Back to Journals » International Journal of General Medicine » Volume 15
Percutaneous Kyphoplasty in Patients with Severe Osteoporotic Vertebral Compression Fracture with and without Intravertebral Cleft: A Retrospective Comparative Study
Authors Liu H, Zhou Q , Shao X, Zhang J, Deng L, Liu T , Yang H
Received 7 April 2022
Accepted for publication 21 June 2022
Published 18 July 2022 Volume 2022:15 Pages 6199—6209
DOI https://doi.org/10.2147/IJGM.S369840
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Scott Fraser
Hao Liu, Quan Zhou, Xiaofeng Shao, Junxin Zhang, Lei Deng, Tao Liu, Huilin Yang
Department of Orthopaedics, The First Affiliated Hospital of Soochow University, Suzhou, 215006, People’s Republic of China
Correspondence: Tao Liu; Huilin Yang, Department of Orthopaedics, The First Affiliated Hospital of Soochow University, No. 899 Pinhai Road, Suzhou, 215006, People’s Republic of China, Email [email protected]; [email protected]
Purpose: To comparatively analyze the clinical and radiographic outcomes of percutaneous kyphoplasty (PKP) in patients with severe osteoporotic vertebral compression fracture (sOVCF) with or without intravertebral cleft (IVC).
Methods: We enrolled a total of 75 patients with sOVCF receiving PKP between January 2016 and December 2018. The patients were divided into the following two groups based on their radiographic findings: with IVC (IVC group) and without IVC (NIVC group). The following radiographic outcomes were determined: anterior vertebral height (AVH), kyphotic angle (KA), lumbar lordosis (LL), sacral slope (SS), pelvic incidence (PI), and pelvic tilt (PT). The clinical functional assessment included Oswestry disability index (ODI) and visual analog scale (VAS) scores.
Results: No significant difference was found between the demographic data of the two groups (P > 0.05). AVH, KA, and LL in both groups were significantly corrected one month after surgery (P < 0.05). There was statistical difference between the two groups in AVH and KA one year and three years after surgery, and in LL and PT three years after surgery (P < 0.05). Compared with the results one month after surgery, AVH, KA, and LL of the IVC group deteriorated significantly one year and three years after surgery, whereas AVH, KA, and LL of the NIVC group deteriorated significantly three years after surgery (P < 0.05). The VAS and ODI scores in both groups decreased significantly one month, one year, and three years after surgery than preoperative results (P < 0.05), and a statistical difference was observed between the two groups three years after surgery (P < 0.05).
Conclusion: PKP can give satisfactory outcomes for the treatment of sOVCF with or without IVC. However, the NIVC group showed better clinical outcomes and could maintain spinal sagittal balance better than the IVC group during long-term follow-up.
Keywords: kyphoplasty, severe osteoporotic vertebral compression fracture, intervertebral cleft, sagittal balance
Introduction
Osteoporosis is a disease characterized by low bone mass and destruction of bone structure, which compromises bone strength and increases the risk of fracture.1 As the age of the population increases, the risks of osteoporosis and osteoporotic fractures also increase in the world population. Osteoporotic vertebral compression fracture (OVCF) is one of the most common complications of osteoporosis. OVCF is a fragile fracture caused by osteoporosis under the action of slight external force or not, causing intractable pain, lowering the quality of life, and also increasing the incidence of systemic complications and mortality.2–4 Severe OVCF (sOVCF) is defined by a two-third or more reduction in vertebral height, which is usually found along with severe kyphosis.5 Percutaneous kyphoplasty (PKP) is a minimally invasive surgical treatment for OVCF, which is effective for rapid pain relief and restores vertebral height.6 This minimally is an excellent minimally invasive technique for OVCF patients with persistent back pain who do not respond well to conventional conservative treatment, and it was performed after MRI detection of vertebral compression fractures. However, many controversies are associated with whether surgical intervention is necessary for patients with sOVCF. Some researchers have reported that a severe collapse fracture of the vertebral body is a relative contraindication for vertebroplasty.7–9 However, some studies have reported that PKP is an effective treatment intervention for sOVCF.10,11
Microfractures occur on the basis of osteoporosis, and such microfractures damage the small arteries supplying blood to the vertebral body, thus affecting the blood supply to the vertebral body, and eventually lead to the occurrence of intravertebral cleft (IVC) sign. The incidence of IVC is found in approximately 7–13% of patients with OVCF,12 and the time of IVC appearance usually varies from several weeks to months after fracture. The pathogenesis of OVCF with IVC is still unclear. Kim et al13 reported that IVC was a result of bone ischemic necrosis in OVCF. OVCF with IVC is different from common OVCF, which may not respond to conservative treatment. Previous studies have reported that PKP was an effective surgical treatment for OVCF with IVC,14,15 and pain reduction and vertebral body height restoration are the main purposes of treating OVCF with PKP.7,16
However, in-depth studies on the long-term results of PKP in sOVCF with IVC and sOVCF without IVC are less, and only a few detailed studies have investigated the effect of PKP on the spinopelvic sagittal balance in OVCF. Therefore, in this retrospective study, we aimed to compare the clinical and radiographic outcomes of PKP in elderly patients with sOVCF with or without IVC.
Materials and Methods
Patients
Inclusion criteria: (1) a single-level compression fracture of the lumbar spine; (2) the fractured vertebrae collapsed to less than one-third of their original height; (3) patients with osteoporosis (T < −2.5) on dual-energy X-ray absorptiometry (DEXA); (4) patients > 60 years of age. (5) the period of fracture within 2 to 6 weeks. (6) patients with obvious back pain, but no symptoms of nerve damage.
Exclusion criteria: (1) other spine-related diseases, such as ankylosing spondylitis, spinal tuberculosis, and spinal tumors; (2) surgical interventions at the spinal alignment during follow-up; (3) patients who died or were unable to complete 36 months of follow-up.
With reference to the strict inclusion and exclusion criteria, 75 patients between January 2016 and December 2018 were enrolled in this study. According to the preoperative CT and MRI of each patient, the three co-authors determined the fracture segment, the degree of fracture compression, and whether there was IVC. If there was IVC, they were divided into IVC group, if not, they were divided into NIVC group. All patients’ data were sourced from the electronic medical record management system of our hospital. This study was approved by the Ethics Committee of our institution.
Surgical Procedure
All patients were placed in the prone position after administering general anesthesia on the surgical table The fractured vertebra was localized with C-arm fluoroscopy. Guidewires were inserted into the fractured vertebral body along the bilateral pedicle. A working cannula was inserted along the guidewire. Balloon tamps were inserted through the cannula and placed in the anterior portion of the vertebral body in the lateral view. The fractured vertebra was reduced by gradually inflating the balloon. This balloon was then deflated and removed. The polymethylmethacrylate (PMMA) cement was slowly inserted into the cavity formed by the balloon. Incremental temperature cement delivery and graded-infusion techniques were applied at our hospital to minimize the leakage rate.17 The cement injection and leakage were monitored through C-arm fluoroscopy during the surgery.
During the follow-up period, all patients performed functional exercises of the back muscles and consumed anti-osteoporosis drugs under the guidance of their doctors.
Radiographic Evaluation
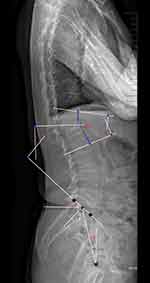
The bone mineral density (BMD) was assessed as a T score in the lumbar spine with DEXA (Discovery Wi, Hologic, America). The radiographic evaluation was conducted by blind method. Three co-authors measured each parameter of the same patient, and the data difference of each parameter was less than 5%, indicating that the measurement of the three co-authors has stability and reliability. The mean value of the three results measured for each parameter was used for analysis. Changes in the anterior vertebral height (AVH), kyphotic angle (KA), lumbar lordosis (LL), sacral slope (SS), pelvic incidence (PI), and pelvic tilt (PT) were measured using lateral X-ray radiographs (Figure 1). The AVH ratio was determined by the ratio of the anterior height of the fractured vertebrae to the average anterior height of the upper and lower adjacent vertebrae. The local KA was defined by Cobb’s method as the angle between the upper endplate of the vertebrae above and the lower endplate of the vertebrae below the fractured segment. The following parameters of the spinopelvic sagittal balance were measured:18 LL was defined as the angle between the upper endplate of the L1 and S1 vertebrae by Cobb’s method. PI was formed by the vertical line to the midpoint of the sacral plate and the line between the midpoint of the sacral plate of the S1 and the centroid of the femoral heads. PT was formed by the angle between the line connecting the midpoint of the sacral endplate with the axis of the femoral heads and the vertical line. SS was defined as the angle formed between the upper endplate of S1 and the horizontal line.
Clinical Evaluation
For the measurement of clinical outcomes, all patients filled out the following questionnaires preoperatively, 1 month after surgery, 1 year after surgery, and at the final follow-up. ODI scores were recorded to assess the patients’ improvement in quality of life and VAS scores for back pain to evaluate the patients’ subjective pain perception (0–10 score, 0 = no pain, 10 = extremely severe pain).19
Statistical Analysis
Statistical analyses were performed with SPSS software (SPSS 23.0, USA). The results were presented as the mean ±standard. The independent sample t-test and the one-way ANOVA were applied for inter-group comparisons at different time points. The repeated-measures ANOVA was applied for intra-group comparisons at different timepoints. P < 0.05 was considered to indicate statistical significance.
Results
Demographic Data
The demographic data of both groups are presented in Table 1. We included a total of 75 elderly patients who received PKP and completed the final follow-up in this study. The average age of the included patients was 70.79 ±5.85 years old. Male patients (25.33%) were less than female patients (74.67%), and L1 (45.33%) was the most common fractured segment in the lumbar spine. No significant difference was found between the two groups with respect to age, gender, and fractured segment (P > 0.05). The mean body mass index (BMI) in the NIVC group was slightly higher than that in the IVC group; however, the difference was not statistically significant (P > 0.05). The mean BMD of all patients was −3.32±0.50, and no significant difference was found between the two groups (P > 0.05). The time between fracture and surgery in the IVC group was slightly higher than that in the NIVC group; however, the difference was not statistically significant (P > 0.05). Many patients had different comorbidities, such as hypertension, diabetes, hyperlipidemia, or smoking habits, in both groups. However, no significant difference was found between the two groups (P > 0.05). The average follow-up duration of all patients was 44.45 ±6.60 months, and no significant difference was found in the follow-up durations between the two groups (P > 0.05).
 |
Table 1 General Characteristics of the Patients |
Radiographic Outcomes
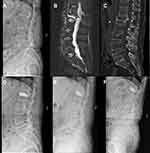
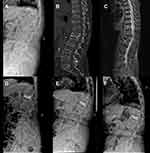
All radiographic data are presented in Table 2. No significant difference was found in the preoperative radiographic data between the two groups, which included AVH, KA, LL, PI, PT, and SS (P > 0.05). AVH, KA, and LL in the two groups were significantly corrected one month after surgery (P < 0.05). A statistically significant difference was found in AVH and KA between the two groups one year and three years after surgery (P < 0.05), and in LL and PT three years after surgery (P < 0.05). Compared with the results of one-month after surgery, AVH, KA, and LL in the IVC group deteriorated significantly at one year and three years after surgery, whereas AVH, KA, and LL in the NIVC group deteriorated significantly three years after surgery (P < 0.05). During follow-up, no significant difference was found in PI and SS between the two groups (P > 0.05). One typical case from both groups is shown in Figures 2 and 3, respectively.
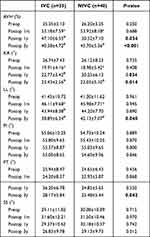 |
Table 2 Comparison of Radiographic Parameters Between the Two Groups |
Functional Outcomes
The functional outcomes are presented in Table 3. No significant difference was found in the preoperative VAS and ODI scores between the two groups (P > 0.05). The VAS and ODI scores in both groups were significantly lower at one month, one year, and three years after surgery than those before surgery (P < 0.05), and a statistically significant difference was found between the two groups at three years after surgery (P < 0.05).
 |
Table 3 Comparison of Functional Outcomes Between the Two Groups |
Discussion
In the present study, both groups achieved satisfactory clinical outcomes after PKP. Compared with the preoperative outcomes, the VAS and ODI scores in the two groups were significantly lower with distinct pain relief, indicating that the quality of life of patients after PKP was significantly improved. We showed that AVH and KA in the two groups were significantly corrected one month after surgery, and they were maintained at final follow-up in patients even though varying degrees of deterioration were observed during the follow-up. The results indicated that PKP was satisfactory for patients with sOVCF in restoring vertebral height and correcting kyphosis angle. No significant difference was found in VAS scores, ODI scores, AVH, and KA between the two groups one month after surgery. Therefore, we assumed that PKP is an effective method for the treatment of sOVCF, and no significant difference was found in the short-term clinical outcomes of sOVCF with or without IVC. This result was consistent with the results of previous studies.14,15
Even though the patients in both groups achieved satisfactory short-term clinical results after PKP, a gradual decrease was found in AVH and KA progression in the two groups. Moreover, AVH and KA in the IVC group progressed more aggressively than those in the NIVC group during the follow-up. The results can be due to the following reasons. The incomplete distribution of bone cement and the decrease in vertebral stiffness in severely compressed vertebrae gradually lead to the decrease in AVH and KA progression post-surgery. Because of extremely severe vertebral compression prior to surgery, the postoperative AVH and KA of the injured vertebrae were still difficult to attain the ideal level of normal vertebrae, which would increase the stress in the injured anterior column of the vertebral body. Lastly, even though all patients in this study underwent an anti-osteoporosis treatment after PKP, the treatment process was slow, and fragile bone could decrease postoperative AVH and KA. Moreover, the maintenance of AVH and KA in the IVC group was worse than those in the NIVC group during follow-up. This may be associated with the spatial distribution of bone cement. In the IVC group, after the bone cement was injected into the vertebral body to concentrate in the vertebral fissure, it hindered the further dispersion of bone cement, which resulted in the massive blocky distribution of bone cement in the vertebral body. In the NIVC group, after the bone cement was injected into the vertebral body to slowly diffuse along the bone trabecula to the whole vertebral body, the bone cement was evenly distributed radially in the vertebral body. However, the bulky distribution of bone cement led to an uneven distribution of vertebral stiffness, which reduced the contact between bone cement and bone. This could easily cause bone loss and even fracture. Some previous studies have also reported this phenomenon.15,20
Spine sagittal balance is one of the most important factors to determine the outcome of spinal treatment interventions. It is the state of maintaining a stable, standing posture with minimal muscle strength.21,22 Patients with OVCF often face severe spinal deformities, especially those with sOVCF. The imbalance in the spinal sagittal plane is mainly due to the gradual deterioration in the spinal deformity, which affects the compensatory ability of the pelvis, spine, and lower limbs. Zhang et al23 reported that OVCF often causes local kyphosis and changes the local sagittal alignment of the spine, and multiple vertebral compression fractures can cause spinal sagittal imbalance. However, it is unknown whether PKP can improve the angular parameters due to vertebral fractures and improve spinal sagittal balance in patients with sOVCF. Zhong et al24 analyzed the effects of PKP on the sagittal balance parameters of 90 patients with OVCF. They reported that PKP improved global sagittal spinal alignment, especially fractures, in the thoracolumbar region. Yokoyama et al25 reported that PKP can improve total sagittal imbalance by alleviating local kyphosis in 21 patients. In this study, the postoperative increase in LL indicated that the reduction of local KA can contribute to the partial correction of sagittal imbalance in patients with sOVCF. Three spinopelvic sagittal balance parameters, namely PI, PT, and SS, did not improve after surgery and their improvement did not appear at the final follow-up in our study. The above results can be due to the following two reasons: The elderly patients are often inactive and lack daily activities post-surgery. Therefore, the effects of PKP on spinopelvic sagittal balance may not be completely observed during the follow-up. Elderly patients with osteoporosis may have a certain degree of spinal deformity even before the vertebral fracture. Therefore, even though PKP restored the height of the fractured vertebral body and corrected KA, the effects of PKP on spinopelvic sagittal balance may not be significant. However, these are only our hypotheses and require deeper research and longer follow-ups for confirmation.
Previous studies have reported that satisfactory clinical outcomes are associated with the restoration of sagittal balance after spinal surgery.26,27 For patients with sOVCF, the reduction of the injured vertebral height can lead to kyphosis progression, which causes an anterior shift in the center of gravity and decreases LL. Posterior rotation of the pelvis occurs and decreases SS and increases PT to compensate for the decrease in LL. The spinal sagittal imbalance was majorly associated with chronic lower back pain in adults.28 An increased KA extends the posterior muscles and causes chronic pain. Sung-Soo et al29 reported that patients with PT improvement showed significantly better VAS and ODI scores than those without improvement. Furthermore, in patients with improved PT, the improvement in clinical outcomes was associated with postoperative LL.30 During the final follow-up, have found a significant difference in LL and PT between the two groups, which may account for the differences in ODI and VAS scores between the two groups during the final follow-up.
This study has many limitations. This is a retrospective comparative study, and the sample size was relatively insufficient. We did not include the types and number of complications in all patients during the follow-up period, and we lacked relevant information about complications. We only included patients with a time of fracture within 2 to 6 weeks to reduce the interference of surgical timing on postoperative efficacy to a certain extent. However, the time of IVC usually varied from several weeks to months after fracture; therefore, we excluded patients with a long time of fracture, which may cause some deviation in the results. Because some images did not contain complete bilateral femoral heads, we mainly determined the position of the center of the femoral heads by observing the morphology of the acetabulum in such cases, which may lead to a certain deviation in the measurement of the PI and PT angle. Therefore, prospective randomized controlled studies are required in the future. Future studies should investigate whether significant differences are present in the complications between the two groups.
Conclusions
In conclusion, PKP can give satisfactory clinical and radiographic outcomes in patients with sOVCF with or without IVC. However, compared to the NIVC group, the IVC group is more prone to have the loss of AVH and progression of KA, and it could be not as good as the NIVC group in maintaining spinal sagittal balance during long-term follow-up. Therefore, we suggest that more attention should be paid to anti-osteoporosis and other rehabilitation treatments after PKP in patients with sOVCF along with IVC.
Abbreviations
OVCF, osteoporotic vertebral compression fracture; sOVCF, severe osteoporotic vertebral compression fracture; PKP, percutaneous kyphoplasty; IVC, intravertebral cleft; NIVC, without intravertebral cleft; DEXA, dual-energy X-ray absorptiometry; BMD, bone mineral density; AVH, anterior vertebral height; KA, kyphotic angle; LL, lumbar lordosis; SS, sacral slope; PI, pelvic incidence; PT, pelvic tilt; ODI, Oswestry disability index; VAS, visual analog scale; BMI, body mass index.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Acknowledgment
The authors would like to thank all the reviewers who participated in the review and MJEditor (www.mjeditor.com) for its linguistic assistance during the preparation of this manuscript. This work was supported by the National Natural Science Foundation of China [82072476]; the Natural Science Foundation of Jiangsu Province [BK20191173]; Youth Science and technology project of rejuvenating health through science and education in Suzhou [KJXW2019010].
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. Hao Liu, Quan Zhou and Xiaofeng Shao have contributed equally to this work and share first authorship.
Disclosure
All authors declare that they have no conflicts of interest in this work.
References
1. Johnston CB, Dagar M. Osteoporosis in older adults. Med Clin North Am. 2020;104(5):873–884. doi:10.1016/j.mcna.2020.06.004
2. Taylor RS, Fritzell P, Taylor RJ. Balloon kyphoplasty in the management of vertebral compression fractures: an updated systematic review and meta-analysis. Eur Spine J. 2007;16(8):1085–1100. doi:10.1007/s00586-007-0308-z
3. Yang HL, Zhao L, Liu J, et al. Changes of pulmonary function for patients with osteoporotic vertebral compression fractures after kyphoplasty. J Spinal Disord Tech. 2007;20(3):221–225. doi:10.1097/01.bsd.0000211273.74238.0e
4. Edidin AA, Ong KL, Lau E, Kurtz SM. Morbidity and mortality after vertebral fractures: comparison of vertebral augmentation and nonoperative management in the medicare population. Spine. 2015;40(15):1228–1241. doi:10.1097/BRS.0000000000000992
5. Peh WC, Gilula LA, Peck DD. Percutaneous vertebroplasty for severe osteoporotic vertebral body compression fractures. Radiology. 2002;223(1):121–126. doi:10.1148/radiol.2231010234
6. Majd ME, Farley S, Holt RT. Preliminary outcomes and efficacy of the first 360 consecutive kyphoplasties for the treatment of painful osteoporotic vertebral compression fractures. Spine J. 2005;5(3):244–255. doi:10.1016/j.spinee.2004.09.013
7. Kim KH, Kuh SU, Chin DK, et al. Kyphoplasty versus vertebroplasty: restoration of vertebral body height and correction of kyphotic deformity with special attention to the shape of the fractured vertebrae. J Spinal Disord Tech. 2012;25(6):338–344. doi:10.1097/BSD.0b013e318224a6e6
8. Nieuwenhuijse MJ, van Erkel AR, Dijkstra PD. Percutaneous vertebroplasty in very severe osteoporotic vertebral compression fractures: feasible and beneficial. J Vasc Interv Radiol. 2011;22(7):1017–1023. doi:10.1016/j.jvir.2011.02.036
9. Cotten A, Boutry N, Cortet B, et al. Percutaneous vertebroplasty: state of the art. Radiographics. 1998;18(2):
10. Lee JH, Lee DO, Lee JH, Lee HS. Comparison of radiological and clinical results of balloon kyphoplasty according to anterior height loss in the osteoporotic vertebral fracture. Spine J. 2014;14(10):2281–2289. doi:10.1016/j.spinee.2014.01.028
11. Lee JK, Jeong HW, Joo IH, Ko YI, Kang CN. Percutaneous balloon kyphoplasty for treatment of very severe osteoporotic vertebral compression fractures: a case-controlled study. Spine J. 2017;18:962–969.
12. Venmans A, Klazen CA, Lohle PN, Mali WP, van Rooij WJ. Natural history of pain in patients with conservatively treated osteoporotic vertebral compression fractures: results from VERTOS II. AJNR Am J Neuroradiol. 2012;33(3):519–521. doi:10.3174/ajnr.A2817
13. Kim YC, Kim YH, Ha KY. Pathomechanism of intravertebral clefts in osteoporotic compression fractures of the spine. Spine J. 2014;14(4):659–666. doi:10.1016/j.spinee.2013.06.106
14. Ai MW, Zhong KL, Wen FN, Yong LC, Qi SH. The existence of intravertebral cleft impact on outcomes of non-acute osteoporotic vertebral compression fractures patients treated by percutaneous Kyphoplasty: a comparative study. J Spinal Disord Tech. 2013;27(3):E88.
15. Li Z, Liu T, Yin P, et al. The therapeutic effects of percutaneous kyphoplasty on osteoporotic vertebral compression fractures with or without intravertebral cleft. Int Orthop. 2018;42:1–7. doi:10.1007/s00264-017-3565-4
16. Hiwatashi A, Westesson PL, Yoshiura T, et al. Kyphoplasty and vertebroplasty produce the same degree of height restoration. AJNR Am J Neuroradiol. 2009;30(4):669–673. doi:10.3174/ajnr.A1442
17. Yang H, Liu H, Wang S, Wu K, Meng B, Liu T. Review of Percutaneous Kyphoplasty in China. Spine. 2016;41(Suppl 19):B52–B58. doi:10.1097/BRS.0000000000001804
18. Cédric B, Jérôme J, Gilles P, Pierre R. Spinopelvic alignment of patients with degenerative spondylolisthesis. Neurosurgery. 2007;61(5):5.
19. Price DD, Mcgrath PA, Rafii A, Buckingham B. The validation of visual analog scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17(1):45–56. doi:10.1016/0304-3959(83)90126-4
20. Fang X, Yu F, Fu S, Song H. Intravertebral clefts in osteoporotic compression fractures of the spine: incidence, characteristics, and therapeutic efficacy. Int J Clin Exp Med. 2015;8(9):16960–16968.
21. Endo K, Suzuki H, Tanaka H, Kang Y, Yamamoto K. Sagittal spinal alignment in patients with lumbar disc herniation. Eur Spine J. 2010;19(3):435–438. doi:10.1007/s00586-009-1240-1
22. Booth KC, Bridwell KH, Lenke LG, Baldus CR, Blanke KM. Complications and predictive factors for the successful treatment of flatback deformity (fixed sagittal imbalance). Spine. 1999;24(16):1712–1720. doi:10.1097/00007632-199908150-00013
23. Zhang YL, Shi LT, Tang PF, Sun ZJ, Wang YH. Correlation analysis of osteoporotic vertebral compression fractures and spinal sagittal imbalance. Orthopade. 2017;46(3):249–255. doi:10.1007/s00132-016-3359-1
24. Cao Z, Wang G, Hui W, Liu B, Liu Z, Sun J. Percutaneous kyphoplasty for osteoporotic vertebral compression fractures improves spino-pelvic alignment and global sagittal balance maximally in the thoracolumbar region. PLoS One. 2020;15(1):e0228341. doi:10.1371/journal.pone.0228341
25. Yokoyama K, Kawanishi M, Yamada M, et al. Postoperative change in sagittal balance after Kyphoplasty for the treatment of osteoporotic vertebral compression fracture. Eur Spine J. 2015;24(4):744–749. doi:10.1007/s00586-014-3678-z
26. Berven SH, Deviren V, Smith JA, Hu SH, Bradford DS. Management of fixed sagittal plane deformity: outcome of combined anterior and posterior surgery. Spine. 2003;28(15):
27. Matsumoto M, Watanabe K, Fujita N. Impact of sagittal spinopelvic alignment on clinical outcomes after decompression surgery for lumbar spinal canal stenosis without coronal imbalance. J Neurosurg Spine. 2015;23(4):451–458. doi:10.3171/2015.1.SPINE14642
28. Korovessis P, Repantis T, Papazisis Z, Iliopoulos P. Effect of sagittal spinal balance, levels of posterior instrumentation, and length of follow-up on low back pain in patients undergoing posterior decompression and instrumented fusion for degenerative lumbar spine disease: a multifactorial analysis. Spine. 2010;35(8):898–905. doi:10.1097/BRS.0b013e3181d51e84
29. Sung-Soo C, Whan E, Eun-Sang K, Sun-Ho L, Mi K, Chong-Suh L. The impact of sagittal balance on clinical results after posterior interbody fusion for patients with degenerative spondylolisthesis: a Pilot study. BMC Musculoskelet Disord. 2011;12(1):69. doi:10.1186/1471-2474-12-69
30. Bourghli A, Aunoble S, Reebye O, Le Huec J. Correlation of clinical outcome and spinopelvic sagittal alignment after surgical treatment of low-grade isthmic spondylolisthesis. Eur Spine J. 2011;20:663–668. doi:10.1007/s00586-011-1934-z
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.