Back to Journals » Infection and Drug Resistance » Volume 14
Performance of Procalcitonin to Distinguish Fungal from Bacterial Infections in Patients with Systemic Lupus Erythematosus
Authors He S, Ma J, Fan C, Tang C, Chen Y, Xie C
Received 10 September 2021
Accepted for publication 2 November 2021
Published 16 November 2021 Volume 2021:14 Pages 4773—4781
DOI https://doi.org/10.2147/IDR.S337871
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Shuangjun He,* Jun Ma,* Chenyu Fan, Chao Tang, Yi Chen, Cuiying Xie
Department of Emergency, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yi Chen; Cuiying Xie
Department of Emergency, Shanghai Jiao Tong University School of Medicine Affiliated Renji Hospital, 2000 Jiangyue Road, Minhang District, Shanghai, 200025, People’s Republic of China
Email [email protected]; [email protected]
Purpose: To evaluate the performance of serum procalcitonin (PCT) concentrations to diagnose fungal infection in patients with systemic lupus erythematosus (SLE).
Patients and Methods: From January 2017 to October 2020, SLE patients hospitalized for serious infection with an identified single bacterial or fungal pathogen, as well as PCT measured within 24h after admission were included. The diagnostic performance of PCT was evaluated independently and in combination with the white blood cell (WBC) count, C-reactive protein (CRP) level, and erythrocyte sedimentation rate (ESR). The analysis included the sensitivity, specificity, positive and negative predictive values, positive and negative likelihood ratios, and the crude and adjusted area under the receiver operating characteristic curve (AUROC).
Results: Sixty-nine patients were included; 26 had a fungal infection (38%) and 43 had a bacterial infection (22 gram-positive and 21 gram-negative). Fungal infection patients were mainly distributed in the respiratory group (88.5%), and bacterial infection distribution were more prevalent in respiratory group (44.2%) and abdominal/urinary group (23.3%). The PCT concentration was significantly lower in fungal infections than bacterial infections (fungal: 0.22 ng/mL, interquartile range [IQR], 0.09– 0.44 vs bacterial: 0.60 ng/mL, IQR, 0.16– 5.74; p = 0.016) and differed significantly between different infection sites (p = 0.022). PCT had better diagnostic performance for predicting fungal infection (AUROC = 0.731) than the WBC count (AUROC = 0.581), the CRP level (AUROC = 0.716), and ESR (AUROC = 0.583). PCT and ESR together had the best diagnostic performance, with 46.2% sensitivity and 88.4% specificity. Further, the AUROC increased compared to PCT alone but was statistically insignificant (p = 0.693).
Conclusion: For SLE patients with serious infection, the PCT concentration had better diagnostic accuracy for predicting fungal infection than the WBC count, the CRP level, and ESR. Combining PCT and ESR obtained the highest AUROC and provided an acceptable discrimination performance.
Keywords: diagnostic markers, emergency department, microorganisms, autoimmune disease
Introduction
Systemic lupus erythematosus (SLE) is a heterogeneous systemic autoimmune disease with protean clinical manifestations.1 The overall survival rate of SLE has dramatically improved over the past decades, partially due to immunosuppressive agents. However, immunological aberrations may increase the susceptibility of SLE patients to infections, becoming a major cause of death.2,3 In one recent population-based study, renal disease, infections, and cardiovascular disease were the top three causes of mortality of SLE patients.4 Patients with these acute events were more likely to seek care in the emergency department (ED), with approximately 40% patients having at least one ED visit per year of SLE.5 Further, infections were the leading cause of ED visits for SLE patients.6
SLE patients are often in an immunosuppressive state compared to non-immunocompromised individuals. Opportunistic infections, for example, fungi, are more common, increasing the challenges of anti-infection. Further, diagnosing a specific pathogen based on symptoms, signs, and clinical imaging alone is difficult. Narrowing the etiology of the pathogen’s species is crucial for the timely administration of appropriate antimicrobials. Therefore, identifying laboratory markers capable of distinguishing pathogenic microorganisms is greatly important.
Procalcitonin (PCT), the precursor of calcitonin, is normally secreted by parafollicular cells (C cells) of the thyroid and neuroendocrine cells in the lungs and the intestine. Under normal physiological conditions, the PCT concentration in healthy individuals is negligible. Therefore, serum PCT may be a tool for differentiating between bacterial and non-bacterial causes of inflammation,7,8 and could also be useful in systemic autoimmune diseases. Brodská et al reported that the PCT concentration in fungal infections was significantly lower than in Gram-positive and Gram-negative bacterial infections.9 However, similar studies have not been performed in individuals with SLE. In a small sample study, lupus patients with bacterial or fungal infections had higher serum PCT concentrations than those with viral infections, but the study did not compare bacterial and fungal infections.10 Further, a Chinese retrospective study reported that fungal infection ranked third in the total number of infections in SLE patients but did not analyze the PCT concentration.11
Therefore, the serum PCT concentrations in SLE patients with infection remains unknown,12–14 along with the difference between bacterial and fungal infections and the predictive value for fungal infection. To address this knowledge gap, we retrospectively analyzed SLE patients with infection to determine if the PCT concentration can differentiate between bacterial and fungal infections.
Methods
Study Design, Setting, and Subjects
This cross-sectional study evaluated SLE patients admitted to Renji Hospital, affiliated with Shanghai Jiao Tong University of Medicine, from the ED between January 2017 and October 2020. SLE patients aged 18 years or older hospitalized for serious infection were screened. Serious infection was defined as a composite of bacterial infections (eg, meningitis, encephalitis, cellulitis, endocarditis, pneumonia, pyelonephritis, septic arthritis, osteomyelitis, and bacteremia) or invasive fungal infections (eg, systemic candidiasis, cryptococcosis, aspergillosis, or Pneumocystis jirovecii) using discharge diagnosis ICD-10 codes from hospital inpatient records. Patients with viral infections were excluded, as it is well-documented that they have little effect on the PCT concentration, and we aimed to distinguish between bacterial and fungal infections. Patients with mixed infections were also excluded because, in this situation, it is difficult to decipher the effect of the infecting pathogen on the PCT level. Other exclusion criteria included other autoimmune diseases, a history of burns, multiple traumas, or surgery in the last three months, malignant tumors, SLE concurrent macrophage activation syndrome, and patients without PCT data within 24 h of admission. The study protocol was approved by the ethics committees of Shanghai Jiao Tong University of Medicine affiliated with Renji Hospital. Written informed consent was not required because all data were analyzed retrospectively and anonymously. The study conforms to the provisions of the Declaration of Helsinki (as revised in 2013).
Infectious Pathogen Identification
The infectious pathogen identification was based on a positive pathogen test from various specimens (eg, blood, sputum, pus, and urine), clear evidence of infection (eg, radiographic presentations on computed tomography, ultrasound, or magnetic resonance imaging), and obvious clinical signs correlating with infection. Molecular diagnostic methods, including next-generation sequencing and real-time quantitative reverse transcription-polymerase chain reaction, were also used to determine the causative pathogens (Pneumocystis jiroveci and Nocardia farcinica, respectively). Additionally, a positive response to the standard anti-infective therapy supported the diagnosis.
The above processes and decisions regarding the pathogenicity of common contaminants, including coagulase-negative staphylococci, Corynebacterium, and Candida species, were judged by an infectious disease physician and two trained physicians to avoid misclassification. The reviewers were blinded to the PCT concentration, and if they disagreed on the pathogen, the final result was decided based on the majority. Individuals were divided into a bacterial group (Gram-negative and Gram-positive subgroups) and a fungal group based on the pathogen.
PCT, C-Reactive Protein (CRP), White Blood Cell (WBC) Count, and Erythrocyte Sedimentation Rate (ESR) Measurements
PCT was measured using a UPT-3A-1200 with automated time-resolved fluorescence immunoassay (Rejing, China) with a 0.02 ng/mL limit of detection. In healthy individuals, the PCT concentration is <0.05 ng/mL in our laboratory. The CRP concentration and WBC count were analyzed in serum using a BC-7500R with high-sensitivity latex-enhanced nephelometry (Mairui, China). ESR was determined by the Westergren method using an established normal range of 0–20 mm/h. Per routine clinical practice, the medical staff ordered a PCT test if the patient was suspected to be admitted to the hospital due to infection. For individuals with multiple PCT tests during the first 24 h of admission, the highest value was used for analysis. A PCT level below the lower limit of detection was replaced by values half of the limit of detection (ie, 0.01 ng/mL).
Data Collection
We used a standardized electronic data extraction form to record data on patient demographics, comorbidities, clinical and laboratory characteristics, the infection site, microbiological test results, medication history, and inflammatory markers. SLE disease activity was assessed using the SLE Disease Active Index 2000 (SLEDAI-2k); a high SLEDAI score represents high disease activity. The current tools to identify infection and the infection severity, such as the sequential organ failure assessment (SOFA) and quick SOFA scores, were also applied as indicated at baseline. Following the Sepsis 3.0 criteria, patients were classified as having sepsis if there was a suspected or documented infection plus an acute increase in the SOFA score (greater than 2 points).
Statistical Analyses
Data are expressed as means ± standard deviations or medians (interquartile ranges [IQR]) and percentages, depending on the variable type. The PCT concentrations were compared between the documented bacterial and fungal groups using the Mann–Whitney U-test, and a difference between multiple infection sites calculated by Kruskal–Wallis nonparametric test. The discriminative ability of the PCT concentration for predicting fungal versus bacterial infections was evaluated by the area under the receiver operating characteristic curve (AUROC) and 95% confidence intervals. The discriminatory ability of the CRP concentration, WBC count, and ESR to predict fungal infection were also evaluated by AUROC independently and combined with the PCT concentration. In parallel, the AUROC was calculated after adjusting for covariates (eg, the SLEDAI score and infection site) affecting the PCT concentration. Cut-off values were determined by the Youden index.
Statistical analyses were performed using the IBM SPSS version 24.0 for Windows (IBM Corp., Armonk, NY, USA). Adjusted AUROC was computed using the R package “RISCA” version. All tests were two-sided, and a p-value of <0.05 was considered statistically significant.
Results
During the study period, a total of 123 patients were screened and 69 patients were included in the final analysis (Figure 1); 22 (32%) had a Gram-positive infection, 21 (30%) had a Gram-negative infection, and 26 (38%) had a fungal infection. Twenty-three (88.5%) fungal infection patients were distributed in the respiratory group, 2 (7.7%) in the abdominal/urinary group and 1 (3.8%) in the other group. Nineteen (44.2%) bacterial infection patients were distributed in the respiratory group, 10 (23.3%) in the abdominal/urinary group, 5 (11.6%) in the skin/soft-tissue and 9 (20.9%) in the other group. Table 1 presents the demographic and clinical characteristics. Patients with a fungal infection had a significantly lower SLEDAI score than those with a bacterial infection (7.54 vs 10.48; p = 0.037), but other baseline characteristics did not differ between the fungal and bacterial groups.
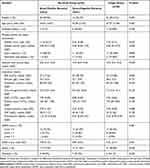 |
Table 1 Baseline Characteristics of Gram-Positive Bacterial, Gram-Negative Bacterial and Fungal Infection Groups |
 |
Figure 1 Study population flow diagram. Abbreviations: SLE, Systemic Lupus Erythematosus; PCT, procalcitonin. |
sTable 1 details the pathogen distributions. Streptococcus spp (n = 11) and Escherichia coli (n = 8) were the most commonly identified bacteria. Of the fungal group, Candida spp (n = 13) was the most common, followed by Aspergillus fumigatus (n = 5), Cryptococcus neoformans (n = 4), Pneumocystis jirovecii (n = 3), and Penicillium marneffei (n = 1).
The PCT concentration in patients with fungal infections (0.22 ng/mL [IQR, 0.09–0.44]) was significantly lower than in patients with Gram-positive (1.43 ng/mL [IQR, 0.14–8.26]; p = 0.049) or Gram-negative (0.57 ng/mL [IQR, 0.20–2.57]; p = 0.033) infections (Figure 2A). The CRP concentration (p = 0.122), WBC count (p = 0.666), and ESR (p = 0.906) did not differ between the bacterial and fungal groups (sFigures 1–3) The PCT concentration significantly differed between different infection sites (p = 0.022) and was the highest in patients with an abdominal or urinary infection, followed by skin and soft tissue and respiratory infections (Figure 2B). In the comparable respiratory infection group, the PCT concentration in patients with fungal and bacterial infection also show difference (P=0.041) (sFigure 4).
The bacterial and fungal infection groups were compared, and the AUROC were plotted based on the derived cut-off values for PCT, CRP, WBC, and ESR (Figure 3). PCT had the highest AUROC, providing a relatively higher diagnostic value than the other three markers (all p-values <0.05). The PCT sensitivity (76.9%) and specificity (58.1%) were the best at a cut-off value of 0.49 ng/mL, and the positive predictive value (PPV) and negative predictive value (NPV) were 52.6% and 80.7%, respectively. After adjusting for the SLEDAI score and infection site, PCT also outperformed the other biomarkers for the discrimination of fungal infections with statistically significant incremental differences (p <0.001) (Table 2).
 |
Table 2 Results of the ROC Analysis for Predicting Fungal Infection |
We also analyzed the AUROC of the markers in varying combinations to increase the diagnostic accuracy. PCT and ESR combined had the highest PPV (70.6%), AUROC (0.688), and the specificity of the combination of PCT and ESR for confirming fungal infections was the highest of all combinations (88.4%) (Table 2); however, the AUROC showed no significant differences, as compared to PCT alone (p = 0.693; Figure 3).
Discussion
This cross-sectional study indicated that the PCT concentration has better diagnostic performance for discriminating fungal from bacterial infections in SLE patients than the CRP concentration, WBC count, and ESR. The results were consistent after accounting for the influence of the SLEDAI score and infection site. The combination of PCT and ESR obtained the highest AUROC and provided an acceptable discrimination performance. However, we do not recommend solely relying on the PCT concentration for diagnostic decisions.
Previous studies have evaluated PCT as an infection biomarker in SLE. One study evaluated patients with SLE and systemic inflammatory response syndrome, demonstrating that the PCT concentration was significantly higher in the infected group than in the non-infected group.15 Further, AlJarhi et al found that PCT levels helped differentiate bacterial infections from disease activity in SLE patients.16 However, these studies excluded fungal infections or were not specifically targeted to fungi. Meanwhile, for SLE patients, fungi are becoming the predominant pathogen and a common reason for hospitalization. Therefore, clarifying the ability of PCT to differentiate fungal and bacterial infections is necessary. To our knowledge, this is the first report aiming to elucidate the role of PCT in SLE patients with serious infections.
In our study, the PCT concentration was significantly lower in the fungal infection group than in the bacterial infection group, in line with the literature regarding critically ill patients.17,18 Although the serum PCT concentration was elevated in fungal and bacterial infections, PCT expression mechanisms differed. Mostly, C-type lectin receptors (CLR) recognize fungi.19 Cytokine secretion (eg, interleukin [IL]-1, IL-10, and IL-6) induced by CLR signaling pathway activation results in a slightly elevated PCT concentration after fungal infection.20 Gram-negative and Gram-positive bacteria are precociously recognized by the innate immune system via toll-like receptor 4 and toll-like receptor 2, leading to higher IL-6 and IL-8 levels and contributing to elevated PCT levels.21 Our results agree with this theory. Thus, we suggest a larger study of the diagnostic value of the PCT concentration for confirmation.
In this study, a PCT concentration of <0.49 ng/mL indicated a fungal infection (with 77% sensitivity, 58% specificity, 53% PPV, and 81% NPV at this cut-off). Our results are partially consistent with those previously reported for diagnosing bacteremia using the PCT concentration (>0.5 ng/mL).7 Furthermore, a systematic review strengthens our findings, suggesting that a PCT concentration of >0.5 ng/mL strongly indicated a bacterial infection in SLE patients.12 However, this review did not compare the PCT concentrations between fungal and bacterial infections because of the high proportion of mixed infections. To avoid this issue, our protocol only included patients with a single-pathogen infection. In contrast, a multicenter retrospective study conducted in an intensive care unit indicated that a PCT threshold of <1.93 ng/mL was optimal for diagnosing candidemia,22 with better sensitivity and specificity than a cut-off of <0.5 ng/mL. This result is not surprising since, in that study, the PCT level in patients with candidemia was higher than in patients in our study with fungal infections (0.73 vs 0.22 ng/mL). That study also had better AUROC curves (AUROC = 0.789) for the PCT assay than in our population. Thus, the discriminative capability differences should be interpreted with caution, considering the different study populations and definitions of fungal infection. By including various fungal species, our results could be more applicable to clinical scenarios.
Early investigators raised the possibility of determining SLE disease activity based on PCT concentrations.14,23 Likewise, we found a significant difference between the groups based on the SLEDAI score. Our study also confirmed that the infection site influences the PCT concentration, in line with the study by Thomas et al.18 Adjusted AUROC compliment unadjusted AUROC by allowing for the control of confounding factors, resulting in a more precise assessment of diagnostic testing. Our findings demonstrated an improvement in the AUROC of PCT (from 0.6740 to 0.7308) after adjusting for the SLEDAI score and infection site, but it was not “excellent” (ie, AUROC >0.8). However, the discriminatory performance of PCT was overall superior to the CRP concentration, WBC count, and ESR. For this reason, we recommend that the PCT concentration be used cautiously as a single biomarker for diagnosing or ruling out fungal infections, and it is no substitute for clinical judgment in cases of severely ill patients or patients with SLE. Combining clinical features and biomarkers will likely add value to the overall diagnostic accuracy.
Candida infections were the most frequent type of fungal infection (50%), followed by Aspergillus and Cryptococcus neoformans. These results differ from previously published findings from China, where Candida was found in only 22.2% of cases (ranking third) and Aspergillus was found in 33.3% (ranking first).24 This may largely be related to the sample size, but the range of variation was small. Our results also deliver useful information about fungus types by assessing the fungal infections in SLE patients.
This study has some limitations. First, it is inherently limited by the retrospective design, and the PCT assay protocol was not strictly unified. Although PCT concentrations are influenced by multiple factors, such as previous antibiotic administration and concurrent macrophage activation syndrome, genuine differences regarding the PCT concentration between fungal and bacterial infections were unlikely to be attenuated through relatively stringent patient selection criteria. Second, we possibly missed some cases since the data was obtained from discharge electronic records, despite our cases being classified based on ICD-10 codes coupled with manual audits. Third, despite knowing which pathogen species affected the PCT concentration, there were too many species to adjust for when calculating the AUROC. Forth, multiple measurement of PCT concentration might contribute to the higher predictive power of fungal infections,25 compared with a single measurement. However, because of the retrospective nature of the data, we have not been able to carry out this analysis. Finally, conclusions drawn from this study can only apply to SLE patients infected with bacteria or fungi alone and cannot be extrapolated to SLE patients with mixed infection.
Conclusion
The PCT concentration is a useful marker for discerning fungal from bacterial infections in patients with SLE; the combination of PCT and ESR yielded the highest AUROC and exhibited an acceptable performance. However, further research is needed to confirm its diagnostic capacity for fungal infections.
Ethics Approval and Consent to Participate
This observational study was conducted according to the terms and regulations of the local institutional review boards (Shanghai Jiao Tong University School of Medicine affiliated Renji Hospital). According to Chinese law, informed consent was not required since this study did not modify the physician’s treatment decisions. The study conformed to the provisions of the Declaration of Helsinki (as revised in 2013).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Li PH, Lau CS. Lupus in the far east: a modern epidemic. Int J Rheum Dis. 2017;20(5):523–525. doi:10.1111/1756-185X.13115
2. Fors Nieves CE, Izmirly PM. Mortality in systemic lupus erythematosus: an updated review. Curr Rheumatol Rep. 2016;18(4):21. doi:10.1007/s11926-016-0571-2
3. Adwan MH, Qasem U, Mustafa KN. In-hospital mortality in patients with systemic lupus erythematosus: a study from Jordan 2002–2017. Rheumatol Int. 2020;40(5):711–717. doi:10.1007/s00296-020-04538-z
4. Moghaddam B, Marozoff S, Li L, et al. All-cause and cause-specific mortality in systemic lupus erythematosus: a Population-based Study. Rheumatology. 2021. doi:10.1093/rheumatology/keab362
5. Garris C, Jhingran P, Bass D, et al. Healthcare utilization and cost of systemic lupus erythematosus in a US managed care health plan. J Med Econ. 2013;16(5):667–677. doi:10.3111/13696998.2013.778270
6. Nagai Y, Yokogawa N, Shimada K, et al. Characteristics and risk factors of an emergency department visit in patients with systemic lupus erythematosus. Rheumatol Int. 2019;39(9):1567–1573. doi:10.1007/s00296-019-04377-7
7. Hoeboer SH, van der Geest PJ, Nieboer D, et al. The diagnostic accuracy of procalcitonin for bacteraemia: a systematic review and meta-analysis. Clin Microbiol Infect. 2015;21(5):474–481. doi:10.1016/j.cmi.2014.12.026
8. Tektonidou MG, Ward MM. Validation of new biomarkers in systemic autoimmune diseases. Nat Rev Rheumatol. 2011;7(12):708–717. doi:10.1038/nrrheum.2011.157
9. Brodska H, Malickova K, Adamkova V, et al. Significantly higher procalcitonin levels could differentiate Gram-negative sepsis from Gram-positive and fungal sepsis. Clin Exp Med. 2013;13(3):165–170. doi:10.1007/s10238-012-0191-8
10. Shin KC, Lee YJ, Kang SW, et al. Serum procalcitonin measurement for detection of intercurrent infection in febrile patients with SLE. Ann Rheum Dis. 2001;60(10):988–989. doi:10.1136/ard.60.10.988
11. Chen D, Xie J, Chen H, et al. Infection in southern Chinese patients with systemic lupus erythematosus: spectrum, drug resistance, outcomes, and risk factors. J Rheumatol. 2016;43(9):1650–1656. doi:10.3899/jrheum.151523
12. Serio I, Arnaud L, Mathian A, et al. Can procalcitonin be used to distinguish between disease flare and infection in patients with systemic lupus erythematosus: a systematic literature review. Clin Rheumatol. 2014;33(9):1209–1215. doi:10.1007/s10067-014-2738-4
13. Lanoix JP, Bourgeois AM, Schmidt J, et al. Serum procalcitonin does not differentiate between infection and disease flare in patients with systemic lupus erythematosus. Lupus. 2011;20(2):125–130. doi:10.1177/0961203310378862
14. Wang J, Niu R, Jiang L, et al. The diagnostic values of C-reactive protein and procalcitonin in identifying systemic lupus erythematosus infection and disease activity. Medicine. 2019;98(33):e16798. doi:10.1097/MD.0000000000016798
15. Echeverri A, Naranjo-Escobar J, Posso-Osorio I, et al. Neutrophil CD64 expression, procalcitonin and presepsin are useful to differentiate infections from flares in SLE patients with SIRS. Lupus. 2018;27(7):1130–1139. doi:10.1177/0961203318763740
16. AlJarhi UM, Sadek KM, Darwish EM, et al. Evaluation of serum presepsin, procalcitonin, copeptin, and high-sensitivity C-reactive protein for differentiating bacterial infection from disease activity in Egyptian patients with systemic lupus erythematosus. Clin Rheumatol. 2021;40(5):1861–1869. doi:10.1007/s10067-020-05471-z
17. Leli C, Ferranti M, Moretti A, et al. Procalcitonin levels in gram-positive, gram-negative, and fungal bloodstream infections. Dis Markers. 2015;2015:701480. doi:10.1155/2015/701480
18. Thomas-Ruddel DO, Poidinger B, Kott M, et al. Influence of pathogen and focus of infection on procalcitonin values in sepsis patients with bacteremia or candidemia. Crit Care. 2018;22(1):128. doi:10.1186/s13054-018-2050-9
19. Plato A, Hardison SE, Brown GD. Pattern recognition receptors in antifungal immunity. Semin Immunopathol. 2015;37(2):97–106. doi:10.1007/s00281-014-0462-4
20. Akin H, Akalin H, Budak F, et al. Alterations of serum cytokine levels and their relation with inflammatory markers in candidemia. Med Mycol. 2015;53(3):258–268. doi:10.1093/mmy/myu084
21. Li S, Rong H, Guo Q, et al. Serum procalcitonin levels distinguish Gram-negative bacterial sepsis from Gram-positive bacterial and fungal sepsis. J Res Med Sci. 2016;21(1):39. doi:10.4103/1735-1995.183996
22. Giacobbe DR, Mikulska M, Tumbarello M, et al. Combined use of serum (1,3)-beta-D-glucan and procalcitonin for the early differential diagnosis between candidaemia and bacteraemia in intensive care units. Crit Care. 2017;21(1):176. doi:10.1186/s13054-017-1763-5
23. Yu J, Xu B, Huang Y, et al. Serum procalcitonin and C-reactive protein for differentiating bacterial infection from disease activity in patients with systemic lupus erythematosus. Mod Rheumatol. 2014;24(3):457–463. doi:10.3109/14397595.2013.844391
24. Fan YC, Li WG, Zheng MH, et al. Invasive fungal infection in patients with systemic lupus erythematosus: experience from a single institute of Northern China. Gene. 2012;506(1):184–187. doi:10.1016/j.gene.2012.06.059
25. Charles PE, Castro C, Ruiz-Santana S, et al. Serum procalcitonin levels in critically ill patients colonized with Candida spp: new clues for the early recognition of invasive candidiasis? Intensive Care Med. 2009;35(12):2146–2150. doi:10.1007/s00134-009-1623-0
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


