Back to Journals » International Journal of General Medicine » Volume 14
Percutaneous Endoscopic Transforaminal Lumbar Interbody Fusion: Technique Note and Comparison of Early Outcomes with Minimally Invasive Transforaminal Lumbar Interbody Fusion for Lumbar Spondylolisthesis
Authors Zhang H , Zhou C, Wang C, Zhu K , Tu Q, Kong M , Zhao C , Ma X
Received 23 December 2020
Accepted for publication 5 February 2021
Published 22 February 2021 Volume 2021:14 Pages 549—558
DOI https://doi.org/10.2147/IJGM.S298591
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Hao Zhang,* Chuanli Zhou,* Chao Wang, Kai Zhu, Qihao Tu, Meng Kong, Chong Zhao, Xuexiao Ma
Department of Spinal Surgery, The Affiliated Hospital of Qingdao University, Qingdao, Shandong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xuexiao Ma
Department of Spinal Surgery, The Affiliated Hospital of Qingdao University, No. 59 Haier Road, Qingdao, Shandong, 266000, People’s Republic of China
Tel +86 18661807895
Email [email protected]
Purpose: To compare the preliminary postoperative outcomes of percutaneous endoscopic transforaminal lumbar interbody fusion (Endo-TLIF) and minimally invasive transforaminal lumbar interbody fusion (MIS-TLIF) in the treatment of lumbar spondylolisthesis.
Methods: Sixty-two patients with single-segment lumbar spondylolisthesis received Endo-TLIF and MIS-TLIF were enrolled in present study. Perioperative parameters, including operation time, estimated blood loss (EBL), interoperative fluoroscopy time, ambulation time and operative complications were recorded, respectively. The results of clinical metrics such as the Visual Analog Scale (VAS) for back and leg pain, the Oswestry Disability Index (ODI) and the Japanese Orthopaedic Association (JOA) score were obtained, respectively. Postoperative fusion rates were assessed by clinical fusion and CT at 12-month after surgery.
Results: No significant differences were found in the demographic data between the two groups. Compared with MIS-TLIF group, Endo-TLIF group had similar operative time, less intraoperative blood loss and shorter ambulation time but longer duration of X-ray radiation. The postoperative VAS scores of back pain, ODI and JOA score were significantly improved comparing with the preoperative scores in two groups, but the Endo-TLIF group showed more significant improvement in the early follow-up (P < 0.05, respectively). There were no significant differences in terms of the interbody fusion rate between the two groups. Meanwhile, no serious postoperative complications were observed in the study.
Conclusion: Compared with MIS-TLIF, Endo-TLIF technique showed relatively faster recovery and better outcomes in terms of early curative effect, especially in 6 months after operation. However, intraoperative repeated fluoroscopy could result in highly cumulative radiation and longer operation time.
Keywords: percutaneous endoscopic transforaminal lumbar interbody fusion; Endo-TLIF, MIS-TLIF, lumbar spondylolisthesis, clinical outcome
Introduction
Lumbar spondylolisthesis is defined as a forward slippage of a lumbar vertebra relative to the next vertebral body and resulting in instability of the segment.1,2 The most common type includes degenerative and isthmic lumbar spondylolisthesis. Degenerative spondylolisthesis is one of the most common degenerative spinal disorder in the aging population, and often associated with lumbar canal stenosis.3,4 Bhalla5 pointed out that the chief goals of surgical treatment include neurologic decompression and stabilization of the vertebral segments with instrumented fusion. Although posterior lumbar interbody fusion (PLIF) technique have good effects, it may damage the paraspinal muscles, resulting in postoperative pain, prolonged hospital stays, protracted rehabilitation programs and heavy financial burden to patients.6,7 These drawbacks of standard PLIF have prompted the development of less-invasive techniques.8–11 Scheufler et al12 mentioned that numerous minimally invasive techniques have been applied for spinal surgery since a novel tubular retractor system was introduced by Foley in 1997.
Harms et al13 first reported transforaminal lumbar interbody fusion (TLIF) technique, which can effectively avoid muscle and nerve root traction injury in 1982. But the technique still questioned by its limited workspace, the field of vision of this surgical procedure and higher incidence of complications.14 Recently, the percutaneous endoscopic transforaminal lumbar interbody fusion (Endo-TLIF) technique was reported to address the variety of spinal disorders by using endoscopic and expandable cages.15,16 Endo-TLIF technique was derived from the percutaneous endoscopic lumbar discectomy (PELD) technique and combined endoscopic visualization, expandable cage and interbody fusion technique.17 In recent years, Endo-TLIF technique has become a hot topic and attracted many spine surgeons on its clinical application.
There were limited studies that evaluated and compared the clinical effects of the Endo-TLIF technique for the treatment of lumbar spondylolisthesis with other minimally invasive lumbar fusion surgeries. So that, surgeons lack reference and clinical experience of percutaneous endoscopic lumbar interbody fusion technique in evaluating preliminary clinical outcomes. Thus, the aims of this study were to demonstrate the clinical efficacies of Endo-TLIF for the treatment of single-segment lumbar spondylolisthesis comparing with MIS-TLIF in detail.
Patients and Methods
Participants
Method approach to retrospective case-controlled study, the Endo-TLIF group compared with the MIS-TLIF group. In total, 62 patients were enrolled in the study between May and August 2019 according to the screening criteria. The procedures were approved by the ethics committee of the Affiliated Hospital of Qingdao University.
The inclusion criteria were defined as follows: (i) Patients over 18-years-old; (ii) Patients who were diagnosed with degenerative or isthmic spondylolisthesis (Meyerding Grade I/II) with or without resultant stenosis at one lumbar level; (iii) Patients who were diagnosed with single segment lumbar instability; (iv) Patients who had neural symptoms (including low back pain, sciatica and extremity symptoms); (v) Patient’s symptoms were not alleviated after 6 months or more of conservative therapy.
The exclusion criteria were defined as follows: (i) Patients could not tolerate surgery because of serious systemic diseases; (ii) Patients who had undergone previous lumbar fusion surgery or the needed treatment for more than one level; (iii) Patients who were mentally incompetent; (iv) Patients who had trauma, arachnoiditis, active infection (local or systemic) or spinal metastasis.
All diagnoses were based on clinical symptoms and neuroradiological imaging manifestations including extension and flexion lateral radiograph, computerized tomography (CT) and magnetic resonance imaging (MRI) scans. To eliminate bias, all patients have received surgery from the one surgeon who had rich clinic surgery experience and skillful operation technology of PELD and MIS-TLIF. Meanwhile, there were only one data collector in present study. Sixty-two patients were divided into 2 groups: the Endo-TLIF group and the MIS-TLIF group. All patients were followed up more than 12 months and no follow up loss in the 2 groups.
Endo-TLIF Technique
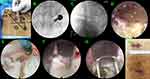
Following induction of general anesthesia, the patients were positioned prone on a radiolucent table Spinal cord monitoring was performed during the procedure to prevent unexpected nerve injury. The entry points of endoscopic working channel and the pedicle screws were marked on the skin via fluoroscopy (Figure 1A). Four guide wires of pedicle screw were placed through cannulated needles that were inserted into the pedicle based on the bilateral pedicle projection (Figure 1B).
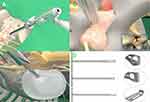
Blunt guiding rod was inserted and put on the surface of the zygapophyseal joint, followed by working channel. The appropriate position for insertion of the Endo-TLIF tubular system (D = 8.5mm; Unin-Tech®, Maoyu, Inc., Shanghai, China) was confirmed by anteroposterior and lateral X-ray views (Figures 1B, C and 2A). Meanwhile, the more details of technique and instruments of Endo-TLIF system were shown in Figure 2. Foraminoplasty and laminectomy were achieved by the removal of part of the articular process and laminae with full-see trephine for 4–8 times under fluoroscopic and endoscopic vision. It is noteworthy that the procedures of foraminoplasty and laminectomy like transforaminal-LIF rather than trans-Kambin TLIF (Figure 2B). A satisfactory foraminoplasty and laminectomy was obtained through trephine and lamina forceps according to preoperative plan for decompression (Figure 1D). Caudal, cephalad, lateral edge of ligament flavum were visible clearly and removed with rongeurs. By turning the working channel from a ventral to dorsal position, the nerve root and dural sac were protected behind the sharp bevel of the working cannula. Next, discectomy, annulus opening, and nerve root decompression were performed under endoscopic visualization (Figure 1E and F). Bilateral discectomy and nerve root decompression should be performed to some patients with neurological symptoms of both lower limbs through the Endo-TLIF technique.
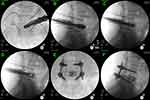
All the procedures of discectomy and endplate preparation were finished under endoscopy by using osteotomes, pituitary rongeurs, inside and outside reamers, curettes, scraper and flexible scraper (Figures 1G and 2C, D). Based on the results of the trial cage, a suitable expandable interbody cage, filling with skeletal particles, was selected and subsequently placed into the disc space through the working tube (Figure 3A and B). The cages were adjusted to a satisfactory position according to intraoperative fluoroscopy and the cages were expanded to increase the rear height by 2 mm to make full contact with the endplate. The cage was expanded through the special access cannula and sufficient bony grafts was placed in the expanded cage through a hollow tube (Figure 3C and D).
Before removing the working channel and endoscopy, the cage position and decompression effect of the nerve root were confirmed again. The bilateral percutaneous pedicle screws were placed by direction of guide wires, and rods were inserted and fixed percutaneously (Figure 3E and F). Finally, a subfascial drainage tube was placed at the wound and the 5 small incisions (1 for Endo-TLIF tubular system, 4 for pedicle screws) were closed directly.
MIS-TLIF Technique
Under the guidance of the C-arm X-ray machine, the surgical level was located and the skin incision was made on the side and the level of pathology. The unilateral muscle (multifidus and longissimus muscles) was bluntly separated from dorsal to ventral, and the Quadrant retractor system was placed on the symptomatic side via the muscular gaps. Under direct visualization, unilateral laminectomy, facetectomy, partial ligament flavum resection, nerve root decompression, excision of the intervertebral disc and placement of a suitable rigid PEEK cage filling with skeletal particles were performed based on the Quadrant dilator. These procedures were performed as routine MIS-TLIF technique that described by Wang et al.18
Clinical Assessment
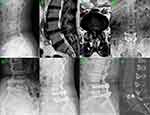
The demographic data included sex, age, body mass index (BMI), segment distribution and spondylolisthesis severity. The preoperative factors of patients including operative time, estimated blood loss (EBL), interoperative fluoroscopy time, ambulation time and operative complications was obtained, respectively. The clinical metrics including the Visual Analog Scale (VAS) for back and leg pain, the Oswestry Disability Index (ODI) and the Japanese Orthopaedic Association (JOA) score preoperatively and 1 week, 3 months, 6 months and 12 months after surgery were obtained to evaluate patients’ postoperative recovery and functional outcomes. Postoperative fusion rates were assessed by clinical fusion and CT scan at 12-month postoperative follow-up. Less than 4° segmental movement at the fused levels on flexion–extension dynamic radiographs and no mechanical low-back pain was considered as the successful clinical fusion. Continuous bone trabecular bridging between intervertebral bodies was considered as the standard of spinal intervertebral fusion19,20 (Figure 4H).
Statistical Analysis
Statistical analysis was performed by using the SPSS 22.0 statistical software package (SPSS Inc., Chicago, IL, USA). The normality of the distribution of measurement data was evaluated using the Kolmogorov–Smirnov test. The normal distribution continuous data from two groups were assessed with t-tests. Categorical variables were analyzed with the χ2 test. A P-value < 0.05 was considered statistically significant.
Results
Demographic Characteristics
The mean age of the 32 (51.6%) patients in the Endo-TLIF group was 53.1±16-years-old and there were 12 males and 20 females. The mean age of the 30 (48.4%) patients in the MIS-TLIF group was 55.7±14.2-years-old and there were 14 males and 16 females. No significant differences were found in the demographic data of all patients in the two groups (P < 0.05, respectively). Further details were listed Table 1.
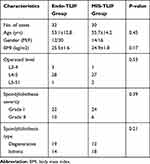 |
Table 1 Demographic Data |
Perioperative Parameters
Compared with the MIS-TLIF group, the Endo-TLIF group had lower estimated intraoperative blood loss, shorter ambulation time and higher intraoperative fluoroscopy time (P < 0.05, respectively). In our study, MIS-TLIF needed at least 7 times X-rays but Endo-TLIF required at least 15 times X-rays. A longer mean operation time was observed in the Endo-TLIF group (202.6±31.4 minutes) than in the MIS-TLIF group (192.1±18.9 minutes), but there were no statistically significant differences between the two groups (P = 0.119). The further perioperative indicators are shown in Table 2.
 |
Table 2 Perioperative Parameters |
Clinical Outcomes
Both groups showed a significant reduction in the VAS scores of back pain and the ODI scores, but the Endo-TLIF group showed a significant better curative effect at the 3-months follow-up (P < 0.05, respectively). At more than 6 months after surgery, both groups showed continuous improvement but with no significant differences between the two groups (P ≥ 0.05). Leg pain VAS scores were similar between the two groups and with no significant differences in the follow-up period (P ≥ 0.05, respectively). JOA scores were used to assess neurologic function, furthermore, the JOA scores on postoperative 1 week and 6 months were significantly higher (P < 0.05, respectively) for Endo-TLIF group. The further results and change trend are shown in Table 3 and Figure 5.
 |
Table 3 Preoperative, Follow-Up VAS, ODI, and JOA Scores |
 |
Figure 5 The Endo-TLIF group had better clinical outcomes than MIS-TLIF group in terms of VAS, ODI and JOA scores. (A) VAS score of back pain. (B) VAS score of leg pain. (C) ODI. (D) JOA score. |
Interbody Fusion Rates
Postoperative radiographic imaging including X-ray radiographs and CT images were taken at 3, 6, and 12 months after surgery (Figure 4). All patients have achieved clinical fusion in two groups. In the Endo-TLIF group, continuous bone trabecular bridging could not be identified in 2 cases based on the CT scan at the 12-month follow-up, and the remaining 60 cases were regarded as interbody fusion. However, it must be noted that there were no significant differences in the interbody fusion rates between two groups.
Complications
There were no severe complications in present study, such as postoperative infections, dura mater tears or nerve root injuries. But one case in the Endo-TLIF group happened that the shorter rod came off from the screw 7 weeks post-surgery and was revised under local anesthesia to change a longer rod.
Discussion
Theoretical Basis and Advantages of Endo-TLIF
As a novel technology, Endo-TLIF technique was the combination of PELD technique and interbody fusion technique. The present study showed that Endo-TLIF offered many advantages over MIS-TLIF for the treatment of spondylolisthesis. The Endo-TLIF technique not only can achieve bilateral direct decompression, interbody cage insertion and pedicle implantation but also less dissection of normal structures. In other words, the muscle, soft tissue and nerve roots can be significantly protected because of the access to procedures and the direct visualization under endoscopy. In our study, the MIS-TLIF technique requires 8–10 cm lengthy skin incision approximately. Although the Wiltse approach with the use of a Quadrant channel can reduce the need for muscular stripping, it still requires partial laminotomy to achieve decompression and fusion.14 Because of the application of endoscopy and progressive tissue dilation, the Endo-TLIF technique can performed with a 1–2 cm-length skin incision and entirely preserve the function of paraspinal muscles (Figure 1H). In the Endo-TLIF group, the mean amount of blood loss was significantly less than MIS-TLIF group (73.0±26.4mL vs. 129.0±31.7mL), and the patients were ambulatory within 12 hours. Compared with MIS-TLIF group, continuous irrigation of normal saline and certain water pressure during the operation may be one of the main factors for reducing blood loss. Furthermore, the technique requires a more accurate angle for working channel placement because the use of smaller tube. But, the height of the iliac crest had some influence on angle of working channel when undergoing in level L5-S1. Former studies showed that the limited amount of muscle dissection and innervation damage are principle factors that can reduce the incidence of low back pain and the recovery time.7 So that, there were significant differences in VAS of back pain, ODI scores and JOA between the two groups in 6 months after surgery (P < 0.05, respectively). That means the patients in Endo-TLIF had faster function recovery than MIS-TLIF group. Although other percutaneous endoscopic lumbar interbody fusion technique and our techniques shared the label of percutaneous Endo-TLIF technique but with difference in several aspects. The difference was not only the methods of screw implantation, working channel system, surgical procedures and details but also the interbody implant cage. Similar to the result of present study, they also reported that Endo-TLIF technique, comparing with MIS-TLIF, has advantages of less surgical trauma, less hidden blood loss, and less postoperative low-back pain.21
Conventional fusion is still the widely accepted standard for managing lumbar degeneration disorders that required the maintenance of spinal stabilization. The distinguishing factor of the Endo-TLIF technique is the endoscopy-based transforaminal posterolateral approach that permits the use of an expandable fusion cage placing in the disk space with limited removal of bone structure.22 Nerve root injury is one of the most serious complications of spinal surgery, so we must pay great attention on each step that may result in damage during operation. Foraminoplasty is a necessary step for enlarging the foramen to provide sufficient space for subsequent procedures and eliminate exiting root injury.15 In addition, the technique is similar but with some differences when undergoing in level L3-4, L4-5 and L5-S1. The level L3-4 and L4-5 has higher intervertebral space so the exiting nerve root do not need to be exposed. However, the L5-S1 level has the opposite option and we prefer to expose the exiting nerve root under endoscope. Similar to the PELD technique, the nerve root can be protected by a working tube with a beveled tip from trepan cutting and cage implantation. There were no cases of exiting nerve root injury in our study. Meanwhile, endplate preparation and expandable cage placement under direct endoscopic visualization provide effective advantages for the protection of nerves and the dural sac.
The application of expandable cages is also one of the advantages of the Endo-TLIF technique. Compared with a rigid PEEK cage, this cage requires less excision of bony structures and smaller size to avoid the excessive pulling of nerve roots.23 Expandable cages are convenient for implantation, and they provide sufficiently high interbody restoration during lumbar spine surgery. Spinal leg pain may result from disc and foraminal collapse, which resulting in compression of the exiting nerve root. In such circumstances, expandable cage implantation can restore intervertebral height to achieve indirect neural decompression and additional foraminal expansion.24 So that, there were no significant differences between the two groups for VAS of leg pain (P ≥ 0.05, respectively). The Varian® cage (Medyssey, Korea) that we used in Endo-TLIF procedure allowed adjustment of the cage’s position even was once expanded. We prefer to place the cage posterior of intervertebral space, the reasons of posterior placement of cage are defined as follow: the posterior placement of cage can help maintain the stabilization of cage in order to avoid the cage loose after rod placement; The bone grate has been placed on the anterior of intervertebral space before cage insertion; The angle and length of instrumentation under endoscope force us to posterior placement of cage, and we also need to observe the rear of cage under endoscope. In addition, the surface of the expandable cage provides stability and decent bone integration with the help of a bone graft within the cage. Endplate preparation is a vital step for achieving satisfactory fusion. In present study, there were no significant differences between the two groups for interbody fusion rates in postoperative 12 months (P = 0.492). The damage to the endplate in the process of cage implantation maybe the main cause of cage subsidence. So that, satisfactory endplate preparation and careful cage implantation were performed in the process of surgery. No cage subsidence was observed in two groups. In addition, percutaneous pedicle screw fixation is necessary in Endo-TLIF, especially for patients with broken endplates or osteoporosis.25
Drawbacks of Endo-TLIF
Endo-TLIF technique offers many advantages, such as limited invasiveness, less blood loss, short recovery time and no need for postoperative drainage. Despite mentioned benefits, there still are some limitations that should be given attention. The locations of the working tube, expandable cage and pedicle screws need to be determined with X-ray during the operation, thus, patients and doctors are exposed to higher doses of radiation (46.3±5.1 vs. 32.2±3.9 seconds, P < 0.05). It is undeniable that the Endo-TLIF technique also requires a steep learning curve that requiring a thorough understanding of foraminal anatomy and rich endoscopic experience.
Limitation of Study
Some limitations of the study must be noted. First, the number of patients in the two groups was relatively small, and all patients in the study were only followed for 1 year. So that, a larger sample with a longer follow-up time are needed to make definitive clinical conclusions. Second, a large amount of physiological saline solution was used to rinse the surgical area which causes hidden blood loss, but the hidden blood loss cannot be calculated accurately. Finally, the height of the iliac crest had some influence on patient selection.
Conclusions
In present study, Endo-TLIF technique has shown great advantages in terms of the early curative effect in patients. As a result of the study, the Endo-TLIF technique seems to be a suitable choice for patients with lumbar spondylolisthesis due to its advantages of being minimally invasive and the fact that it can even be performed under local anesthesia. However, intraoperative repeated fluoroscopy usually results in highly cumulative radiation and longer operation time.
Data Sharing Statement
The data sets used and/or analyzed in the current study are available from the corresponding authors upon reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the ethics committee of the Affiliated Hospital of Qingdao University. Written informed consent was obtained from all participants. Meanwhile, all personal details were erased before analysis to cover patient data and comply with the Declaration of Helsinki.
Consent for Publication
All authors have read and approved the content, and they agree to submit it for consideration for publication in the journal.
Acknowledgments
The authors thank all the staff members in the Affiliated Hospital of Qingdao University.
These authors contributed equally to this project and manuscript preparation: Hao Zhang and Chuanli Zhou.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81672200, 81871804) and National Key Research and Development Project (CN) (2019YFC0121400).
Disclosure
The authors confirm that they have no conflicts of interest for this work or with respect to the manuscript content or funding.
References
1. De Kunder SL, Van Kuijk SMJ, Rijkers K, et al. Transforaminal lumbar interbody fusion (TLIF) versus posterior lumbar interbody fusion (PLIF) in lumbar spondylolisthesis: a systematic review and meta-analysis. Spine J. 2017;17(11):1712–1721. doi:10.1016/j.spinee.2017.06.018
2. Bydon M, Alvi MA, Goyal A. Degenerative lumbar spondylolisthesis: definition, natural history, conservative management, and surgical treatment. Neurosurg Clin N Am. 2019;30(3):299–304. doi:10.1016/j.nec.2019.02.003
3. Koreckij TD, Fischgrund JS. Degenerative spondylolisthesis. J Spinal Disord Tech. 2015;28(7):236–241. doi:10.1097/bsd.0000000000000298
4. Ferrero E, Guigui P. Current trends in the management of degenerative lumbar spondylolisthesis. EFORT Open Rev. 2018;3(5):192–199. doi:10.1302/2058-5241.3.170050
5. Bhalla A, Bono CM. Isthmic lumbar spondylolisthesis. Neurosurg Clin N Am. 2019;30(3):283–290. doi:10.1016/j.nec.2019.02.001
6. Li D, Hai Y, Meng X, Yang J, Yin P. Topping-off surgery vs posterior lumbar interbody fusion for degenerative lumbar disease: a comparative study of clinical efficacy and adjacent segment degeneration. J Orthop Surg Res. 2019;14(1):197. doi:10.1186/s13018-019-1245-3
7. Fan SW, Hu ZJ, Fang XQ, Zhao FD, Huang Y, Yu HJ. Comparison of paraspinal muscle injury in one-level lumbar posterior inter-body fusion: modified minimally invasive and traditional open approaches. Orthop Surg. 2010;2(3):194–200. doi:10.1111/j.1757-7861.2010.00086.x
8. Weber BR, Grob D, Dvorak J, Muntener M. Posterior surgical approach to the lumbar spine and its effect on the multifidus muscle. Spine. 1997;22(15):1765–1772. doi:10.1097/00007632-199708010-00017
9. Elias WJ, Simmons NE, Kaptain GJ, Chadduck JB, Whitehill R. Complications of posterior lumbar interbody fusion when using a titanium threaded cage device. J Neurosurg. 2000;93(1 Suppl):45–52. doi:10.3171/spi.2000.93.1.0045
10. Kawaguchi Y, Matsui H, Tsuji H. Back muscle injury after posterior lumbar spine surgery. A histologic and enzymatic analysis. Spine. 1996;21(8):941–944. doi:10.1097/00007632-199604150-00007
11. Goldstein CL, Macwan K, Sundararajan K, Rampersaud YR. Perioperative outcomes and adverse events of minimally invasive versus open posterior lumbar fusion: meta-analysis and systematic review. J Neurosurg Spine. 2016;24(3):416–427. doi:10.3171/2015.2.Spine14973
12. Scheufler KM, Dohmen H, Vougioukas VI. Percutaneous transforaminal lumbar interbody fusion for the treatment of degenerative lumbar instability. Neurosurgery. 2007;60(4Suppl 2):
13. Harms J, Rolinger H. A one-stager procedure in operative treatment of spondylolistheses: dorsal traction-reposition and anterior fusion. Z Orthop Ihre Grenzgeb. 1982;120(3):343–347. doi:10.1055/s-2008-1051624
14. Wu AM, Hu ZC, Li XB, et al. Comparison of minimally invasive and open transforaminal lumbar interbody fusion in the treatment of single segmental lumbar spondylolisthesis: minimum two-year follow up. Ann Transl Med. 2018;6(6):105. doi:10.21037/atm.2018.02.11
15. Wu J, Liu H, Ao S, et al. Percutaneous endoscopic lumbar interbody fusion: technical note and preliminary clinical experience with 2-year follow-up. Biomed Res Int. 2018;2018:5806037. doi:10.1155/2018/5806037
16. Yang J, Liu C, Hai Y, et al. Percutaneous endoscopic transforaminal lumbar interbody fusion for the treatment of lumbar spinal stenosis: preliminary report of seven cases with 12-month follow-up. Biomed Res Int. 2019;2019:3091459. doi:10.1155/2019/3091459
17. Syed H, Voyadzis JM. True percutaneous transforaminal lumbar interbody fusion: case illustrations, surgical technique, and limitations. J Neurol Surg a Cent Eur Neurosurg. 2016;77(4):344–353. doi:10.1055/s-0035-1558821
18. Wang HW, Hu YC, Wu ZY, et al. Minimally invasive transforaminal lumbar interbody fusion and unilateral fixation for degenerative lumbar disease. Orthop Surg. 2017;9(3):277–283. doi:10.1111/os.12345
19. Rothman SL, Glenn WV
20. Chafetz N, Cann CE, Morris JM, Steinbach LS, Goldberg HI, Ax L. Pseudarthrosis following lumbar fusion: detection by direct coronal CT scanning. Radiology. 1987;162(3):803–805. doi:10.1148/radiology.162.3.3809497
21. Ao S, Zheng W, Wu J, et al. Comparison of Preliminary clinical outcomes between percutaneous endoscopic and minimally invasive transforaminal lumbar interbody fusion for lumbar degenerative diseases in a tertiary hospital: is percutaneous endoscopic procedure superior to MIS-TLIF? A prospective cohort study. Int J Surg. 2020;76:136–143. doi:10.1016/j.ijsu.2020.02.043
22. Morgenstern R, Morgenstern C. Percutaneous transforaminal lumbar interbody fusion (pTLIF) with a posterolateral approach for the treatment of degenerative disk disease: feasibility and preliminary results. Int J Spine Surg. 2015;9:41. doi:10.14444/2041
23. Lee SH, Erken HY, Bae J. Percutaneous transforaminal endoscopic lumbar interbody fusion: clinical and radiological results of mean 46-month follow-up. Biomed Res Int. 2017;2017:3731983. doi:10.1155/2017/3731983
24. Shen J. Fully endoscopic lumbar laminectomy and transforaminal lumbar interbody fusion under local anesthesia with conscious sedation: a case series. World Neurosurg. 2019;127:e745–e750. doi:10.1016/j.wneu.2019.03.257
25. Osman SG. Endoscopic transforaminal decompression, interbody fusion, and percutaneous pedicle screw implantation of the lumbar spine: a case series report. Int J Spine Surg. 2012;6:157–166. doi:10.1016/j.ijsp.2012.04.001
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.