Back to Journals » Infection and Drug Resistance » Volume 11
Peptide dendrimers as “lead compounds” for the treatment of chronic lung infections by Pseudomonas aeruginosa in cystic fibrosis patients: in vitro and in vivo studies
Authors Pompilio A , Geminiani C , Mantini P , Siriwardena TN, Di Bonaventura I , Reymond JL, Di Bonaventura G
Received 22 March 2018
Accepted for publication 4 May 2018
Published 11 October 2018 Volume 2018:11 Pages 1767—1782
DOI https://doi.org/10.2147/IDR.S168868
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Joachim Wink
Arianna Pompilio,1,2 Cristina Geminiani,1,2 Paolo Mantini,1,2 Thissa N Siriwardena,3 Ivan Di Bonaventura,3 Jean Louis Reymond,3 Giovanni Di Bonaventura1,2
1Department of Medical, Oral, and Biotechnological Sciences, G d’Annunzio University of Chieti-Pescara, Chieti 66100, Italy; 2Center of Excellence on Aging and Translational Medicine, G d’Annunzio University of Chieti-Pescara, Chieti, Italy; 3Department of Chemistry and Biochemistry, University of Bern, Bern, Switzerland
Aim: In the present work, the potential of the D-enantiomeric dendrimers dG3KL and dTNS18 was evaluated in relation to tobramycin (Tob), for the development of novel antibacterials to treat Pseudomonas aeruginosa chronic lung infections in patients with cystic fibrosis.
Results: The activity of dendrimers against planktonic P. aeruginosa cells was less than Tob against three of the four strains tested (median minimum inhibitory concentration [MIC] 8 vs 1 µg/mL, respectively), but 32-fold higher against the PaPh32 strain isolated at posttransplantation stage. Results from comparative minimum bactericidal concentration/MIC evaluation and time–kill assay suggested a bactericidal mechanism for all test agents. Subinhibitory concentrations of both dendrimers and Tob significantly affected biofilm formation by all strains in a dose-dependent manner, although the PaPh26 strain, isolated during the chronic stage of infection, was particularly susceptible to dendrimers. The activity of dendrimers against preformed P. aeruginosa biofilm was generally comparable to Tob, considering both dispersion and viability of biofilm. Particularly, exposure to the test agent at 10 × MIC caused significant biofilm death (>90%, even to eradication), though with strain-specific differences. Single administration of dendrimers or Tob at 10 × MIC was not toxic in Galleria mellonella wax-moth larvae over 96 hours. However, contrarily to Tob, dendrimers were not protective against systemic infection caused by P. aeruginosa in G. mellonella. Kinetics of P. aeruginosa growth in hemolymph showed that bacterial load increased over time in the presence of dendrimers.
Conclusion: Overall, our findings indicated that dG3KL and dTNS18 peptide dendrimers show in vitro activity comparable to Tob against both P. aeruginosa planktonic and biofilm cells at concentrations not toxic in vivo. Further studies are warranted to explore different dosages and to increase the bioavailability of the peptides to solve the lack of protective effect observed in G. mellonella larvae.
Keywords: cystic fibrosis, chronic infection, Pseudomonas aeruginosa, dendrimer peptides, biofilm
Introduction
CF patients are prone to chronic infection of the respiratory tract, which ultimately leads to pulmonary failure, the primary cause of death in this patient population.1 Pseudomonas aeruginosa is the most prevalent respiratory pathogen in adult CF patients, where it is the main cause of morbidity and mortality.2
Whereas P. aeruginosa eradication cannot be achieved once the infection is fully established in a CF patient, the aim of antimicrobial therapy is a reduction in bacterial density in the respiratory tract.3 In the absence of exacerbation, maintenance treatment with inhaled antibiotics is used, due to the high concentrations reached at the site of infection and the minimal systemic effects. Despite the demonstrated efficacy of regimes based on cycles of nebulized Tob in reduction of bacterial loads, decreasing antibiotic effects are observed over time.4 This unfortunate outcome may be explained not only by the extraordinary capacity of P. aeruginosa to develop resistance through chromosomal mutations5 but also by its high ability to adapt to the CF pulmonary environment by the formation of biofilms, cellular aggregations embedded in extracellular polymeric substances inherently resistant to antibiotic therapy, and host immunity.6 This scenario is further complicated by evidence that at the site of infection, pathogens grow in highly viscous sputum whose composition (eg, extracellular DNA, lipids, proteins) affects both delivery and functionality of antibiotics.7 As such, adequate treatment options are limited and new compounds with potent antibiofilm activity are needed urgently.
The potential of using AMPs as a valid alternative to conventional antibiotics has been recently recognized and studied.8,9 In fact, their generally fast and strong antimicrobial activity also directed toward multidrug-resistant microorganisms, together with antibiofilm activity and the evidence that they are less prone to induce development of resistance compared to conventional antibiotics, make AMPs lead compounds for anti-infective agent development.10–14
Dendrimers are hyperbranched polymeric molecules with an almost-perfect geometrical three-dimensional architecture that confers them functionality completely different from linear polymers.15 The potential of using dendrimers as antimicrobial agents has been recognized over the last 10–15 years. Although they can use different mechanisms of action, generally associated with the multivalence of the branched scaffold, they typically cause increased membrane permeability and consequent bacterial lysis following electrostatic interactions with the negatively charged bacterial membrane of both Gram-positive and Gram-negative bacteria.16,17
In this context, two very interesting dendrimers have been recently obtained: G3KL, a third-generation dendrimer consisting of 37 amino acids, whose structure contains dipeptides (lysine and leucine) coupled via lysine residue, which proved to be active also against carbapenemase-producing P. aeruginosa and Acinetobacter baumannii clinical strains;18,19 and the second-generation dendrimer TNS18, consisting of 18 amino acids with the presence of a lipid, similarly to polymyxin’s structure, displaying a broader antibacterial activity spectrum compared with G3KL, since it also includes MRSA.20 Despite their activity against MDR clinical strains, both dendrimers show lower stability than d-enantiomeric dendrimers in the presence of serum.19,20
The aim of the present study was to evaluate for the first time the potential of the metabolically more stable d-enantiomeric dendrimers dG3KL and dTNS18 as compounds for the development of novel antibacterials to treat lung infections caused by P. aeruginosa in patients affected by CF. Four P. aeruginosa strains, representative of different stages of CF infection, were selected to assess both dendrimers for in vitro activity against planktonic and biofilm cells, as well as in vivo toxic potential and protective effects against P. aeruginosa systemic infection in the Galleria mellonella invertebrate.
Methods
Bacterial strains and growth conditions
The four P. aeruginosa strains tested in the present study were isolated from sputum samples collected by CF patients at different stages of infection: PaPh13 and PaPh14 at the early stage, PaPh26 at the chronic stage, and PaPh32 at the posttransplantation stage. PaPh32 was the only strain showing a mucoid phenotype. All strains were stored at –80°C in a Microbank system (Pro-Lab Diagnostics, Toronto, Canada) until use, when they were aerobically grown at 37°C in TSB (Thermo Fisher Scientific, Waltham, MA, USA), and then twice on MHA (Thermo Fisher Scientific) to check for contamination and regain the original phenotype. All assays were carried out using a standardized bacterial inoculum. Briefly, P. aeruginosa colonies grown overnight on MHA were resuspended in TSB and incubated for 16 hours at 37°C under agitation (150 rpm). Suspension was adjusted to an OD550 of 1 (corresponding to 1–5×108 CFU/mL) and then diluted 1:10 in TSB. Inoculum size was confirmed by cell-viability count on each experiment.
Peptide dendrimers
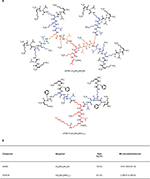
The chemical structure and characteristics of dG3KL and dTNS18 peptide dendrimers are summarized in Figure 1. Synthesis was performed at the Department of Chemistry and Biochemistry, University of Berne with a CEM Liberty Blue automated microwave synthesizer under solid-phase peptide-synthesis conditions at 0.25 mmol using Rink amide SpheriTide (0.19 mmol/g) resin. The resin was swollen in DCM–DMF 1:1 mixture and transferred to a reaction vessel from an HT loader. Deprotection was performed with 20% (v:v) piperidine in DMF for 2 minutes at room temperature and 3 minutes at 75°C. Unprotected amino groups of resin or amino acids were acylated with 3 mL 0.2 M amino acid solution in DMF, 2 mL 1.0 M DIC in DMF, and 2 mL 1.0 M Oxyma Pure (ethyl cyanohydroxyiminoacetate) in DMF for 5 minutes at 75°C. Synthesis was programmed for one deprotection and one coupling (G0), one deprotection and two couplings (G1), one deprotection and four couplings (G2), and two deprotections and eight couplings (G3).
A lipid chain was attached to the dTNS18 with modifications. Fluorenylmethyloxycarbonyl (Fmoc)-d-Lys(Alloc)-OH was attached first to the resin. Before deprotection of the last Fmoc-group, the Alloc protecting group was removed under dry conditions with 0.25 eq Pd(PPh3)4 and 25 eq PhSiH3 in 10 mL dry DCM. This step was repeated twice with washing of the resin with twice-dried DCM in between. After the second cycle, the resin was washed for 1 hour twice with DCM and the lipid chain attached to dendrimer with carboxylic acid (5 eq/amine), Oxyma Pure (5 eq/amine), and DIC (5 eq/amine) in N-methyl-2-pyrrolidone and stirred once overnight and once for 2 hours at room temperature.
Final deprotection was done in 20% piperidine in DMF (2×10 minutes) manually after the synthesis. The resin was washed twice with MeOH and dried under vacuum before cleavage was carried out using TFA–triisopropylsilane–H2O (94:5:1 v:v:v) over 4.5 hours. After filtration, the peptide was precipitated with 50 mL ice cold TBME, centrifuged at 4,400 rpm for 15 minutes, and washed twice with TBME. For purification, the crude peptide was subjected to preparative RP-HPLC. Elution solutions were Milli-Q deionized water containing 0.1% TFA, Milli-Q deionized water–acetonitrile (10:90 v:v) containing 0.1% TFA, and purified compound obtained as TFA salt after lyophilization.
Tob
Tob as powder with known potency was from Sigma-Aldrich Co. (St Louis, MO, USA). For each test agent, a stock solution was prepared at 10 mg/mL in Milli-Q reagent water, 0.22 µm-filtered, and finally stored at −80°C until use.
MIC and MBC measurement
The lowest concentration of the test agent that completely inhibited visible bacterial growth (MIC) was determined in CAMHB (BD, Franklin Lakes, NJ, USA) by microdilution according to Clinical Laboratory Standards Institute guidelines.21 P. aeruginosa ATCC 27853 was chosen as the quality-control strain in each batch of tests. Following MIC reading, the lowest concentration of the test agent killing at least 99.99% of the original inoculum (MBC) was measured by plating 100 µL broth from clear wells on MHA plates and incubation at 37°C for 24 hours for CFU count.22
Time–kill assay
Kinetics of both peptide dendrimers and Tob activity against P. aeruginosa was evaluated by broth macrodilution. Briefly, the standardized inoculum (1–2×106 CFU/mL) was exposed to the test agent at MIC in CAMHB and incubated at 37°C in a spectrophotometric reader (SpectraMax Plus 384; Molecular Devices LLC, Sunnyvale, CA, USA). OD550 readings were performed every 15 minutes up to 24 hours, when a viable-cell count was performed by seeding the entire content of the wells where no growth was recorded.
Screening for biofilm formation
Each P. aeruginosa strain was evaluated for the ability to form biofilm over 48 hours using a microtiter-plate method. Briefly, 200 µL standardized inoculum was aliquoted in each well of a 96-well polystyrene, flat-bottomed, tissue-culture-treated microtiter (Kartell, Milan, Italy) and statically incubated at 37°C. Following 24 and 48 hours’ incubation, biofilm samples were washed twice with PBS (EuroClone, Pero, Italy), scraped with a pipette tip following 5 minutes’ exposure to 100 µL trypsin-EDTA 0.25% (Sigma-Aldrich), and then underwent tenfold dilutions for colony count onto MHA.
In vitro activity against biofilm formation
In each well of a 96-well flat-bottomed polystyrene microtiter plate, 5 µL standardized inoculum was added to 100 µL CAMHB containing test agent at concentrations equal to 1/2×, 1/4×, and 1/8× MIC. After incubation at 37°C for 24 hours, nonadherent bacteria were removed by washing twice with 100 µL sterile PBS (pH 7.2). Slime and adherent cells were fixed by incubation for 1 hour at 60°C and stained for 5 minutes at room temperature with 100 µL 1% crystal violet solution (Sigma-Aldrich). Wells were then rinsed with distilled water and dried at 37°C for 30 minutes. Biofilms were destained by treatment with 100 µL 33% glacial acetic acid (Sigma-Aldrich) for 15 minutes and OD492 measured (Sunrise; Tecan, Männedorf, Switzerland). The low cutoff was represented by approximately three SDs above the mean OD492 of control wells (containing medium alone without bacteria). The percentage of inhibition was calculated as (1 – OD492 of test/OD492 of untreated control) ×100.23
In vitro activity against preformed biofilm
Biofilm was allowed to grow for 24 hours in each well of a 96-well microtiter plate, as described in the “Screening for biofilm formation” section. Next, biofilm was exposed to each test agent at concentrations of 1–16× MIC and prepared in CAMHB. Following 24-hour exposure at 37°C, the effect against mature biofilm was evaluated both by crystal violet (dispersion of biofilm biomass) and colony count (biofilm viability) assays.
In vivo toxicity assay
The toxicity of dendrimers and Tob was comparatively assessed in G. mellonella wax-moth larvae.24 For each group, 20 larvae weighing 250–350 mg were injected using a Hamilton syringe (Sigma-Aldrich) directly into the hemocoel via the left proleg with 10 µL test agent at a concentration of 10 × MIC in distilled H2O. Control larvae were inoculated with distilled water only. Larvae were incubated at 37°C, and the number of dead caterpillars was counted every 24 hours until 96 hours, considering as dead those unresponsive to touch.24
In vivo protection studies
Firstly, an inoculum test was performed to determine the optimum inoculum for staggered larval killing (ie, LD50 at 24 hours postinoculation). G. mellonella larvae (n=20/group) were infected with several infectious doses (102, 103, 104, 105, and 106 CFU/larvae) via a 10 µL injection into the left proleg, then incubated at 37°C and scored for survival at 24, 48, 72, and 96 hours. Control larvae were administrated with vehicle (PBS) only.
Next, in the protection studies, larvae (n=20/group) were infected with P. aeruginosa strains, each at relative LD50 (5×105 CFU/larva and 104 CFU/larva, respectively, for PaPh26 and PaPh32 strains; 10 CFU/larva both for PaPh13 and PaPh14 strains). After 30 minutes, a second injection into the hemocoel via the right proleg of dendrimer or Tob was administered, each at 10 × MIC. Control larvae were infected, but exposed to H2O only. Larvae were incubated at 37°C and scored for survival at 24, 48, 72, and 96 hours.
Kinetics of bacterial growth in hemolymph
G. mellonella larvae were infected with P. aeruginosa and 30 minutes later administered dendrimer or Tob at 10 × MIC, as previously described. At 4 and 12 hours posttreatment, G. mellonella larvae were anesthetized by placing them in ice for 5 minutes until no leg movement could be observed, and then, hemolymph was collected following incision between two segments near the head. Quantification of P. aeruginosa within pooled hemolymph samples from five larvae was done by preparing serial dilutions and enumerating by total viable count onto cetrimide agar (Thermo Fisher Scientific). Saturated thiourea was added to hemolymph to prevent melanization.
Statistical analysis
All experiments were performed at least in triplicate and repeated on at least two occasions. Differences between MIC or MBC values were considered significant for discrepancies >1-log2 concentration steps. Statistical significance of differences was evaluated using ANOVA followed by Tukey’s multiple-comparison test (for normally distributed data), unpaired t-test (kinetics of bacterial growth in hemolymph), or by Fisher’s exact test (proportions). Survival curves were analyzed using the log-rank (Mantel–Cox) test. Statistical analysis of results was conducted with GraphPad Prism 4.00 (GraphPad Software, Inc., La Jolla, CA, USA), considering as statistically significant P<0.05.
Results
Activity against planktonic cells: MIC, MBC
Susceptibility-test results are summarized in Table 1. Dendrimers dG3KL and dTNS18 showed comparable activity (median MIC and MBC 8 µg/mL), except for the PaPh13, strain whose MIC and MBC values were significantly (>1 log2) lower for dTNS18. Tob was significantly more active compared to the tested peptides (median MIC 1 µg/mL), although strain-dependent activity was observed. In particular, Tob was less active than either dendrimer against PaPh32, showing an MIC value ≥32-fold higher than both. MBC values for both peptides and Tob were within 1-log2 dilution compared to respective MIC values, suggesting bactericidal activity for all test agents.
Time–kill
The effect of both peptide dendrimers on P. aeruginosa growth was assessed comparatively to Tob, monitoring bacterial growth over 24 hours following a single exposure at MIC (Figure 2). Viable cell counts were performed after 24 hours’ incubation to check for eradication. Exposure to dG3KL allowed regrowth for P. aeruginosa PaPh14 and PaPh32 strains only, after 21 and 11 hours’ incubation, respectively. Bacterial counts performed after 24 hours’ incubation showed eradication of PaPh13, but the presence of PaPh26 in low numbers (<350 CFU/mL). In contrast, no growth was observed following dTNS18 exposure, regardless of the strain considered. Cell-viability counts performed following 24 hours’ incubation confirmed the eradication of PaPh14 and PaPh32 strains, whereas PaPh13 and PaPh26 were found to be present, although at very low concentrations (<50 CFU/mL). Tob caused a delay in bacterial growth of 13 and 8 hours for PaPh13 and PaPh14 strains, respectively. No growth was observed over 24 hours for PaPh26, although a bacterial load of 3.5×102 CFU/mL was achieved.
Screening for biofilm formation
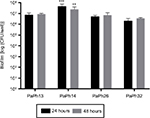
Kinetics of biofilm formation onto polystyrene by P. aeruginosa strains were evaluated by viable cell count, and results are summarized in Figure 3. All strains produced biofilm throughout 48 hours, although the cellularity of PaPh14 was significantly higher than other strains, regardless of the incubation time considered (4.7+2.9×107 and 2.5+1.6×107 CFU/well, respectively, for 24 and 48 hours; P<0.01 vs other strains). Since the cellularity of biofilm did not change significantly over 48 hours, to evaluate the activity of peptides on mature biofilm, a biofilm preformed for 24 hours was exposed to each peptide for a further 24 hours.
Effect of subinhibitory concentrations against biofilm formation
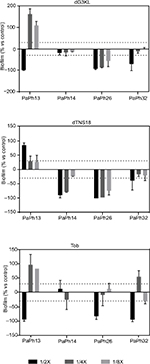
To evaluate the effect of peptides in preventing biofilm formation by P. aeruginosa, subinhibitory concentrations of each peptide were tested against biofilm formation, and results are shown in Figure 4. Considering the proportion of cases where biofilm formation was significantly reduced in the presence of the test agent, comparable activity was observed for both dendrimers and Tob, with 50% (six of 12 cases) for dTNS18, 41.6% (five of 12) for dG3KL, and 33% (four of 12) for Tob. Reductions were significantly higher in the presence of 1/2 × MIC (75% vs 25% and 25%, respectively, for 1/2 ×, 1/4 ×, and 1/8 × MIC; P<0.05), thus indicating a dose-dependent antibiofilm effect. Particularly susceptible to the effect of dendrimers was PaPh26, isolated during the chronic stage of infection, whose biofilm formation was affected regardless of the concentration tested. Although in a minor number of cases, exposure to peptides at sub-MIC caused a significant increase in biofilm-biomass formation compared to untreated control samples. This effect was not dependent on dose or strain considered.
Biofilm dispersion caused by inhibitory concentrations
The effect of peptides at MIC and its multiples on the dispersion of preformed biofilm by P. aeruginosa was assessed comparatively to Tob, and results are shown in Figure 5. Considering the number of cases where preformed biofilm was dispersed significantly following treatment, Tob caused more of reductions compared to dendrimers, although differences were not statistically significant (60% vs 35% and 35%, respectively). The effect was not dependent on the concentration tested. In contrast, strain-dependent activity was observed for both Tob (PaPh13 and PaPh26) and dG3KL (PaPh13). In a comparable number of cases, the exposure to Tob or peptides caused a significant increase of biofilm biomass formation compared to untreated control samples. Increases were comparable for all molecules (35% vs 30% vs 26.7%, respectively, for dTNS18, dG3KL, and Tob). The effect was not dependent on both concentration or strain tested.
Effect against viability of preformed biofilm
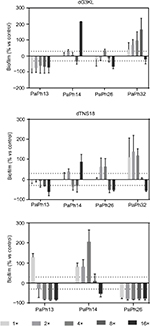
The effect of peptides at bactericidal concentration (10 × MIC) on the viability of preformed biofilm by P. aeruginosa was evaluated comparatively to Tob using viable cell counts, and results are shown in Figure 6. Exposure to Tob always caused a significant reduction in biofilm viability compared to unexposed controls (killing ≥99.96%). This effect was generally comparable to that provoked by peptides (killing range 90.2%–100% and 90.6%–100%, respectively, for dG3KL and dTNS18), with the exception of PaPh14, whose biofilm cellularity was not affected by exposure to peptides compared to unexposed controls, but decreased significantly following treatment with Tob (1.6±0.1×107 vs 1.4±0.5×107 vs 1.4±0.2×103 CFU/well, respectively, for dG3KL, dTNS18, and Tob; P<0.001).
In vivo toxicity
To assess the toxic potential associated to the bactericidal dose used in biofilm assays, G. mellonella larvae were administered 10 × MIC of each peptide and Tob and a survival curve plotted, as shown in Figure 7. Overall, no significant differences were observed in the survival rate of peptide- or Tob-administrated larvae by log-rank (Mantel–Cox) test. In particular, on day 1 PE, both peptides caused the same mortality rate observed in control unexposed larvae (two of 40 larvae, 5%). The same mortality rate was observed in Tob-exposed larvae on day 2 PE, where dG3KL and dTNS18 provoked death in 15% and 10% of the larvae population. On day 3 PE, the mortality rate associated with Tob increased to 10%. No death was observed on day 4 PE.
In vivo infection assays
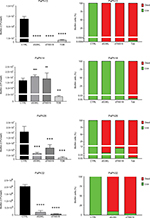
To determine whether G. mellonella larvae were a suitable model to study the protective effect of peptide dendrimers against P. aeruginosa infection, we first defined its infection characteristics. The effect of infection with P. aeruginosa PaPh13, PaPh14, PaPh26, and PaPh32 on G. mellonella larvae survival is shown in Figure 8. Overall, our results show that G. mellonella was susceptible to P. aeruginosa infection. Particularly, P. aeruginosa PaPh13 and PaPh14 were the most virulent strains, causing killing ≥97.5% within 24 hours, regardless of dose administered. In contrast, the killing rate was significantly dependent on the number of cells injected in a dose-dependent manner for P. aeruginosa PaPh26 and PaPh32. PaPh32 caused a death rate ≥95% when tested at doses of 106 and 105 CFU/larva, whereas a comparable killing rate was achieved by PaPh26 only at 106 CFU/larva. Based on these results, we chose the infectious doses to be used in protective studies: 10 CFU/larva (PaPh13 and PaPh14), 5×105 CFU/larva (PaPh26), and 104 CFU/larva (PaPh32).
In vivo protective studies
The protective effect of peptides against P. aeruginosa infection was evaluated comparatively to Tob in G. mellonella by monitoring survival over 96 hours following administration of each molecule at 10 × MIC (Figure 9). Treatment with peptides provided no protection against P. aeruginosa infection, as indicated by mortality rates comparable with the positive control (infected but not treated). In contrast, treatment with Tob allowed significant protection against P. aeruginosa infection compared to untreated larvae, regardless of the strain tested (P<0.01, P<0.001, and P<0.0001, respectively, for PaPh13, PaPh14, and PaPh26 vs controls).
Kinetics of bacterial growth in hemolymph
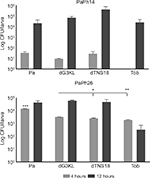
The antibacterial activity of dendrimers was assessed over 12 hours compared to Tob in pooled hemolymph from G. mellonella larvae infected with PaPh14 and PaPh26, selected on the basis of their different virulence, as previously described in the “In vivo infection assays” section (Figure 10). Overall, bacterial load increased over time in the presence of dendrimers, regardless of the strain tested. A similar trend was observed for Tob, except for PaPh26, where a decrease was observed. The kinetics of bacterial killing were strain-dependent.
No significant differences were observed between treated and control groups in larvae infected by PaPh14. Conversely, when larvae were infected with PaPh26, the bacterial load measured at 4 hours posttreatment in control larvae was significantly higher than in treated larvae, regardless of the molecules considered (1.5±0.01×104 vs 3.4±0.1×103, 2.6±0.2×103, and 1.9±0.1×103 CFU/larva, respectively for untreated, dG3KL-treated, dTNS18-treated, and Tob-treated larvae; P<0.0001). Further, P. aeruginosa concentration in dG3KL-infected larvae was significantly higher compared with dTNS18 (P<0.05) and Tob (P<0.01). Following 12 hours, the bacterial load was reduced after exposure to Tob compared to untreated larvae, although not to a statistically significant extent.
Discussion
Representing the defense system of multicellular organisms,25–27 AMPs offer an interesting alternative to antibiotic therapy in addressing antibiotic-resistant strains, especially in CF patients, where increasing incidence of MDR P. aeruginosa strains poses significant challenges for clinicians.28 These peptides typically contain up to 50 amino acids with many basic residues (lysine or arginine) and at least 30% hydrophobic side chains29 and act mostly by folding into amphipathic conformations, inducing membrane disruption, similarly to various peptidomimetic systems.30–32
Amino-acid-sequence variations in linear and cyclic AMPs have been explored widely, although alternative topologies of the peptide chain have not been studied extensively for AMP design. Dendrimer topology has only been used as a tool to achieve multivalence of preexisting AMP sequences or single amino acids, typically by attachment to a dendritic polylysine tree.33,34 By exploring unusual multibranched topologies of the peptide chain, such as peptide dendrimers and bicyclic peptides, and extending on previous reports that multivalent display of AMPs on a polylysine tree can increase their antimicrobial activity,33,34 Stach et al and Siriwardena et al recently identified two AMPs, dG3KL and dTNS18, with broad antibacterial activity, including MDR strains.19,20
dG3KL is a third-generation dendrimer consisting of 37 amino acids with a lysine–leucine dipeptide repeated across the dendrimer branches.19 The leucine residues not only enhance the hydrophobicity of G3KL but also enable medium-dependent conformational changes, which might contribute to the antimicrobial effect.19 dTNS18 is a second-generation dendrimer of 18 amino acids with the presence of a lipid, similar to polymyxin structure.20 Both peptides are stable in serum, and their mechanism of action consists in the interaction with the negatively charged lipids of the membrane, enabling its disruption with consequent rapid bacterial killing. Further, their activity is not affected by the lipopolysaccharide layer, an efficient barrier against hydrophobic compounds, as indicated by unchanged activity against lipopolysaccharide-mutant strains of P. aeruginosa.19,20
In this study, we evaluated (using both in vitro and in vivo assays) for the first time the potential of dG3KL and dTNS18 as compounds for the development of novel antibacterials to treat lung infections caused by P. aeruginosa in CF patients. With this aim, four P. aeruginosa strains were selected and isolated at different stages of CF infection: PaPh13 and PaPh14 at the early stage, PaPh26 at the chronic stage, and PaPh32, the only one showing a mucoid phenotype, at the posttransplantation stage.
First, dendrimers were evaluated comparatively to Tob for in vitro activity against planktonic cells of P. aeruginosa CF strains. Our results indicated that both dendrimers showed comparable activity against most P. aeruginosa strains (median MIC 8 µg/mL), although dTNS18 was more effective against PaPh13, since it was active at a concentration 3-log2 lower than dG3KL. Tob was generally more active than dendrimers (median MIC 1 µg/mL), although strain-dependent activity was observed. In the case of PaPh32 – the only one showing a mucoid phenotype among strains tested – both dendrimers were at last 32-fold more active than Tob, as indicated by MIC values. This finding is particularly relevant, since P. aeruginosa isolated from CF patients often harbors a mucoid phenotype, related to the overproduction of extracellular polysaccharides, among which alginate contributes to the chronicity of infections, since it affects the activity of several classes of antibiotics.35
MBC values were always within 1-log2 dilution compared to MIC for both dendrimers and Tob, indicating a probable bactericidal mechanism of action at the site of infection. Time–kill results confirmed a bactericidal effect particularly relevant in the case of dendrimers, which caused significantly more delayed regrowth compared to Tob and even eradication. Our results are in agreement with Stach et al and Siriwardena et al,19,20 who reported on dG3KL and dTNS18 activity against MDR P. aeruginosa strains, although they observed a relevant strain-dependent effect, as suggested by the wide range in MIC values (4–32 µg/mL).
During chronic colonization, pathogens adapt over time to cope with changing selection pressures, coinfecting species, and antibiotic therapies.35 In the case of the CF lung, these adaptations are induced by environmental pressures, such as inflammatory responses, hypoxia, nutrient deficiency, osmolarity, low pH, and repeated cycles of antibiotic therapy. Several studies have clearly shown that the long-term persistence of P. aeruginosa in the airways of CF patients is associated with complex and finely tuned mechanisms of adaptation.36–40 Among these, a highly successful survival strategy of P. aeruginosa, as well as for other CF pathogens,41,42 involves the production of biofilms, multilayered microbial communities adhered to a substratum whose formation is finely regulated by a “quorum-sensing” system comprising networks of genes and regulators.43 In this state, bacteria are surrounded by a dense extracellular polymeric matrix, mainly constituted of bacterial polysaccharides, which protects them against the inflammatory defense mechanism (mainly phagocytosis) and prevents penetration throughout the biofilm of antibiotic agents, making them 100- to 1,000-fold more tolerant to antimicrobial agents than planktonic counterparts.44,45
Confirming the high ability of P. aeruginosa to adapt to the CF lung, all strains tested in the present study were able, though with striking differences, to form stable biofilm over 48 hours using a microtiter plate. The potential of dendrimers for prophylactic use in preventing biofilm formation was assessed at doses lower than MIC using the crystal violet assay to measure biofilm biomass. Comparative evaluation of the percentage of cases where exposure to the test agent caused a significant reduction in biofilm biomass compared to controls indicated that both peptides exhibited activity similar to Tob. Antibiofilm activity was dose-dependent, with maximum activity observed following exposure to 1/2 × MIC, and also strain-specific, with chronic PaPh26 the most susceptible among those tested.
Exposure to peptides at sub-MIC values also caused a significant increase in biofilm-biomass formation compared to untreated control samples, although in a minor number of cases. As previously observed for some antibiotics46–48 and human LL37 and bovine BMAP27 and BMAP28 cathelicidins,10,49 biofilm-viability enhancement in the presence of subinhibitory concentrations of peptides might be due to the upregulation of specific pathways using antimicrobial compounds as activating signals or to the triggering of a bacterial stress response inducing the bacteria to develop biofilm as a resistance form. These findings highlight once again the common need to modulate precisely the amount of antibiotic compound to be administered, in order to avoid detrimental side effects during antimicrobial therapy.
To assess their therapeutic potential for the treatment of chronic biofilm-associated P. aeruginosa infections in the CF lung, dG3KL and dTNS18 were also tested at multiples of MIC against preformed P. aeruginosa biofilm. At first, we evaluated the effect on the dispersal of biofilm biomass, consisting of both cells and extracellular matrix, by crystal violet assay. Exposure to Tob caused dispersal at a percentage higher than dendrimers, although differences were not significant. Next, we evaluated cell-viability to assess the effect of exposure to 10 × MBC on the viability of preformed biofilm. Both dendrimers were effective in reducing the viability of preformed biofilms, even until eradication (killing rate 90.2%–100%), with the exception of PaPh14, whose biofilm was intrinsically resistant. In contrast to dendrimers, Tob was effective against all strains tested, with a killing rate near eradication (≥99.96%). Inherent resistance shown by P. aeruginosa PaPh14 biofilm to peptides deserves further study to elucidate the underlying mechanisms. We can speculate that dendrimer activity could be affected by the high cellularity of PaPh14 biofilm, the highest among strains tested. Further, excessive amounts of extracellular DNA – an important component in P. aeruginosa biofilm50,51 – could sequester dendrimers, due to its high affinity with cationic AMPs.52
The clinical relevance of the antibiofilm effects exhibited in vitro by dendrimers was further investigated by assessing toxic potential associated with bactericidal concentrations we used against preformed biofilms. Although cultured cells are often used to estimate chemical doses for subsequent rodent acute-toxicity tests, this model cannot simulate pharmacokinetics associated with metabolism of the compound, making it poorly predictive. G. mellonella – the greater wax moth – has been recently introduced as an alternative and highly predictive model to study bacterial diseases, antimicrobial drug testing, and acute toxicity of drugs.53–56 Although this invertebrate does not have lungs, it has been frequently used to gain new knowledge in the pathogenesis of human lung infections, including those observed in CF patients.56,57 Benefits associated with the use of G. mellonella over traditional mammalian models (low costs, presence of sophisticated cellular and humoral defenses very similar to innate immunoresponses of mammals, larvae can be easily maintained and infected by injection without anesthesia, not subject to ethical limitations of mammalian models, larvae can be maintained at 37°C, well suited for study of human pathogens) makes this model useful for prescreening evaluation of the efficacy of antimicrobial agents, thus lowering the number of test agents proceeding to confirmatory tests in rodent models.57 In the present study, we administered G. mellonella larvae with 10 × MIC of each peptide and compared with Tob, and a survival curve over 4 days was plotted. Overall, our results indicated that dendrimers were not toxic, since no significant differences were observed in survival rates of peptide- or Tob-administrated larvae, as indicated by the log-rank (Mantel–Cox) test. In accordance with our findings, Stach et al19 found that dG3KL combined good broad-spectrum activity with >200-fold selectivity against hemolysis.
The development of new antimicrobial agents requires confirmation of their efficacy in vivo. In this context, it was recently found that TNS18 shows promising activity in a murine infection model with MDR clinical isolates of A. baumannii and Escherichia coli, providing the first evidence that AMPs might be amenable to in vivo use.20 In spite of their good in vitro antibacterial activity and lack of in vivo toxicity, neither dendrimer was able to protect G. mellonella larvae against systemic infection caused by P. aeruginosa. Tob showed a protective effect against P. aeruginosa infection compared to untreated larvae, regardless of the strain tested. The inability of peptides to provide protection against P. aeruginosa infection might be explained by the scarce stability of the peptides in G. mellonella, since they were inactive already within 4 hours posttreatment, as suggested by kinetic results of bacterial growth in hemolymph. Peptide inactivation is probably not due to degradation by host or bacterial proteases, since both dG3KL and dTNS18 contain only d-amino acids. Confirming the stability of dG3KL, Stach et al recently reported that contrarily to its precursor G3KL, it is not degraded in the presence of human serum.19 Numerous other factors could be responsible for the lack of pronounced activity following therapy with dendrimers, including the virulence of the strains used and/or the in vivo distribution and bioavailability of the peptide.
Conclusion
The results from the present study indicate for the first time that dG3KL and dTNS18 peptide dendrimers have good in vitro activity against both P. aeruginosa planktonic and biofilm cells at concentrations not toxic in vivo. Despite these promising results, the lack of protective effect observed in G. mellonella larvae suggests that these peptides have some pharmacokinetic limitations that need to be identified and eliminated in order to improve their effectiveness. Further studies are warranted to explore different dosages and to increase the bioavailability of the peptides. Finally, it is worth considering that the rapid synthesis and use of standard amino acids only (especially in the case of dTNS18, only half the size of dG3KL), as well as the slow resistance development,19 present further significant advantages for the potential development of AMPs as a new class of antimicrobial agents.
Abbreviation list
AMPs, antimicrobial peptides
CAMHB, cation-adjusted Mueller–Hinton broth
CF, cystic fibrosis
DCM, dichloromethane
DIC, N,N’-diisopropyl carbodiimide
DMF, N,N-dimethylformamide
MBC, minimum bactericidal concentration
MDR, multidrug-resistant
MHA, Mueller–Hinton agar
MIC, minimum inhibitory concentration
MRSA, methicillin-resistant Staphylococcus aureus
TFA, trifluoroacetic acid
TSB, trypticase soy broth
tBME, tert-butyl methyl ether
Tob, tobramycin
PE, postexposure
LD50, lethal dose 50
Acknowledgments
The authors thank Ersilia Fiscarelli (Bambino Gesù Hospital of Rome) for providing us with strains. This study was financially supported by a grant from G. d’Annunzio University of Chieti-Pescara (FAR – ex60%; 2016). Dr IDB is an employee in Pepscan, Lelystad, The Netherlands.
Author contributions
All authors contributed toward data analysis and drafting and revising the paper and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Ciofu O, Hansen CR, Høiby N. Respiratory bacterial infections in cystic fibrosis. Curr Opin Pulm Med. 2013;19(3):251–258. | ||
Cox MJ, Allgaier M, Taylor B, et al. Airway microbiota and pathogen abundance in age-stratified cystic fibrosis patients. PLoS One. 2010;5(6):e11044. | ||
Cantón R, Máiz L, Escribano A, et al. Spanish consensus on the prevention and treatment of Pseudomonas aeruginosa bronchial infections in cystic fibrosis patients. Arch Bronconeumol. 2015;51(3):140–150. | ||
Ramsey BW, Pepe MS, Quan JM, et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. N Engl J Med. 1999;340(1):23–30. | ||
Kerem E. Cystic fibrosis: priorities and progress for future therapies. Paediatr Respir Rev. 2017;24:14–16. | ||
Olsen I. Biofilm-specific antibiotic tolerance and resistance. Eur J Clin Microbiol Infect Dis. 2015;34(5):877–886. | ||
Hunt BE, Weber A, Berger A, Ramsey B, Smith AL. Macromolecular mechanisms of sputum inhibition of tobramycin activity. Antimicrob Agents Chemother. 1995;39(1):34–39. | ||
Aoki W, Ueda M. Characterization of antimicrobial peptides toward the development of novel antibiotics. Pharmaceuticals (Basel). 2013;6(8):1055–1081. | ||
Roscia G, Falciani C, Bracci L, Pini A. The development of antimicrobial peptides as new antibacterial drugs. Curr Protein Pept Sci. 2013;14(8):641–649. | ||
Mardirossian M, Pompilio A, Crocetta V, et al. In vitro and in vivo evaluation of BMAP-derived peptides for the treatment of cystic fibrosis-related pulmonary infections. Amino Acids. 2016;48(9):2253–2260. | ||
Batoni G, Maisetta G, Brancatisano FL, Esin S, Campa M. Use of antimicrobial peptides against microbial biofilms: advantages and limits. Curr Med Chem. 2011;18(2):256–279. | ||
Kang SJ, Park SJ, Mishig-Ochir T, Lee BJ. Antimicrobial peptides: therapeutic potentials. Expert Rev Anti Infect Ther. 2014;12(12):1477–1486. | ||
Pompilio A, Crocetta V, Scocchi M, et al. Potential novel therapeutic strategies in cystic fibrosis: antimicrobial and anti-biofilm activity of natural and designed α-helical peptides against Staphylococcus aureus, Pseudomonas aeruginosa, and Stenotrophomonas maltophilia. BMC Microbiol. 2012;12:145. | ||
Pompilio A, Scocchi M, Pomponio S, et al. Antibacterial and anti-biofilm effects of cathelicidin peptides against pathogens isolated from cystic fibrosis patients. Peptides. 2011;32(9):1807–1814. | ||
Mccarthy TD, Karellas P, Henderson SA, et al. Dendrimers as drugs: discovery and preclinical and clinical development of dendrimer-based microbicides for HIV and STI prevention. Mol Pharm. 2005;2(4):312–318. | ||
García-Gallego S, Franci G, Falanga A, et al. Function oriented molecular design: dendrimers as novel antimicrobials. Molecules. 2017;22(10):E1581. | ||
Scorciapino MA, Serra I, Manzo G, Rinaldi AC. Antimicrobial dendrimeric peptides: structure, activity and new therapeutic applications. Int J Mol Sci. 2017;18(3):E542. | ||
Pires J, Siriwardena TN, Stach M, et al. In vitro activity of the novel antimicrobial peptide dendrimer G3KL against multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2015;59(12):7915–7918. | ||
Stach M, Siriwardena TN, Köhler T, van Delden C, Darbre T, Reymond JL. Combining topology and sequence design for the discovery of potent antimicrobial peptide dendrimers against multidrug-resistant Pseudomonas aeruginosa. Angew Chem Int Ed Engl. 2014;53(47):12827–12831. | ||
Siriwardena TN, Stach M, He R, et al. Lipidated peptide dendrimers killing multidrug-resistant bacteria. J Am Chem Soc. 2018;140(1):423–432. | ||
Clinical Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. 26th ed. Wayne (PA): CLSI; 2016. | ||
Clinical Laboratory Standards Institute [webpage on the Internet]. Methods for determining bactericidal activity of antimicrobial agents; approved guideline. 1999. Available from: http://demo.nextlab.ir/getattachment/096a51d4-1530-4f81-92c0-f5477c584b9b/CLSI-M26-A.aspx. Accessed July 31, 2018. | ||
Stepanović S, Vuković D, Hola V, et al. Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS. 2007;115(8):891–899. | ||
Desbois AP, Coote PJ. Utility of greater wax moth larva (Galleria mellonella) for evaluating the toxicity and efficacy of new antimicrobial agents. Adv Appl Microbiol. 2012;78:25–53. | ||
Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415(6870):389–395. | ||
Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3(3):238–250. | ||
Fjell CD, Hiss JA, Hancock RE, Schneider G. Designing antimicrobial peptides: form follows function. Nat Rev Drug Discov. 2011;11(1):37–51. | ||
Stefani S, Campana S, Cariani L, et al. Relevance of multidrug-resistant Pseudomonas aeruginosa infections in cystic fibrosis. Int J Med Microbiol. 2017;307(6):353–362. | ||
Nguyen LT, Haney EF, Vogel HJ. The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol. 2011;29(9):464–472. | ||
Chongsiriwatana NP, Patch JA, Czyzewski AM, et al. Peptoids that mimic the structure, function, and mechanism of helical antimicrobial peptides. Proc Natl Acad Sci U S A. 2008;105(8):2794–2799. | ||
Tew GN, Scott RW, Klein ML, Degrado WF. De novo design of antimicrobial polymers, foldamers, and small molecules: from discovery to practical applications. Acc Chem Res. 2010;43(1):30–39. | ||
Hayouka Z, Chakraborty S, Liu R, Boersma MD, Weisblum B, Gellman SH. Interplay among subunit identity, subunit proportion, chain length, and stereochemistry in the activity profile of sequence-random peptide mixtures. J Am Chem Soc. 2013;135(32):11748–11751. | ||
Tam JP, Lu YA, Yang JL. Antimicrobial dendrimeric peptides. Eur J Biochem. 2002;269(3):923–932. | ||
Mintzer MA, Dane EL, O’Toole GA, Grinstaff MW. Exploiting dendrimer multivalency to combat emerging and re-emerging infectious diseases. Mol Pharm. 2012;9(3):342–354. | ||
Cullen L, McClean S. Bacterial adaptation during chronic respiratory infections. Pathogens. 2015;4(1):66–89. | ||
Folkesson A, Jelsbak L, Yang L, et al. Adaptation of Pseudomonas aeruginosa to the cystic fibrosis airway: an evolutionary perspective. Nat Rev Microbiol. 2012;10(12):841–851. | ||
Sousa AM, Pereira MO. Pseudomonas aeruginosa diversification during infection development in cystic fibrosis lungs: a review. Pathogens. 2014;3(3):680–703. | ||
Kirisits MJ, Prost L, Starkey M, Parsek MR. Characterization of colony morphology variants isolated from Pseudomonas aeruginosa biofilms. Appl Environ Microbiol. 2005;71(8):4809–4821. | ||
Clark ST, Diaz Caballero JD, Cheang M, et al. Phenotypic diversity within a Pseudomonas aeruginosa population infecting an adult with cystic fibrosis. Sci Rep. 2015;5:10932. | ||
Oliver A. Mutators in cystic fibrosis chronic lung infection: prevalence, mechanisms, and consequences for antimicrobial therapy. Int J Med Microbiol. 2010;300(8):563–572. | ||
Pimentel de Araujo FP, D’Ambrosio F, Camilli R, et al. Characterization of Streptococcus pneumoniae clones from paediatric patients with cystic fibrosis. J Med Microbiol. 2014;63(Pt 12):1704–1715. | ||
Pompilio A, Pomponio S, Crocetta V, et al. Phenotypic and genotypic characterization of Stenotrophomonas maltophilia isolates from patients with cystic fibrosis: genome diversity, biofilm formation, and virulence. BMC Microbiol. 2011;11:159. | ||
Singh PK, Schaefer AL, Parsek MR, Moninger TO, Welsh MJ, Greenberg EP. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature. 2000;407(6805):762–764. | ||
Sriramulu DD, Lünsdorf H, Lam JS, Römling U. Microcolony formation: a novel biofilm model of Pseudomonas aeruginosa for the cystic fibrosis lung. J Med Microbiol. 2005;54(Pt 7):667–676. | ||
Mah TF, O’Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9(1):34–39. | ||
Kaplan JB. Antibiotic-induced biofilm formation. Int J Artif Organs. 2011;34(9):737–751. | ||
Wu S, Li X, Gunawardana M, et al. Beta-lactam antibiotics stimulate biofilm formation in non-typeable Haemophilus influenzae by up-regulating carbohydrate metabolism. PLoS One. 2014;9(7):e99204. | ||
Hsu CY, Lin MH, Chen CC, et al. Vancomycin promotes the bacterial autolysis, release of extracellular DNA, and biofilm formation in vancomycin-non-susceptible Staphylococcus aureus. FEMS Immunol Med Microbiol. 2011;63(2):236–247. | ||
Limoli DH, Rockel AB, Host KM, et al. Cationic antimicrobial peptides promote microbial mutagenesis and pathoadaptation in chronic infections. PLoS Pathog. 2014;10(4):e1004083. | ||
Bjarnsholt T, Jensen PØ, Fiandaca MJ, et al. Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatr Pulmonol. 2009;44(6):547–558. | ||
Montanaro L, Poggi A, Visai L, et al. Extracellular DNA in biofilms. Int J Artif Organs. 2011;34(9):824–831. | ||
Hale JD, Hancock RE. Alternative mechanisms of action of cationic antimicrobial peptides on bacteria. Expert Rev Anti Infect Ther. 2007;5(6):951–959. | ||
Allegra E, Titball RW, Carter J, Champion OL. Galleria mellonella larvae allow the discrimination of toxic and non-toxic chemicals. Chemosphere. 2018;198:469–472. | ||
Megaw J, Thompson TP, Lafferty RA, Gilmore BF. Galleria mellonella as a novel in vivo model for assessment of the toxicity of 1-alkyl-3-methylimidazolium chloride ionic liquids. Chemosphere. 2015;139:197–201. | ||
Correa W, Manrique-Moreno M, Behrends J, et al. Galleria mellonella native and analogue peptides Gm1 and ΔGm1. II: anti-bacterial and anti-endotoxic effects. Biochim Biophys Acta. 2014;1838(10):2739–2744. | ||
Tsai CJ, Loh JM, Proft T. Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence. 2016;7(3):214–229. | ||
López Hernández YL, Yero D, Pinos-Rodríguez JM, Gibert I. Animals devoid of pulmonary system as infection models in the study of lung bacterial pathogens. Front Microbiol. 2015;6:38. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.