was the cost per HSIL+ (CIN2+) case identified in the first round of screening. Age was expressed as the median and interquartile range because it was not normally distributed. Differences between categorical variables were compared using the chi-squared (χ2) test. Statistical analysis was performed using SPSS 24.0 (IBM, New York, NY, USA), and P<0.05 was considered statistically significant.
Results
Characteristics of the Study Population
A total of 10,910 women were recruited for this study from January 2020 to January 2022. Among these, 983 women were excluded because they met the exclusion criteria, and 9927 eligible participants were included in the analysis. They provided a sample of exfoliated cervical cells and a completed questionnaire. The median age of the participants was 43 years (interquartile range: 36–50). Of the 9927 women with both cytology and HR-HPV mRNA screening results, 8.1% (807/9927) had a positive cytology result (ASCUS or worse), and 13.1% (1305/9927) were infected with HR-HPV mRNA. Of these, 13.5% (176/1305) were HPV16 mRNA positive, and 7.4% (97/1305) were HPV18/45 positive. Among women with a cytological diagnosis of ASCUS or worse, HR-HPV mRNA positivity was 73.4% (592/807). Of the 1520 women who tested positive for either screening test, 907 (59.7%) underwent colposcopy (Figure 1).
The prevalence of HR-HPV mRNA infection was analyzed in ten different age subgroups (Figure 3). The highest prevalence of HR-HPV mRNA was found in the 21–25 years age group (22.7%), followed by the 61–65 years age group (22.3%). The lowest prevalence was in the 41–45 years age group (11.2%). The overall age-specific HR-HPV mRNA prevalence showed a U-shaped distribution. The age-specific HPV16 mRNA prevalence was similarly U-shaped, but the HPV18/45 mRNA prevalence was highest in women >65 years old (2.8%) and lowest in women 21–25 years old (0.8%).
Detection Rate of HSIL and the Above Lesions
In this study, 613 women indicted for the procedures by the study protocol did not undergo colposcopy and biopsy because of loss to follow-up. In addition, among those who were judged to have inadequate colposcopy due to unclear exposure of the cervical squamous-columnar junction, 86 women underwent additional endocervical scraping (ECC) to determine the presence of cervical lesions. Ultimately, 9314 women had histopathological results for the follow-up analysis.
A total of 217 (2.3%) women were identified as CIN2+. The HR-HPV infection rate of cervical CIN2+ women was significantly higher than that of normal/LSIL women (97.2% vs 5.6%, P<0.001). The age of first sex, previous pregnancy, weekly alcohol use and smoking did not differ between CIN2+ and normal/LSIL women (P=0.202, 0.236, 0.735, 0.527, respectively, Table 1).
 |
Table 1 Characteristics of the Participants in This Study. (N=9314) |
Comparison of the Efficacy of Different Screening Methods
Positive screening by cytology was defined as ASCUS or worse (ASCUS+). Positive screening for HR-HPV mRNA was defined as positive for any type of HR-HPV mRNA infection. The comparative efficacy of the two screening methods in detecting HSIL+ (CIN2+/CIN3+) is shown in Table 2. The sensitivity of the HR-HPV mRNA assay to detect CIN2+ and CIN3+ was 97.2% and 97.9%, respectively, which was significantly higher than that of cytology at 82.9% (P<0.001) and 88.6% (P=0.002). The specificity of the HR-HPV mRNA assay to detect CIN2+ and CIN3+ was 94.4% and 93.7%, respectively, slightly lower than that of cytology at 95.1% and 94.5%.
 |
Table 2 Comparison of Performance of Different Screening Assays. (N=9145) |
Comparison of the Efficacy of the Different Referral Strategies
HR-HPV mRNA primary and co-testing primary screening strategies identified 200 and 205 CIN2+ women in the initial screening, higher than the number for cytology primary screening (179 women). HR-HPV mRNA primary and co-testing primary screening had the highest sensitivity for identifying CIN2+/CIN3+, significantly higher than cytology primary screening (CIN2+: PHPV-Cy=0.002, PCo-Cy<0.0001; CIN3+: PHPV-Cy=0.048, PCo-Cy=0.013). However, the specificity of the three screening strategies to identify CIN2+/CIN3+ was similar (CIN2+: PHPV-Cy=0.803, PCo-Cy=0.114; CIN3+: PHPV-Cy=0.470, PCo-Cy=0.045). This study found that the HR-HPV mRNA screening strategy had the lowest colposcopy referral rate (5.4%), with only 2.5 colposcopy visits per identified CIN2+ woman. For the analysis of screening cost, it was found that the medical cost consumption of the HR-HPV mRNA strategy in one round of screening was significantly lower than that of the cytology screening (P=0.029) and co-testing (P<0.001) strategies (Table 3).
 |
Table 3 Comparison of Performance of Different Triage Strategies. (N=9145) |
Discussion
This study evaluated the efficacy of HR-HPV E6/E7 mRNA and liquid-based cytology assays for screening and three different triage strategies in a hospital-based opportunistic screening cohort of 10,910 women in China. We found that the HR-HPV mRNA assay has the highest sensitivity and good specificity in detecting CIN2+/CIN3+ lesions.
Previously, large population-based studies in China reported HR-HPV infection rates ranging from 9.9% to 27.5%.24 In this study, the HR-HPV mRNA positive rate was 13.1%, which is at the low end of the range of reported HPV infection rates in China. There may be two reasons for the low infection rate. First, this study tested for E6/E7 mRNA of HR-HPV, and previous studies have found that the positive rate of HR-HPV E6/E7 mRNA is lower than that of HR-HPV DNA.25 Second, Fujian Province may not be a high prevalence population for HR-HPV in the Chinese region.
The International Agency for Research on Cancer (IARC) study reported a U-shaped distribution of HR-HPV prevalence in developing countries. Specifically, the prevalence of HR-HPV peaks among women under 25 years of age and then slowly declines to a trough at 45–55 years of age. It then slowly rises, reaching a second peak among women over 55 years of age.7,26 Similarly, a large population-based study conducted in 37 cities in China in 2015 reported a “bimodal” pattern of HR-HPV prevalence, with peaks occurring at ages 15–19 and 50–60 years.27
In the present study, a similar U-shaped pattern of HR-HPV mRNA infection was observed, although the largest, second peak occurred at ages >60 years. This finding can be explained by the fact that postmenopausal women are at high risk for HPV infection. Possible reasons for this include the following: postmenopausal women and their sexual partners may have altered patterns of sexual behavior; atrophy of the cervical and vaginal mucosa may make these women more vulnerable to microdamage, immunosuppression, and persistent viral infection; and they may not be vaccinated. Therefore, postmenopausal women are a critical population to target for the prevention and treatment of cervical cancer.
The advantages of HR-HPV testing are that it is objective, the results are available in a short period of time, and it is easily reproducible. HPV testing can detect precancerous cervical lesions much earlier than cytology. Negative results of HR-HPV testing have been reported to predict a low risk of future CIN2+ and may allow such individuals to reduce their frequency of screening.14,28,29 Theoretically, HR-HP mRNA is only produced after integration of the HPV viral genome and it may represent an active HR-HPV infection, so detection of HR-HPV mRNA transcripts may provide greater specificity for CIN2+.30
HR-HPV mRNA testing has received attention in recent years, with several studies comparing its performance in cervical cancer screening to HPV DNA testing. For example, one study15 enrolled 9451 women aged 30–60 years attending routine cervical cancer screening in Germany and compared an RNA-based test with a DNA-based test and found no statistically significant difference in the sensitivity of the two in detecting CIN2+ or CIN3+ lesions. However, the HR-HPV mRNA test had a higher specificity and positive predictive value than the HR-HPV DNA assay. HR-HPV RNA-based assays only detect actively infected cells, whereas DNA-based assays cannot distinguish between intracellular and extracellular viral DNA, leading to the possibility that the results are influenced by contamination with extracellular viral particles. In line with this, in other earlier studies, RNA-based HR-HPV assays were slightly less sensitive in detecting CIN2+.31 In addition, more recent reports have shown equal32,33 or higher sensitivity for HR-HPV mRNA testing compared to DNA testing.18,34 After long-term follow-up, women who tested negative for HR-HPV mRNA were found to have a fairly low risk of future HSIL+, as were women who tested negative for DNA-based tests.35,36 In this study, the HR-HPV mRNA assay showed good performance in opportunity-based cervical cancer screening with a high sensitivity of 97.2% and a high specificity of 94.4%. Therefore, the HR-HPV mRNA assay could be suitable for primary cervical cancer screening.
The current strategy in China is mainly to perform annual cytological screening. The reported detection rate of cervical precancerous lesions and cervical cancer by cytology screening is only 11.57/100,000 in China.37,38 CIN2+ was found in 217 (2.3%) of the women included in this study, among whom the HR-HPV mRNA assay identified 211 women with CIN2+ compared to 180 women with the cytology assay. The HR-HPV mRNA assay had higher sensitivity in identifying CIN2+/CIN3+ than the cytology assay (P<0.001, P=0.002). Therefore, the poor sensitivity of cytology requires the development of more accurate screening methods. Previous studies found HR-HPV testing to be a more sensitive screening method than cytology, and HR-HPV mRNA assays to further improve the detection of CIN2+ cases have been developed and evaluated.31–34
In this study, the CIN2+ detection rate for co-testing screening strategy was 2.2%. The detection rate of CIN2+ by the HR-HPV mRNA primary screening strategy was 2.1%, but the detection rate by the cytology primary screening strategy was only 1.9%. The sensitivity and specificity of the HR-HPV primary screening strategy for CIN2+/CIN3+ identification were similar to those of the co-testing screening strategy (sensitivity: P=0.336, P=0.394; specificity: P=0.183, P=0.198), but the sensitivity was significantly higher than that of the cytology primary screening strategy. Given that HR-HPV mRNA primary screening has a lower referral rate for colposcopy (P<0.001) and a lower screening cost (P=0.029) than cytology primary screening strategies, it is more often recommended for cervical cancer screening in China. Because of the higher cost of co-testing screening, it may only be applicable to economically developed areas of China. Cost is an important factor to consider when developing a screening strategy, especially in low- and middle-income countries where resources are relatively scarce.39 Even though the HR-HPV mRNA primary screening strategy is low cost, the number of CIN2+ cases it identifies is equal to or higher than other, more expensive screening strategies. This may be a more cost-effective cervical cancer screening strategy for China, which has low screening coverage and a large population.
Although the sensitivity of primary cytological screening strategies in women over 25 years of age is low, primary cytological screening strategies are still recommended for cervical cancer screening in women under 25 years of age. This is because women aged 21–24 have a high prevalence of HPV infection.5 This study also found a high prevalence of HPV mRNA in women aged 21–24, but women in this age group have a low incidence of cervical lesions.40 Therefore, ASCCP guidelines still recommend cytological screening as a recommended strategy for women under 25 years of age.23
This study has some limitations that need to be considered. First, when analyzing the age-specific HR-HPV prevalence, few women older than 65 were analyzed. Second, 40.3% of women who were indicated for referral for colposcopy did not attend it. Third, longitudinal follow-up should be performed in women with negative results for both cytology and HR-HPV mRNA.
Conclusions
In summary, the HR-HPV mRNA assay is more sensitive than cytology for cervical cancer screening, with good specificity. The results of this study suggest that an HR-HPV mRNA primary screening strategy is a suitable alternative for cervical cancer screening in China.
Data Sharing Statement
The data used and/or analyzed in the present study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
We confirm that our study complies with the Declaration of Helsinki. The study protocol was approved by the ethics committee of Fujian Maternal and Child Health Hospital (approval number: 2020YJ239). Informed consent in our study was waived because of its retrospective nature and anonymous analysis.
Acknowledgments
We thank all participants of this study and the staff of the laboratory and medical record section from Fujian Maternal and Child Health Hospital for their help with technical assistance and information service.
Funding
This work was supported by the Fujian Provincial Maternity and Child Hospital Natural Science Foundation (grant no. YCXM19-07).
Disclosure
The authors declare that they have no competing interests.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
3. Tabibi T, Barnes JM, Shah A, Osazuwa-Peters N, Johnson KJ, Brown DS. Human Papillomavirus vaccination and trends in cervical cancer incidence and mortality in the US. JAMA Pediatr. 2022;176(3):313–316. doi:10.1001/jamapediatrics.2021.4807
4. Arbyn M, Weiderpass E, Bruni L, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8(2):e191–e203. doi:10.1016/S2214-109X(19)30482-6
5. Sun P, Song Y, Ruan G, et al. Clinical validation of the PCR-reverse dot blot human papillomavirus genotyping test in cervical lesions from Chinese women in the Fujian province: a hospital-based population study. J Gynecol Oncol. 2017;28(5):e50. doi:10.3802/jgo.2017.28.e50
6. Wang Y, Meng Y, Li W, et al. Prevalence and characteristics of hrHPV infection among 414,540 women: a multicenter study in Central and Eastern China. J Cancer. 2019;10(8):1902–1908. doi:10.7150/jca.30157
7. de Sanjosé S, Diaz M, Castellsagué X, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007;7(7):453–459. doi:10.1016/S1473-3099(07)70158-5
8. Babi A, Issa T, Issanov A, et al. Prevalence of high-risk human papillomavirus infection among Kazakhstani women attending gynecological outpatient clinics. Int J Infect Dis. 2021;109:8–16. doi:10.1016/j.ijid.2021.06.006
9. Zou Z, Fairley CK, Ong JJ, et al. Domestic HPV vaccine price and economic returns for cervical cancer prevention in China: a cost-effectiveness analysis. Lancet Glob Health. 2020;8(10):e1335–e1344. doi:10.1016/S2214-109X(20)30277-1
10. Tang HP, Cai D, Kong YQ, et al. Cervical cytology screening facilitated by an artificial intelligence microscope: a preliminary study. Cancer Cytopathol. 2021;129(9):693–700. doi:10.1002/cncy.22425
11. Hu SY, Zhao XL, Zhao FH, et al. Implementation of visual inspection with acetic acid and Lugol’s iodine for cervical cancer screening in rural China. Int J Gynaecol Obstet. 2022;24:232.
12. Denny L, Kuhn L, De Souza M, Pollack AE, Dupree W, Wright TC
13. Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136(2):178–182. doi:10.1016/j.ygyno.2014.12.022
14. Zhang J, Zhao Y, Dai Y, et al. Effectiveness of high-risk human papillomavirus testing for cervical cancer screening in china: a multicenter, open-label, randomized clinical trial. JAMA Oncol. 2021;7(2):263–270. doi:10.1001/jamaoncol.2020.6575
15. Iftner T, Becker S, Neis KJ, et al. Head-to-head comparison of the RNA-based Aptima Human Papillomavirus (HPV) assay and the DNA-based hybrid capture 2 HPV test in a routine screening population of women aged 30 to 60 years in Germany. J Clin Microbiol. 2015;53(8):2509–2516. doi:10.1128/JCM.01013-15
16. Weston G, Dombrowski C, Harvey MJ, et al. Use of the Aptima mRNA high-risk human papillomavirus (HR-HPV) assay compared to a DNA HR-HPV assay in the English cervical screening programme: a decision tree model based economic evaluation. BMJ Open. 2020;10(3):e031303. doi:10.1136/bmjopen-2019-031303
17. Dombrowski CA, Weston GM, Descamps PP, Izopet PJ, Adams EJ, Adams E. Health economic evaluation of an mRNA high-risk human papillomavirus (HR-HPV) assay versus a DNA HR-HPV assay for the proposed French cervical screening programme. Medicine. 2022;101(29):e29530. doi:10.1097/MD.0000000000029530
18. Kuroki H, Sakamoto J, Shibata T, Takakura M, Sasagawa T. Comparison of Aptima and hybrid capture-2 HPV tests and Pap test in the referral population in Japan. J Med Virol. 2021;93(8):5076–5083. doi:10.1002/jmv.26865
19. Basu P, Banerjee D, Mittal S, et al. Sensitivity of APTIMA HPV E6/E7 mRNA test in comparison with hybrid capture 2 HPV DNA test for detection of high risk oncogenic human papillomavirus in 396 biopsy confirmed cervical cancers. J Med Virol. 2016;88(7):1271–1278. doi:10.1002/jmv.24453
20. Ge Y, Christensen P, Luna E, et al. Aptima human papillomavirus E6/E7 mRNA test results strongly associated with risk for high-grade cervical lesions in follow-up biopsies. J Low Genit Tract Dis. 2018;22(3):195–200. doi:10.1097/LGT.0000000000000393
21. Heideman DA, Hesselink AT, van Kemenade FJ, et al. The Aptima HPV assay fulfills the cross-sectional clinical and reproducibility criteria of international guidelines for human papillomavirus test requirements for cervical screening. J Clin Microbiol. 2013;51(11):3653–3657. doi:10.1128/JCM.01517-13
22. Obstetrics and Gynecology. Practice bulletin No. 157 summary: cervical cancer screening and prevention. Obstet Gynecol. 2016;127(1):185–187. doi:10.1097/AOG.0000000000001256
23. Perkins RB, Guido RS, Castle PE, et al. 2019 ASCCP risk-based management consensus guidelines committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24(2):102–131. doi:10.1097/LGT.0000000000000525
24. Li J, Huang R, Schmidt JE, Qiao YL. Epidemiological features of Human Papillomavirus (HPV) infection among women living in Mainland China. Asian Pac J Cancer Prev. 2013;14(7):4015–4023. doi:10.7314/APJCP.2013.14.7.4015
25. Mousavi AS, Pouryasin A, Yarandi F, et al. Assessment of cervical cancer molecular-based screening tools; HPV-DNA detection versus E6/E7 mRNA testing; first report of a prospective cohort study among Iranian women. Iran J Public Health. 2020;49(9):1734–1742.
26. Liao G, Jiang X, She B, et al. Multi-infection patterns and co-infection preference of 27 human papillomavirus types among 137,943 gynecological outpatients across China. Front Oncol. 2020;10:449. doi:10.3389/fonc.2020.00449
27. Wang R, Guo XL, Wisman GB, et al. Nationwide prevalence of human papillomavirus infection and viral genotype distribution in 37 cities in China. BMC Infect Dis. 2015;15(1):257. doi:10.1186/s12879-015-0998-5
28. Ronco G, Dillner J, Elfström KM, et al.; International HPV screening working group. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383(9916):524–532. doi:10.1016/S0140-6736(13)62218-7.
29. Zhao Y, Bao H, Ma L, et al. Real-world effectiveness of primary screening with high-risk human papillomavirus testing in the cervical cancer screening programme in China: a nationwide, population-based study. BMC Med. 2021;19(1):164. doi:10.1186/s12916-021-02026-0
30. Zhang Q, Dong L, Hu S, et al. Risk stratification and long-term risk prediction of E6 oncoprotein in a prospective screening cohort in China. Int J Cancer. 2017;141(6):1110–1119. doi:10.1002/ijc.30807
31. Monsonego J, Hudgens MG, Zerat L, et al. Evaluation of oncogenic human papillomavirus RNA and DNA tests with liquid-based cytology in primary cervical cancer screening: the FASE study. Int J Cancer. 2011;129(3):691–701. doi:10.1002/ijc.25726
32. Nieves L, Enerson CL, Belinson S, et al. Primary cervical cancer screening and triage using an mRNA human papillomavirus assay and visual inspection. Int J Gynecol Cancer. 2013;23(3):513–518. doi:10.1097/IGC.0b013e318280f3bc
33. Giorgi Rossi P, Ronco G, Mancuso P, et al.; NTCC2 Working Group. Performance of HPV E6/E7 mRNA assay as primary screening test: results from the NTCC2 trial. Int J Cancer. 2022;151(7):1047–1058. doi:10.1002/ijc.34120
34. Monsonego J, Hudgens MG, Zerat L, Zerat JC, Syrjänen K, Smith JS. Risk assessment and clinical impact of liquid-based cytology, oncogenic human papillomavirus (HPV) DNA and mRNA testing in primary cervical cancer screening (the FASE study). Gynecol Oncol. 2012;125(1):175–180. doi:10.1016/j.ygyno.2012.01.002
35. Reid JL, Wright TC Jr, Stoler MH, et al. Human papillomavirus oncogenic mRNA testing for cervical cancer screening: baseline and longitudinal results from the CLEAR study. Am J Clin Pathol. 2015;144(3):473–483. doi:10.1309/AJCPHVD7MIP3FYVV
36. Iftner T, Neis KJ, Castanon A, et al. Longitudinal clinical performance of the RNA-based Aptima Human Papillomavirus (AHPV) assay in comparison to the DNA-based hybrid capture 2 HPV test in two consecutive screening rounds with a 6-year interval in Germany. J Clin Microbiol. 2019;57(1):e01177–18. doi:10.1128/JCM.01177-18
37. Gu XY, Zheng RS, Sun KX, et al. Incidence and mortality of cervical cancer in China, 2014. Zhonghua Zhong Liu Za Zhi. 2018;40(4):241–246. Chinese. doi:10.3760/cma.j.issn.0253-3766.2018.04.001
38. Guo M, Xu J, Du J. Trends in cervical cancer mortality in China from 1989 to 2018: an age-period-cohort study and Joinpoint analysis. BMC Public Health. 2021;21(1):1329. doi:10.1186/s12889-021-11401-8
39. Mezei AK, Armstrong HL, Pedersen HN, et al. Cost-effectiveness of cervical cancer screening methods in low- and middle-income countries: a systematic review. Int J Cancer. 2017;141(3):437–446. doi:10.1002/ijc.30695
40. Beachler DC, Tota JE, Silver MI, et al. Trends in cervical cancer incidence in younger US women from 2000 to 2013. Gynecol Oncol. 2017;144(2):391–395. doi:10.1016/j.ygyno.2016.11.031
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.