Back to Journals » Clinical Ophthalmology » Volume 14
Performance and Safety of the Extended Depth of Focus Implantable Collamer® Lens (EDOF ICL) in Phakic Subjects with Presbyopia
Authors Packer M , Alfonso JF , Aramberri J, Elies D, Fernandez J, Mertens E
Received 14 July 2020
Accepted for publication 20 August 2020
Published 18 September 2020 Volume 2020:14 Pages 2717—2730
DOI https://doi.org/10.2147/OPTH.S271858
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Mark Packer, 1 Jose F Alfonso, 2 Jaime Aramberri, 3 Daniel Elies, 4 Joaquin Fernandez, 5 Erik Mertens 6
1Packer Research Associates, Boulder, CO, USA; 2Ophthalmology Institute Fernández Vega, Oviedo, Asturias, Spain; 3Opthalmology Clinic Miranza Begitek, San Sebastian, Spain; 4Institute of Ocular Microsurgery, Barcelona, Spain; 5Qvision-Hospital Vithas Virgen del Mar, Almeria, Spain; 6Medipolis, Antwerp, Belgium
Correspondence: Mark Packer
Packer Research Associates, Boulder, CO, USA
Tel +1 541 915 0291
Email [email protected]
Purpose: To evaluate the performance and safety of the Extended Depth of Focus Implantable Collamer® Lens (EDOF ICL) for improvement of uncorrected near, intermediate and distance visual acuity in phakic subjects with myopia and presbyopia.
Design: Prospective multicenter study.
Methods: Presbyopic subjects who required an EDOF ICL in the range of − 0.50 D to − 18.00 D, exhibited ≤ 0.75 D refractive astigmatism and required from +1.00 to +2.50 D reading add were implanted bilaterally. Assessments at 6 months included uncorrected near, intermediate and distance visual acuities, defocus curves, contrast sensitivity, responses to the National Eye Institute Refractive Error Quality of Life Questionnaire and a Task Assessment Questionnaire.
Results: A total of 34 subjects completed the study. Investigators targeted emmetropia in all eyes. Mean binocular uncorrected near, intermediate and distance visual acuities measured logMAR − 0.01 ± 0.05 (20/20), − 0.02 ± 0.08 (20/19) and 0.07 ± 0.10 (20/23), respectively. Mean monocular uncorrected near, intermediate and distance visual acuities measured logMAR 0.068 ± 0.09 (20/23), 0.062 ± 0.10 (20/23) and 0.16 ± 0.12 (20/29). There were no clinically or statistically significant differences in contrast sensitivity between baseline and 6 months under any testing conditions. Subjects reported significant improvements in measures of vision-related quality of life and ability to perform tasks at all distances without glasses or contact lenses. Overall, satisfaction with the EDOF ICL was high: postoperatively, 91.2% of subjects were satisfied with their vision.
Conclusion: This multicenter, prospective clinical investigation demonstrated the ability of the EDOF ICL to correct myopia and presbyopia, resulting in improvement of uncorrected near, intermediate and distance visual acuity without compromising the quality of vision. The EDOF ICL allowed subjects to perform tasks of daily living without glasses or contact lenses. Subjects reported significant improvements in quality of life with high levels of spectacle independence and satisfaction.
Keywords: presbyopia, myopia, phakic refractive lens, implantable Collamer lens
Corrigendum for this paper has been published
Introduction
Presbyopia, the age-related decline in focusing ability, reduces the quality of life.1 Presbyopia corrected with glasses is also associated with decreased quality of life, “similar to that of treated hypertension, for the average person with the condition.”2 The quality of life for patients with monovision is still worse than the quality of life for younger emmetropic subjects.1 Multifocal and extended depth of focus pseudophakic intraocular lenses may increase spectacle independence; however, refractive lens exchange increases the risk of retinal detachment.3
The STAAR EVO+ Visian™ Implantable Collamer® Lens with Aspheric Optic, referred to as the Extended Depth of Focus Implantable Collamer Lens or EDOF ICL (STAAR Surgical, Monrovia, California), represents a novel approach to the surgical correction of refractive error and presbyopia in phakic patients, and includes a combination of the most advanced elements of the ICL platform, including an increased optic diameter, the central port (KS-AquaPORT®) and an aspheric design which is theoretically intended to provide up to about 2.0 D extended depth of focus.4 Published studies have demonstrated the safety and effectiveness of the EVO and EVO+ ICLs for the correction of myopia and astigmatism.5 The objective of this prospective multicenter study was to evaluate the performance and safety of the EDOF ICL for the improvement of uncorrected near, intermediate and distance vision in phakic subjects with presbyopia.
Materials and Methods
Subjects at 5 clinical sites in Spain and 1 site in Belgium were enrolled if they were from 40 to 60 years of age, required an ICL power of −0.50 D to −18.00 D, needed from +1.00 to +2.50 D reading add and exhibited ≤ 0.75 D refractive astigmatism in both eyes. Subjects were excluded if they had anterior chamber depth < 2.8 mm measured from the corneal endothelium to the anterior lens capsule, anterior chamber angle < Shaffer Grade III, low or abnormal endothelial cell density, previous ocular surgery or irregular astigmatism.
A complete ophthalmic examination was performed at baseline and subjects returned for 5 postoperative visits at 1 day, 1 week, 1 month, 3 months and 6 months. All visual acuities were recorded using the M&S Clinical Trial Suite (M&S Technologies, Inc., Niles, IL). This system comprises automated digital visual acuity and contrast sensitivity testing algorithms which can be customized specifically to clinical protocol requirements, including defocus testing. Near visual acuity was measured at 40 cm and intermediate visual acuity was measured at 80 cm. Corneal white to white distance and anterior chamber depth were measured according to each investigator’s preferred methodology, eg, Orbscan II (Bausch & Lomb, Rochester, New York), IOL Master 700 (Carl Zeiss Meditec, Jena, Germany) or Lenstar 900 (Haag Streit, Koniz, Switzerland). The size and optical power of the EDOF ICL were determined according to the manufacturer’s specifications based on the preoperative manifest refraction, white to white distance and anterior chamber depth. Investigators targeted postoperative emmetropia with an acceptable variation of ± 0.50 D manifest refraction spherical equivalent in all eyes. The same aspheric design was implanted in all subjects irrespective of their preoperative add requirements.
Endothelial cell density was measured with the Konan Cell-Check Specular Microscope (Konan Medical, Irvine, CA). Postoperatively, ICL vault was measured with the Visante AS-OCT (Carl Zeiss Meditec, Dublin, CA). Patient-reported outcomes were assessed with the National Eye Institute Refractive Error Quality of Life Questionnaire (NEI-RQL 42).6 Scores for subscales were calculated according to the description provided by Hays and Spritzer.7 In addition, a Subjective Task Assessment Questionnaire evaluated spectacle independence and patient satisfaction.8
The surgical procedure was the same as that for the EVO+ ICL with the central port design.9 Briefly, after two angled paracentesis incisions were constructed at 6 and 12 o’clock, 2% hydroxypropylmethylcellulose ophthalmic viscosurgical device (OVD) was instilled in the anterior chamber and a 3.2 mm temporal clear corneal incision was constructed. The EDOF ICL was injected into the anterior chamber and the footplates were positioned posterior to the iris. Following complete removal of the OVD by irrigation the wound self-sealed. Subjects were prescribed a standard regimen of topical anti-inflammatory and antibiotic agents.
The primary performance endpoint was achievement of monocular uncorrected near visual acuity of Snellen equivalent 20/40 or better at 40 cm 6 months after implantation in equal to or greater than 75% of implanted eyes. Fleming’s two-stage design was used to optimize the sample size while allowing for early termination of the trial for futility or performance. It was expected that 90% of eyes would achieve the performance endpoint and the sample size was therefore conservatively determined based on an 87.5% responder rate. Based on Fleming’s two-stage design, 27 subjects (54 eyes) were enrolled prior to the interim analysis. In case the study was not terminated due to futility or reaching the performance endpoint, an additional 16 subjects (32 eyes) would have been enrolled for a total of 43 patients (86 eyes). To allow for 10% attrition over 6 months, a total of 48 subjects (96 eyes) would have been enrolled for assessment of the primary and secondary performance endpoints and the primary safety endpoint in case the study was not terminated after the first stage. This design yielded a one-sided type I error probability of 2.5% and a power of 80% if the true response rate was 87.5%.
Results
A total of 35 subjects, 11 males and 24 females, enrolled in the study and were implanted bilaterally with the EDOF ICL. In one 44-year-old female subject with preoperative manifest refraction spherical equivalent −0.63 D OD and −0.50 D OS, and preoperative uncorrected distance visual acuity 20/25 OU, the study lenses were explanted due to dissatisfaction with distance vision. There were no complaints of dysphotopsia. Following uncomplicated bilateral explantation prior to the 6-month visit, this subject experienced complete resolution. The remaining 34 subjects completed the 6-month follow up. These subjects had a mean age of 48.7 ± 3.9 (41–59) years. Additional preoperative characteristics are provided in Table 1.
 |
Table 1 Preoperative Characteristics |
Performance
The primary objective of the study was the achievement of uncorrected near visual acuity of Snellen equivalent 20/40 or better at 40 cm at 6 months after implantation. A total of 66 (97.1%) eyes achieved uncorrected near visual acuity of 20/40 or better at the 6-month follow-up visit. The lower 95% exact confidence limit of the responder rate was 89.8%, exceeding the threshold responder rate of 75% that was set to determine the trial success.
Mean monocular uncorrected distance visual acuity (n = 68) at 6 months postoperative measured logMAR 0.16 ± 0.12 (0.00–0.40), a mean improvement of 10.4 ± 3.0 lines from preoperative; mean binocular uncorrected distance visual acuity measured logMAR 0.07 ± 0.10 (−0.06–0.30), a mean improvement of 10.0 ± 2.8 lines from preoperative. The distributions of preoperative and postoperative monocular and binocular uncorrected distance visual acuity are provided in Figures 1 and 2. Mean monocular uncorrected near visual acuity at 6 months measured logMAR 0.068 ± 0.09 (−0.06–0.36), a mean improvement of 6.2 ± 4.0 lines from preoperative; mean binocular uncorrected near visual acuity measured −0.01 ± 0.05 (−0.10–0.10), a mean improvement of 6.1 ± 4.0 lines from preoperative. Mean monocular uncorrected intermediate visual acuity at 6 months measured logMAR 0.062 ± 0.10 (−0.10–0.38), a mean improvement of 7.8 ± 3.5 lines from preoperative; mean binocular uncorrected intermediate visual acuity measured logMAR −0.02 ± 0.08 (−0.20–0.14), a mean improvement of 7.8 ± 3.6 lines from preoperative. Distributions of preoperative and postoperative monocular and binocular uncorrected near and intermediate visual acuities are provided in Figures 3–6. Binocular uncorrected visual acuity of 20/32 or better at all 3 testing distances was achieved by 91.2% of subjects.
 |
Figure 1 Preoperative and postoperative monocular uncorrected distance visual acuity. |
 |
Figure 2 Preoperative and postoperative binocular uncorrected distance visual acuity. |
 |
Figure 3 Preoperative and postoperative monocular uncorrected near visual acuity. |
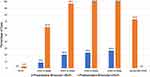 |
Figure 4 Preoperative and postoperative binocular uncorrected near visual acuity. |
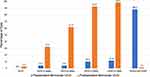 |
Figure 5 Preoperative and postoperative monocular uncorrected intermediate visual acuity. |
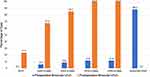 |
Figure 6 Preoperative and postoperative binocular uncorrected intermediate visual acuity. |
The mean postoperative add power for best near visual acuity was +0.49 ± 0.51 (0.00–1.50) D, representing a mean decrease of 1.19 ± 0.48 D from the preoperative mean add power of 1.66 ± 0.44 D. The mean postoperative manifest refraction spherical equivalent measured −0.67 ± 0.55 (−2.25–0.75) D. A scatterplot of intended versus achieved correction is provided in Figure 7.
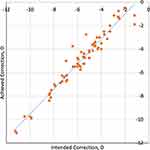 |
Figure 7 Scatterplot of intended versus achieved postoperative manifest spherical equivalent refraction. |
In order to further elucidate the performance of the EDOF ICL vis a vis severity of presbyopia at baseline, the performance of those eyes and subjects which not only required a reading add but also exhibited distance corrected near visual acuity of 20/40 or worse at baseline was examined. For these monocular and binocular cohorts of 21 eyes and 7 subjects, respectively, distributions of preoperative and postoperative monocular and binocular distance corrected near and intermediate visual acuities are provided in Figures 8–11. The improvement in defocus range for the binocular cohort is shown in Figure 12. The magnitude of the increased range of useful defocus is 1.01 ± 1.23 D, extending from approximately −1.5 D to −2.5 D at the 20/32 level.
 |
Figure 8 Preoperative and postoperative monocular distance corrected near visual acuity for the cohort with preoperative monocular distance corrected near visual acuity 20/40 or worse. |
 |
Figure 9 Preoperative and postoperative binocular distance corrected near visual acuity for the cohort with preoperative binocular distance corrected near visual acuity 20/40 or worse. |
 |
Figure 10 Preoperative and postoperative monocular distance corrected intermediate visual acuity for the cohort with preoperative monocular distance corrected near visual acuity 20/40 or worse. |
 |
Figure 11 Preoperative and postoperative binocular distance corrected intermediate visual acuity for the cohort with preoperative binocular distance corrected near visual acuity 20/40 or worse. |
 |
Figure 12 Binocular defocus curve for cohort of subjects with baseline binocular distance corrected near visual acuity 20/40 or worse. |
Safety
At 6 months postoperatively, 42 eyes (61.7%) demonstrated monocular corrected distance visual acuity 20/20 or better, and 33 subjects (97.1%) demonstrated binocular corrected distance visual acuity 20/20 or better. All eyes demonstrated monocular corrected distance visual acuity 20/32 or better. No eye lost more than 1 line of monocular corrected distance visual acuity; 25 eyes lost 1 line, 42 eyes were unchanged, and 1 eye gained 1 line. Similarly, no subject lost more than 1 line of binocular corrected distance visual acuity; 8 subjects lost 1 line, 25 subjects were unchanged, and 1 subject gained 1 line.
Graphs of preoperative and postoperative monocular and binocular photopic and mesopic contrast sensitivity with and without glare are provided in Figures 13A–D and 14A–D. There were no clinically or statistically significant differences in contrast sensitivity between baseline and 6 months under any testing conditions.
 |
Figure 13 (A–D) Preoperative and postoperative monocular photopic contrast sensitivity without (A) and with (B) glare, and monocular mesopic contrast sensitivity without (C) and with (D) glare. |
 |
Figure 14 (A–D) Preoperative and postoperative binocular photopic contrast sensitivity without (A) and with (B) glare, and binocular mesopic contrast sensitivity without (C) and with (D) glare. |
The mean postoperative endothelial cell density at 6 months measured 2560 ± 331 (2099–3300) cells/mm2, representing a 3.7% decrease from baseline. Postoperatively, mean vault measured 591.6 ± 230.0 (178.0–1160.0) μm at 1 day, 533.0 ± 223.7 (183.0–1120.0) μm at 1 week, 502.7 ± 232.5 (160.0–1160.0) μm at 1 month, 484.8 ± 222.7 (107.0–1160.0) μm at 3 months and 476.5 ± 222.4 (118.0–1120.0) μm at 6 months. Mean intraocular pressure measured 11.7 ± 3.1 (5.0–21.0) mm Hg at 1 day, 14.4 ± 3.7 (9.0–26.0) mm Hg at 1 week, 14.4 ± 2.7 (10.0–23.0) mm Hg at 1 month, 14.4 ± 2.6 (9.0–21.0) mm Hg at 3 months and 14.0 ± 2.5 (10.0–20.0) mm Hg at 6 months.
No intraoperative or postoperative complications occurred. There were no cases of pupillary block, pigment dispersion, glaucoma, anterior subcapsular opacity or cataract.
Patient-Reported Outcomes
Vision-related quality of life was evaluated using language-adapted versions of the NEI-RQL 42 Questionnaire. This 42-item instrument has 13 subscales covering specific aspects of vision-related quality of life. Each scale has a score from 0 to 100. A higher score indicates a better self-reported quality of life. In this study, subjects reported significant improvements in near vision, dependence on correction, activity limitations, appearance, worry, expectations, satisfaction with correction and suboptimal correction (Table 2). Other indicators of quality of vision, including symptoms, diurnal fluctuation and clarity of vision, did not change significantly. A significant decrease in vision-related quality of life was observed in only a single subscale, glare.
 |
Table 2 Preoperative and Postoperative NEI-RQL Subscale Scores |
Subjects completed a Subjective Task Assessment Questionnaire preoperatively and at 6 months postoperatively. Subjects were asked a series of questions to evaluate their ability to perform certain tasks in everyday life without visual aids (glasses or contact lenses). There was a series of 5 tasks each for distance, intermediate and near vision activities. For each specific distance, the ability to perform the same 5 tasks was evaluated for both “Good Light” and “Dim Light.” Grading was evaluated per the following scale: “Yes, with ease (2)”, “Yes with difficulty (1)” and “No (0)”. Therefore, the range of possible outcomes for each distance under a specific lighting condition was 0 (worst) to 10 (best). Summaries of the Subjective Task Assessment responses for both good and dim light are provided in Figure 15A–B. On average, subjects reported large improvements in their ability to perform tasks without correction at all distances in both good and dim light. Overall, satisfaction with the EDOF ICL was high: postoperatively, 31 subjects (91.2%) were satisfied with their vision.
 |
Figure 15 Preoperative and postoperative mean Task Assessment Questionnaire scores in good (A) and dim (B) light. |
Discussion
This multicenter prospective clinical investigation in phakic presbyopic patients with preoperative manifest refraction spherical equivalent ranging from −1.13 D to – 11.25 D has demonstrated the ability of the EDOF ICL to correct myopia and presbyopia, resulting in improvements of uncorrected near, intermediate and distance visual acuities and increased ability to perform tasks of daily living without correction and without compromise to the quality of vision. The mean increased range of binocular defocus for patients with distance corrected near visual acuity 20/40 or worse at baseline was approximately one diopter, demonstrating the effectiveness of the EDOF ICL for the correction of presbyopia. Some younger subjects with early presbyopia might not have achieved as much benefit initially but would be expected to have increasing benefit over time as their presbyopia inexorably worsens. On the other hand, these younger subjects may be expected to perform well because of their natural residual accommodative ability.
Overall, binocular uncorrected visual acuity of 20/32 or better was achieved by 91.2% of subjects at all testing distances. There were no clinically or statistically significant changes in contrast sensitivity at any spatial frequency under any testing conditions. Subjects reported significant improvements in measures of vision-related quality of life, including near vision and dependence on correction; subjects also reported large improvements in their ability to perform tasks without correction at all distances in both good and dim light. Only a single measure of vision-related quality of life, glare, worsened postoperatively. Although glare has been reported with the ICL with the central port design as well as with other extended depth of focus lens designs,10,11 in this study mesopic and photopic contrast sensitivity with glare were not diminished postoperatively.
Limitations of this study include the absence of illumination data during the measurement of achieved vault; authors have noted variability of vault with varying degrees of illumination.12 In addition, the tendency towards a more myopic spherical equivalent may be related in part to the challenge of determining the manifest refraction endpoint with an extended depth of focus optical design. Finally, preoperative astigmatism was limited to ≤ 0.75 D, so the behavior of the lens in eyes with greater degrees of astigmatism was not evaluated.
Recently, authors have reported 2-year outcomes in 17 eyes of 10 patients aged 38 to 50 years with a hydrophilic acrylic diffractive trifocal posterior chamber phakic refractive lens for the correction of high myopia and presbyopia.13 For implantation of the lens, “an iridectomy was performed using a 23-gauge vitrectome at the periphery of the iris at a 12 o’clock position.” In this small series, mean monocular postoperative uncorrected distance visual acuity measured 0.11 logMAR (0.03 to 0.17), while mean monocular uncorrected near visual acuity measured Jaeger 1.4 ± 1.7 at approximately 50 cm (the authors noted that “2 eyes read J1 already in the baseline”). The authors reported 4 events of elevated intraocular pressure, and endothelial cell loss of 9.7%. Pigment dispersion on the anterior lens surface was observed in 6 (40%) of 15 eyes. While the distance visual acuity reported in this study is comparable to that reported in the present study, different testing conditions for measuring near visual acuity make comparisons with the present study challenging. For example, in the present study, all visual acuities were measured with a high-resolution, automated system calibrated for both distance and luminance which presents randomized ETDRS letters to the subject and adheres to ANSI and ISO standards.14 In the study of the hydrophilic acrylic diffractive trifocal lens, “Near visual acuities were assessed using the Jaeger chart,” which has previously been described as “not suitable for research purposes.”15
Other approaches to the correction of refractive error and presbyopia include excimer laser monovision, presbyopic excimer laser ablation and refractive lens exchange. Schallhorn et al have reported results for monovision LASIK, with from 77.5% to 90.5% of subjects achieving 20/20 or better binocular uncorrected distance visual acuity and 95.6% to 100% of subjects achieving 20/40 or better binocular uncorrected near visual acuity, depending on their baseline refractive category.16 However, Stival et al have noted that
Limitations of monovision include compromising visual function, such as reduced low contrast and contrast sensitivity, inability to incorporate an intermediate vision correction without compromising distance or near vision, small-angle esotropic shift, and reduced stereopsis.17
Reinstein et al have reported 99% 20/25 or better binocular uncorrected distance visual acuity and 95% J3 (20/40) or better binocular uncorrected near visual acuity utilizing laser blended vision.18 They reported enhancement rates of 12% for emmetropic patients, 19% for myopes, and 22% for hyperopes. Multifocal ablation has provided another treatment for presbyopia. Ang et al reported that 100% of subjects achieved 20/25 or better binocular uncorrected distance visual acuity and 93% achieved J2 (20/30) or better binocular uncorrected near visual acuity with Supracor (Bausch Health, Rochester, NY).19 At 6 months, 6% of eyes lost two or more lines of corrected distance visual acuity; the reported enhancement rate was 5.7%.
Refractive lens exchange “may be preferred in presbyopia with early crystalline lens changes.”16 Schallhorn et al reported 84.2% to 90.7% monocular uncorrected distance visual acuity 20/20 or better and 95.7% to 98.9% monocular uncorrected near visual acuity 20/40 or better with a pseudophakic extended depth of focus IOL.16 However, the risk of retinal detachment is an important consideration in refractive lens exchange. Laube et al reported a high incidence of pseudophakic retinal detachment among patients 50 to 54 years of age in their series (5.39%).20
Conclusions
The EDOF ICL represents a new option for the surgical correction of myopia and presbyopia which spares both the cornea and the crystalline lens. Patients reported significant improvements in measures of vision-related quality of life, increased ability to perform tasks of daily living without glasses or contact lenses at all distances in both good and dim light with high levels of spectacle independence and satisfaction. The EDOF ICL has a favorable safety profile, with no significant loss of best-corrected visual acuity, no decrease in contrast sensitivity and no complications requiring surgical intervention or refractive enhancement. This study demonstrates that the EDOF ICL provides correction of myopia and presbyopia without compromising the quality of vision in patients who desire vision over a continuous range for improved uncorrected near, intermediate and distance visual acuity with increased spectacle independence and enhanced quality of life.
Data Sharing Statement
No further data beyond those provided will be shared.
Ethics Approval and Informed Consent
This clinical investigation was registered on clinicaltrials.gov (NCT03499821) and was conducted in accordance with the United States Code of Federal Regulations for the Clinical Investigation of Medical Devices in Human Subjects, the International Conference on Harmonisation Good Clinical Practice and all applicable local regulations. The protocol was approved by the Universiteit Antwerpen Ethisch Comite, Edegem, Belgium and the Comité Etico de Investigacion Clinica de la Comunidad Autonoma del Pais Vasco, Vitoria, Spain. The research followed the tenets of the Declaration of Helsinki and informed consent was obtained from all subjects after explanation of the nature and possible consequences of the study.
Disclosure
Dr. Packer: Advisor (Advanced Vision Science [Santen], Alcon, Amaros Medical, Aquea Health, Bausch + Lomb, Cassini Technologies, ClearSight, International Biomedical Devices, Keranova, Lensar, LensGen, PhysIOL, Precision for Medicine, Presbia USA, Promedica International, Rayner, Refocus Group, STAAR Surgical, Tarsus Pharmaceuticals, Visant Medical); equity owner (Aerie Pharmaceuticals, Amaros Medical, Aquea Health, Cassini Technologies, ClearSight, Digital Surgery Systems, International Biomedical Devices, Ira, Keranova, LensGen, Refocus Group, STAAR Surgical, Tarsus Pharmaceuticals, TrueVision, Visant Medical). Dr. Alfonso: Research funding (STAAR Surgical). Dr. Aramberri: Advisor (STAAR Surgical, Alcon, Johnson & Johnson, Zeiss). Dr. Elies: Advisor (STAAR Surgical). Dr. Fernandez: Advisor (Medicontur, Carl Zeiss Meditec); research funding (Bausch + Lomb); speakers bureau (STAAR Surgical, Oculus). Dr Mertens: Advisor (PhysIOL, Zeiss, Allotex, VSY, MircoSurgical Technology, Excel-lens, Novoxel, CSO, Ellex, Hoya, Medicontur); equity owner (Excel-lens, Novoxel, STAAR Surgical). The authors report no other conflicts of interest in this work.
References
1. McDonnell PJ, Lee P, Spritzer K, Lindblad AS, Hays RD. Associations of presbyopia with vision-targeted health-related quality of life. Arch Ophthalmol. 2003;121(11):1577–1581. doi:10.1001/archopht.121.11.1577
2. Luo BP, Brown GG, Luo SC, Brown MM. The quality of life associated with presbyopia. Am J Ophthalmol. 2008;145(4):618–622. doi:10.1016/j.ajo.2007.12.011
3. Srinivasan B, Leung HY, Cao H, Liu S, Chen L, Fan AH. Modern phacoemulsification and intraocular lens implantation (refractive lens exchange) is safe and effective in treating high myopia. Asia Pac J Ophthalmol (Phila). 2016;5(6):438–444. doi:10.1097/APO.0000000000000241
4. Pinto et al. United States Patent Application Publication No. 2016/0193037 A1; July 7, 2016.
5. Packer M. The implantable Collamer lens with a central port: review of the literature. Clin Ophthalmol. 2018;12:2427–2438. doi:10.2147/OPTH.S188785
6. Hays RD, Mangione CM, Ellwein L, Lindblad AS, Spritzer KL, McDonnell PJ. Psychometric properties of the National Eye Institute-Refractive Error Quality of Life instrument. Ophthalmology. 2003;110(12):2292–2301. doi:10.1016/j.ophtha.2002.07.001
7. Hays RD, Spritzer KL National Eye Institute Refractive Error Quality of Life Instrument (NEI-RQL-42TM), Version 1.0: a manual for use and scoring. Available from: https://www.nei.nih.gov/sites/default/files/nei-pdfs/rql-42man1.pdf.
8. Garza EB, Gomez S, Chayet A, Dishler J. One-year safety and efficacy results of a hydrogel inlay to improve near vision in patients with emmetropic presbyopia. J Refract Surg. 2013;29(3):166–172. doi:10.3928/1081597X-20130129-01
9. Fernández-Vega-Cueto L, Lisa C, Esteve-Taboada JJ, Montés-Micó R, Alfonso JF. Implantable collamer lens with central hole: 3-year follow-up. Clin Ophthalmol. 2018;12:2015–2029. doi:10.2147/OPTH.S171576
10. Martínez-Plaza E, López-Miguel A, Fernández I, Blázquez-Arauzo F, Maldonado MJ. Effect of central hole location in phakic intraocular lenses on visual function under progressive headlight glare sources. J Cataract Refract Surg. 2019;45(11):1591–1596. doi:10.1016/j.jcrs.2019.06.022
11. Coassin M, Di Zazzo A, Antonini M, Gaudenzi D, Afflitto GG, Kohnen T. Extended depth-of-focus intraocular lenses: power calculation and outcomes [published online ahead of print, 2020 Jun 22]. J Cataract Refract Surg. 2020. doi:10.1097/j.jcrs.0000000000000293
12. González-López F, Bilbao-Calabuig R, Mompeán B, Luezas J, Ortega-Usobiaga J, Druchkiv V. Determining the potential role of crystalline lens rise in vaulting in posterior chamber phakic collamer lens surgery for correction of myopia. J Refract Surg. 2019;35(3):177–183. doi:10.3928/1081597X-20190204-01
13. Stodulka P, Slovak M, Sramka M, Polisensky J, Liska K. Posterior chamber phakic intraocular lens for the correction of presbyopia in highly myopic patients. J Cataract Refract Surg. 2020;46(1):40–44. doi:10.1097/j.jcrs.0000000000000033
14. M&S technologies’ Clinical Trial Suite (CTS) now available with DataRight module. Available from: https://www.newswire.com/news/m-s-technologies-clinical-trial-suite-cts-now-available-with-dataright-5256766.
15. Radner W. Reading charts in ophthalmology. Graefes Arch Clin Exp Ophthalmol. 2017;255(8):1465–1482. doi:10.1007/s00417-017-3659-0
16. Schallhorn SC, Teenan D, Venter JA, et al. Monovision LASIK versus presbyopia-correcting IOLs: comparison of clinical and patient-reported outcomes. J Refract Surg. 2017;33(11):749–758. doi:10.3928/1081597X-20170721-03
17. Stival LR, Figueiredo MN, Santhiago MR. Presbyopic excimer laser ablation: a review. J Refract Surg. 2018;34(10):698–710. doi:10.3928/1081597X-20180726-02
18. Reinstein DZ, Carp GI, Archer TJ, Gobbe M. LASIK for presbyopia correction in emmetropic patients using aspheric ablation profiles and a micro monovision protocol with the Carl Zeiss Meditec MEL 80 and VisuMax. J Refract Surg. 2012;28:531–541. doi:10.3928/1081597X-20120723-01
19. Ang RE, Cruz EM, Pisig AU, Solis M, Reyes RM, Youssefi G. Safety and effectiveness of the SUPRACOR presbyopic LASIK algorithm on hyperopic patients. Eye Vis. 2016;3:33. doi:10.1186/s40662-016-0062-6
20. Laube T, Brockmann C, Lehmann N, Bornfeld N. Pseudophakic retinal detachment in young-aged patients. PLoS One. 2017;12(8):e0184187. doi:10.1371/journal.pone.0184187
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
