Back to Journals » Cancer Management and Research » Volume 12
Performance of a Nomogram Based on the Integration of Inflammation Markers with Tumor Staging in Prognosis Prediction of Stage III Colorectal Cancer
Authors Wang L, Xiao J, Li MZ, Teng WH, Jia J, Lin L, Liu S, Ye X, Zang WD, Chen Y
Received 28 May 2020
Accepted for publication 24 July 2020
Published 7 August 2020 Volume 2020:12 Pages 7077—7085
DOI https://doi.org/10.2147/CMAR.S263577
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Lin Wang,1,* Jun Xiao,2,* Min-Zhe Li,3 Wen-Hao Teng,2 Jing Jia,1 Lu Lin,1 Sheng Liu,2 Xing-ming Ye,1 Wei-Dong Zang,2 Ying Chen1
1Central Laboratory, Fujian Cancer Hospital & Fujian Medical University Cancer Hospital, Fuzhou 350000, People’s Republic of China; 2Department of Gastrointestinal Surgery, Fujian Cancer Hospital & Fujian Medical University Cancer Hospital, Fuzhou 350000, People’s Republic of China; 3General Surgery Department, Beijing Chao-Yang Hospital, Capital Medical University, Beijing 100000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wei-Dong Zang
Department of Gastrointestinal Surgery, Fujian Cancer Hospital & Fujian Medical University Cancer Hospital, Fuzhou 350000, People’s Republic of China
Email [email protected]
Ying Chen
Central Laboratory, Fujian Cancer Hospital & Fujian Medical University Cancer Hospital, Fuzhou 350000, People’s Republic of China
Email [email protected]
Introduction: The aim of the present study was to evaluate a nomogram model for predicting the 5-year overall survival (OS) in lymph node-metastatic colorectal cancer (CRC) patients by combining inflammation markers with some traditional prognostic factors.
Methods: A total of 399 patients with stage III (pTXN1-3M0) CRC operated from January 2007 to December 2012 were enrolled in this retrospective study. All patients underwent D2 lymphadenectomy in the hospital. A prognostic nomogram based on the integration of traditional prognostic factors and NLR (neutrophil-to-lymphocyte ratio) and PLR (platelet-to-lymphocyte ratio) was established and compared with the nomogram based on the traditional prognostic factors alone. ROC curves were further applied to verify the predictive accuracy of the established model.
Results: Both NLR (P=0.00) and PLR (P=0.01) predicted the 5-year OS. In multivariate analysis, age, T3 category, T4 category, N2 category, N3 category, Pgp (P-glycoprotein), NLR and PLR are proven to be independent (all P≤ 0.05). The established nomogram showed better predictive power than that of traditional profile (c-index: 0.66 versus 0.63) in both training and validation cohorts. External assessment by ROC curve analysis demonstrated that the established model had a good prediction accuracy of 5-year OS in stage III CRC patients, with area under curve values of 0.657 and 0.629 in training and validating sets, respectively.
Conclusion: A nomogram based on the integration of traditional prognostic factors and inflammatory markers (NLR and PLR) could provide more precise long-term prognosis information for lymph node-metastatic CRC patients than the model based on traditional profile alone. This model might be useful for clinical application in personalized evaluation.
Keywords: nomogram, TNM staging system, lymph node metastasis, colorectal cancer, prognosis
Introduction
Colorectal cancer (CRC) is the second most frequently diagnosed cancer in females and the third in males, accounting for approximately 1 in 10 cancer cases and cancer-related deaths.1 For non-metastatic CRC, D2 radical resection and regional lymphadenectomy are currently the main curative therapeutic approach. Clinically, conventional TNM staging is the key determining factor for the prognostic outcome of CRC patients. However, the TNM classification system only evaluates the clinicopathologic features of cancer patients, and the limitation of TNM-based prognosis role for CRC has been reported,2 particular for patients with positive lymph node metastasis (stage III).3 To date, several studies have shown that a combination of tumor biomarkers or treatment parameters with TNM staging significantly enhanced the predictive power of clinical outcome in CRC patients.3–5
Previous studies have reported that cancer progression and tumorigenesis are closely related to inflammation and are reflected by the host inflammation response.6 This response could be represented by changes in white blood cell count and acute-phase proteins. Recently, the pretreatment levels of platelet-to-lymphocyte ratio (PLR) and the neutrophil-to-lymphocyte ratio (NLR), known inflammation markers, have been demonstrated as potential prognostic predictors in CRC.7–9 Together these results suggested that systemic inflammation can be an additional factor that affects the prognosis of cancer patients. However, whether the combination of these inflammation markers with conventional TNM staging could enhance the predictive power for long-term survival in CRC patients remains unclear.
Nomograms are widely used to estimate prognosis in oncology and can estimate an individual numerical probability of a clinical event by incorporating various prognostic determinants. Recent studies have reported a rapid development of nomograms as prognostic tools in various types of malignancy.10−16 This study was designed to evaluate the prognostic value of the combination of traditional prognostic factors with inflammation markers NLR and PLR in stage III CRC patients using the nomogram tool.
Methods
Patients
This retrospective study included 399 consecutive CRC cases who underwent D2 lymphadenectomy at the Department of Gastrointestinal Surgery, Fujian Cancer Hospital & Fujian Medical University Cancer Hospital from January 2007 to December 2012. All CRC patients were diagnosed as pTXN1-3M0 based on the American Joint Committee on Cancer (AJCC) 8th edition of the classification of TNM. Patients were excluded if they had received any cancer-specific pretreatment or concurrent hematologic, autoimmune, or infectious diseases. The flow chart for the study design is shown in Figure 1.
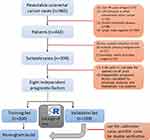 |
Figure 1 A flow chart for the study design. |
Dataset
The Ethics Committee of Fujian Medical University Cancer Hospital approved the study and waived the need for patient’s informed consent, as the present study only retrospectively analyzed the existing data and do not include any personal confidential data or involve any commercial interests. All procedures were performed in accordance with the Declaration of Helsinki. The retrieved information from patient record includes demographic characteristics, clinicopathological features and circulating blood counts at the first assessment before operation. Based on the node grouping of the 8th classification of TNM, the number of metastatic lymph nodes was also counted. The NLR and PLR ratios were calculated by dividing the total number of neutrophils or platelet counts by the absolute number of lymphocyte counts. Follow-up information was gathered from the records of the hospital. The duration was calculated from the date of the surgery to the last date of follow-up (1st January 2019).
Statistical Analysis
SPSS version 19.0 (IBM Corp, Armonk, NY, USA) was used to perform the statistical analyses. The distributions of baseline characteristics were compared using either unpaired t test or ANOVA test. The cut-off values of NLR and PLR were determined and analyzed using X-tile program in terms of survival.
The Kaplan-Meier approach was used to generate the 5-year overall survival (OS) curves and the Log rank test was used to compare the difference of variables. Covariates that showed significance (P<0.05) in univariate analysis were entered into the Cox regression model for multivariate analyses. P<0.05 was considered statistically significant.
Based on the influence weight of each factor, nomogram model was developed by R 4.0. Novel prognostic nomograms for OS and AJCC 8th staging system were established. Concordance index (C-index), the receiver operating characteristic (ROC) curve was further used to evaluate predictive performance.17 The values of P<0.05 were considered statistically significant.
Results
Patients’ Information
A total of 399 patients categorized as pTXN1-3M0 CRC who had undergone D2 radical resection qualified for the study. The patient group included 235 male and 164 female patients, with an age ranging from 20 to 87 years old (Table 1). Among the 399 patients, the average follow-up time was 87.6 months, ranging from 63 to 114 months. The 5-year OS rate was 64.02%.
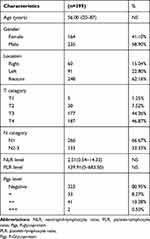 |
Table 1 Demographic Data |
Identification of Optimal Cut-Off Values for NLR and PLR
According to the X-tile plots, the optimal cut-off point for NLR was 3.8 (Figure 2A and B) and PLR was 115.5 (Figure 2D and E). As shown in Figure 2C, the 5-year OS rate of CRC patients in the NLR-low group (less than 3.8) was 66.66% compared with 44.26% in the NLR-high group (more than 3.8). In the PLR-low group (less than 115.5), the 5-year OS rate was 72.48%, while the OS rate was 58.91% in the PLR-high group (more than 115.5) (Figure 2F).
Identification of Prognostic Factors
To evaluate the association of the individual clinicopathological parameters and pretreatment levels of NLR and PLR with prognosis, univariate analyses were performed. Age, T category, N category, NLR and PLR level, Pgp (P-glycoprotein) and tumor location were significantly associated with 5-year OS, while gender showed no association (all P value <0.05, Table 2).
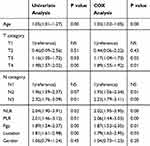 |
Table 2 Univariate and Cox Multivariate Analyses of Prognostic Factors for Overall Survival |
The seven covariates that showed significance were entered into the multivariate Cox analysis. Age, T category, N category, NLR and PLR level, Pgp and tumor location were independent prognostic factors (all P value <0.05, Table 2).
Nomogram Established for Predicting Prognosis of CRC Patients
To evaluate the predictive power of the established nomogram for prognosis, approximately half of the patients (200, 50.12%) were assigned to a training set, while the other 199 cases were assigned to the validation set. Both subgroups had comparable patient demographics and clinicopathologic characteristics (Table 3).
 |
Table 3 Demographics and Clinicopathologic Characteristics of Stage III CRC Patients in the Training Set and Validation Set |
The new nomogram model was created and compared: one was a combination of inflammation markers (NLR and PLR level) and the traditional prognostic factors, including TNM stage system (T category and N category), patient basic information (age, and tumor location) and Pgp status, and the other was only based on the traditional prognostic factors.
The new nomogram was initially established in the training set by bootstrap validation, resulting in an unadjusted C-index of 0.63 (assessment by the traditional prognostic factors) and a bootstrap-adjusted C-index of 0.66 (assessment by the new nomogram model). In the validating set, the C-index was 0.66, which is kept in line with the training set. As shown in Figure 3, nomograms enabled the prediction of a 5-year probability of death for a patient on the lowest scale by summing up the total points assigned to each variable that was indicated at the top of scale.
Calibration curves for three models revealed the calibration plot for the probability of 1, 3 and 5-year OS had a great agreement with the actual observed outcomes (Figure 4A–C).
Internal Validation of Predictive Models by ROC Curve
The predictive accuracy of the established models for 5-year OS was further assessed by ROC curves. The values of area under curve (AUC) were 0.657, and 0.629 in the training set (Figure 5A), the validation set (Figure 5B), respectively.
Discussion
Previous studies have suggested the advantages of the nomogram model in cancer prognosis prediction, particularly when combined with other disease characteristics rather than TNM staging alone.18 A previous report on CRC indicated that the prognosis assessment of node-positive CRC patients by TNM classification is suboptimal.5 Both NLR and PLR inflammation markers have demonstrated prognostic values in previous studies.19,20 In the present study, we evaluated the new nomogram model for prognosis prediction in stage III CRC patients. We found that both PLR and NLR were independent prognostic factors affecting lymph node metastasis in CRC. We also showed that the established the new nomogram model had higher predictive power than traditional TNM staging for 5-year survival (C-index: 0.62 for the traditional prognostic factors and 0.66 for the new nomogram model).
The correlation between NLR and prognosis in patients with CRC has been demonstrated in several studies,11–13 although cut-off values varied. In contrast, the role of PLR remains controversial.21 This difference may be ascribed to the choice of the PLR cut-off point and the heterogeneous nature of the study population. In the present study, we identified optimal cut-off values of inflammatory biomarkers using a robust graphical tool of X-tile program. Moreover, the study population was limited to stage III CRC patients, which was more homogeneous than those of previous studies. Thus, we believe that our results are reliable. The worse prognosis associated with higher PLR might also reflect an important role of platelet dominant inflammation response in the process of lymph node metastasis in CRC, except for the neutrophil dominant inflammation effects that were confirmed by NLR in a previous study.22 Moreover, we also found that T4 stage and N stages were independent prognosis factors in stage III CRC patients, which is consistent with the latest NCCN guideline recommendations23 and previous reports.24,25
P-glycoprotein (P-GP), a member of the ATP binding box (ABC) superfamily, extrudes chemotherapeutic drugs out of cells. In the present study, Pgp was also an independent prognostic variable for survival. This observation was not consistent with the findings reported by Tokunaga et al.26 This discrepancy might result from differences in the patient characteristics or treatment regimen.
Based on the above findings, a nomogram was constructed based on the integration of NLR and PLR with tumor staging features. Using training and validating sets, we found a great agreement between the predictive probability for the 5-year OS with the actual observed outcomes, indicating the good reliability of our established model. The value of adjusted C-index for this model is comparable that in a similar recent report27 and was significantly higher than that the C-index based on TNM staging features (0.58). To further confirm the performance of the new nomogram model, an additional statistical tool of ROC curve was applied. The result also showed that the AUC values were 0.657 and 0.629 for the training set and the validation set, respectively. The data show the new model has good and stabled accuracy as an evaluation system.
This study had several limitations. First, the sample size is small, which may limit the statistical power of this study. Second, this model only included TNM staging, inflammation markers (NLR and PLR level), inflammation markers (NLR and PLR level), patient basic information (age, and tumor location) and immunohistochemical index (Pgp status), but ignored other potential factors such as differentiation grade of tumor and other factors. Thus, this nomogram is worth for further improvement by including other parameters.
Conclusion
We construct a new prognostic evaluation model based on TNM staging, pretreatment inflammation markers (NLR and PLR level), patient basic information (age, and tumor location) and Pgp status for stage III CRC patients. The nomogram integrated with both markers into some traditional prognostic factors could enhance the prediction accuracy of 5-year outcome in these patients. Since NLR and PLR are easily available parameters that could be obtained from routine blood counts, the model might have clinical value for prognosis purpose in CRC patients.
Acknowledgments
We thank Edanz Group for editing a draft of this manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Hari DM, Leung AM, Lee JH, et al. AJCC Cancer Staging Manual 7th edition criteria for colon cancer: do the complex modifications improve prognostic assessment? J Am Coll Surg. 2013;217(2):181–190. doi:10.1016/j.jamcollsurg.2013.04.018
3. Kawada H, Kurita N, Nakamura F, et al. Incorporation of apical lymph node status into the seventh edition of the TNM classification improves prediction of prognosis in stage III colonic cancer. Br J Surg. 2014;101(9):1143–1152. doi:10.1002/bjs.9548
4. Nagtegaal ID, Gosens MJ, Marijnen CA, et al. Combinations of tumor and treatment parameters are more discriminative for prognosis than the present TNM system in rectal cancer. J clin oncol. 2007;25(13):1647–1650. doi:10.1200/jco.2005.05.4825
5. Costi R, Beggi F, Reggiani V, et al. Lymph node ratio improves TNM and Astler-Coller’s assessment of colorectal cancer prognosis: an analysis of 761 node positive cases. J Gastrointestinal Surg. 2014;18(10):1824–1836. doi:10.1007/s11605−014−2591−4
6. Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi:10.1038/nature07205
7. Mallappa S, Sinha A, Gupta S, et al. Preoperative neutrophil to lymphocyte ratio >5 is a prognostic factor for recurrent colorectal cancer. Colorectal Dis. 2013;15(3):323–328. doi:10.1111/codi.12008
8. Li MX, Liu XM, Zhang XF, et al. Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review and meta-analysis. Int J Cancer. 2014;134(10):2403–2413. doi:10.1002/ijc.28536
9. Galizia G, Lieto E, Zamboli A, et al. Neutrophil to lymphocyte ratio is a strong predictor of tumor recurrence in early colon cancers: a propensity score-matched analysis. Surgery. 2015;158(1):112–120. doi:10.1016/j.surg.2015.02.006
10. Yoon WS, Yang DS, Lee JA, et al. Validation of nomograms for survival and metastases after hysterectomy and adjuvant therapy in uterine cervical cancer with risk factors. Biomed Res Int. 2017;2017:2917925. doi:10.1155/2017/2917925
11. Azumi M, Suda T, Terai S, et al. Prognostic impact of indocyanine green plasma disappearance rate in hepatocellular carcinoma patients after radiofrequency ablation: a prognostic nomogram study. Internal Med. 2017;56(9):1001–1007. doi:10.2169/internalmedicine.56.7278
12. Chen H, Sui X, Yang F, et al. Nomograms for predicting recurrence and survival of invasive pathological stage IA non-small cell lung cancer treated by video assisted thoracoscopic surgery lobectomy. J Thorac Dis. 2017;9(4):1046–1053. doi:10.21037/jtd.2017.03.130
13. Di Filippo F, Di Filippo S, Ferrari AM, et al. Erratum to: elaboration of a nomogram to predict nonsentinel node status in breast cancer patients with positive sentinel node, intraoperatively assessed with one step nucleic amplification: retrospective and validation phase. J Exp Clin Cancer Res. 2017;36(1):69. doi:10.1186/s13046−017−0540−2
14. Jing C-Y, Fu Y-P, Zheng S-S, et al. Prognostic nomogram for patients with hepatocellular carcinoma underwent adjuvant transarterial chemoembolization following curative resection. Medicine. 2017;96(11):e6140. doi:10.1097/md.0000000000006140
15. Kim CH, Lee SY, Kim HR, et al. Nomogram prediction of anastomotic leakage and determination of an effective surgical strategy for reducing anastomotic leakage after laparoscopic rectal cancer surgery. Gastroenterol Res Pract. 2017;2017:4510561. doi:10.1155/2017/4510561
16. Li Q, Dai W, Li Y, et al. Nomograms for predicting the prognostic value of serological tumor biomarkers in colorectal cancer patients after radical resection. Sci Rep. 2017;7:46345. doi:10.1038/srep46345
17. Altman DG, Vergouwe Y, Royston P, et al. Prognosis and prognostic research: validating a prognostic model. BMJ (Clinical Research Ed). 2009;338:b605. doi:10.1136/bmj.b605
18. Sternberg CN. Are nomograms better than currently available stage groupings for bladder cancer? J clin oncol. 2006;24(24):3819–3820. doi:10.1200/jco.2006.07.1290
19. Lu J, Dai Y, Xie JW, et al. Combination of lymphovascular invasion and the AJCC TNM staging system improves prediction of prognosis in N0 stage gastric cancer: results from a high-volume institution. BMC Cancer. 2019;19(1):216. doi:10.1186/s12885−019−5416−8
20. Lin JX, Wang W, Lin JP, et al. Preoperative tumor markers independently predict survival in stage III gastric cancer patients: should we include tumor markers in AJCC Staging? Ann Surg Oncol. 2018;25(9):2703–2712. doi:10.1245/s10434−018−6634-z
21. Pedrazzani C, Mantovani G, Fernandes E, et al. Assessment of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and platelet count as predictors of long-term outcome after R0 resection for colorectal cancer. Sci Rep. 2017;7(1):1494. doi:10.1038/s41598−017−01652−0
22. Aoyama T, Takano M, Miyamoto M, et al. Pretreatment neutrophil-to-lymphocyte ratio was a predictor of lymph node metastasis in endometrial cancer patients. Oncology. 2019;96(5):259–267. doi:10.1159/000497184
23. Benson AB, Venook AP, Al-Hawary MM, et al. NCCN guidelines insights: colon cancer, Version 2.2018. J Natl Compreh Cancer Net. 2018;16(4):359–369. doi:10.6004/jnccn.2018.0021
24. Tsuchida K, Saeki H, Takahashi S, et al. [Appropriate range of lymph node dissection in elderly patients with colorectal cancer]. Gan to Kagaku Ryoho. 2017;44(12):1211–1213.
25. Ikuta D, Miyake T, Shimizu T, et al. Fibrosis in metastatic lymph nodes is clinically correlated to poor prognosis in colorectal cancer. Oncotarget. 2018;9(51):29574–29586. doi:10.18632/oncotarget.25636
26. Tokunaga Y, Hosogi H, Hoppou T, et al. Effects of MDR1/P-glycoprotein expression on prognosis in advanced colorectal cancer after surgery. Oncol Rep. 2001;8(4):815–819. doi:10.3892/or.8.4.815
27. Li C, Pei Q, Zhu H, et al. Survival nomograms for stage III colorectal cancer. Medicine. 2018;97(49):e13239. doi:10.1097/md.0000000000013239
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




