Back to Journals » Drug Design, Development and Therapy » Volume 15
Pentamidine Inhibits Ovarian Cancer Cell Proliferation and Migration by Maintaining Stability of PTEN in vitro
Received 15 March 2021
Accepted for publication 12 June 2021
Published 1 July 2021 Volume 2021:15 Pages 2857—2868
DOI https://doi.org/10.2147/DDDT.S311187
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Tin Wui Wong
Yi Wu,1 Zhong Zhang,2 Zuqiang Kou3
1Department of Pathogenic Biology, Shenyang Medical College, Shenyang City, Liaoning Province, 110004, People’s Republic of China; 2Department of Pathology, Shenyang Medical College, Shenyang City, Liaoning Province, 110004, People’s Republic of China; 3Department of Logistics, Shenyang Ligong University, Shenyang City, Liaoning Province, 110004, People’s Republic of China
Correspondence: Yi Wu
Department of Pathogenic Biology, Shenyang Medical College, Shenyang City, Liaoning Province, 110004, People’s Republic of China
Tel/Fax +86-24-62215720
Email [email protected]
Purpose: Pentamidine is an anti-protozoal cationic aromatic diamidine drug and has been reported to exhibit anticancer properties. We aimed to identify the effect of pentamidine on proliferation and migration of human ovarian cancer (OC) cell lines and the related mechanisms.
Methods: HO8910 and Caov3 ovarian cancer cells were treated with pentamidine. MTS and colony formation assays were used to detect the proliferation ability of cells. The migration of cells was detected using wound healing and transwell assays. The protein levels of PTEN, phosphorylated Akt, Akt, N-cadherin, E-cadherin and snail were detected by Western blotting. Immunoprecipitation and Western blotting were used to detect ubiquitination levels of PTEN.
Results: Our findings revealed that pentamidine inhibited both proliferation and migration of OC cells. Further investigation found that pentamidine increased the protein expression of PTEN and reduced phosphorylation levels of AKT in OC cells. Pentamidine treatment modulated PTEN stability through the ubiquitin/proteasome pathway. In addition, pentamidine inhibited the expression of N-cadherin and snail, and increased E-cadherin expression in a dose-dependent manner.
Conclusion: Pentamidine is involved in the maintenance of PTEN protein stability and suppresses proliferation and migration of OC cells.
Keywords: pentamidine, ovarian cancer, PTEN
Introduction
As of 2018, ovarian cancer (OC) was the seventh most common cancer worldwide in women, with around 240,000 new cases.1 Ovarian cancer is the second most common malignancy after breast cancer in women over the age of 40, particularly in developed countries.1 At present, the clinical treatment options for OC mainly include surgery, radiation therapy, chemotherapy and targeted therapy or their combination. Although there have been many innovations in the treatment of ovarian cancer in the past decades, five-year relative survival is below 45% and, unlike other common cancer types, the proportion of women who die from their disease has not improved substantially over time.2 Thus, exploring novel molecular therapy and new drug is necessary and urgent.
PTEN (phosphatase and tensin homologue deleted on chromosome ten), also known as TEP1 (TGFβ-regulated and epithelial cell-enriched phosphatase) or MMAC1 (mutated in multiple advanced cancers), is a well-known tumor suppressor gene.3 PTEN is a dual phosphatase with activity on lipids and proteins which can dephosphorylate phosphatidylinositol (3,4,5)-trisphosphate (PIP3) to phosphatidylinositol (4,5)-trisphosphate (PIP2) and antagonize the phosphatidylinositol 3-kinase (PI3K)/Akt oncogenic pathway, thereby inhibiting cell proliferation, survival and migration.3,4 Recent studies showed that chemotherapy drugs, such as cisplatin, doxorubicin and paclitaxel, could induce tumor cells apoptosis through enhanced PTEN activity and overexpression of PTEN is able to enhance the anti-tumor effect of chemotherapy drugs in many malignant tumors including OC.5 PTEN expression was precisely regulated on its post-transcriptional modifications, including ubiquitylation. Loss or mutation of PTEN has frequently been found in ovarian cancer and is associated with the worse prognosis of cancer patients.6 Therefore, pharmacological control of PTEN expression and activity might be an important strategy for cancer treatment.
Pentamidine is a positively charged aromatic diamine that has a long history of its involvement in the clinical treatment of African trypanosomiasis, antimonial-resistant leishmaniasis and babesiosis as well as the prophylaxis of pneumocystis carinii pneumonia in acquired immune deficiency syndrome (AIDS) patients for several decades.7–11 Recently, several reports suggested that pentamidine has antitumor potential. In fact, pentamidine has been shown to have anti-proliferative effects on various human cancer cell types such as melanoma, prostate, ovarian, colon, breast, lung, and cervical cancers in vitro.12–15 However, anticancer targets of pentamidine and molecular mechanism have not been fully defined in OC.
In this study, our findings demonstrated that pentamidine suppresses cell proliferation and migration in ovarian cells. Moreover, we provided evidence to show pentamidine modulates PTEN stability by ubiquitin/proteasome pathway in OC cells. Furthermore, pentamidine treatment upregulates epithelial markers and downregulated mesenchymal markers in OC cells. Therefore, increasing PTEN stability with pentamidine may serve as its anti-cancer underlying molecular mechanism in OC.
Materials and Methods
Chemicals and Antibodies
Pentamidine was obtained from MedChemExpress (Shanghai China). Cycloheximide (CHX) was obtained from Sigma-Aldrich (St. Louis, MO, USA). MG132 was obtained from Cell Signaling Technology (Beverly, MA, USA).
Antibodies against Phospho-AKT (Ser473, Cat. #. 4060T, 1:1000), AKT (Cat. #. 4691T, 1:1000), E-Cadherin (Cat. #. 3195T, 1:1000) and snail (Cat. #. 3879P, 1:1000) were obtained from Cell Signaling Technology. Antibodies against PTEN (Cat. #. 22034-1-AP, 1:2000), N-Cadherin (Cat. #. 62219-1-lg, 1:2000), GAPDH (Cat. #. 60004-1-lg, 1:20,000) and ubiquitin (Cat. #. 10201-2-AP, 1:500) were obtained from Proteintech.
Cell Lines and Cell Culture
The human ovarian cancer cell lines HO8910 and Caov3 were obtained from American Type Culture Collection (ATCC). HO8910 cells were cultured in RPMI1640 (GIBCO-BRL) and Caov3 cells were cultured in Dulbecco's modified Eagle’s medium (DMEM). The culture medium was supplemented with 10% fetal bovine serum (FBS), 100 units/mL streptomycin and penicillin at 37°C under 5% (v/v) CO2.
Cell Proliferation Assay
Cells were seeded into 96-well plates at a density of 3×103 cells/well and cultured overnight. Then, cells were treated with different concentrations of pentamidine for 24 h. Finally, growth viability of cells was assayed using the one solution cell proliferation assay (MTS assay) (Promega).
Colony Formation Assay
The cells were seeded in 6-well plates at the density of 1000 cells/well and incubated overnight. After culturing the cells with different concentrations of pentamidine for 7 days, the colonies were fixed with 4% paraformaldehyde and stained with crystal violet. The numbers of cell colonies were counted using the ImageJ software.
Wound Healing Assay
Cells were seeded into 6-well culture plates. When the cells reached confluent, cells were washed with phosphate buffer saline (PBS) and cultured with or without pentamidine treatment in RPMI1640 medium or DMEM medium without serum to minimize cell proliferation. Then, a sterile plastic pipette tip was used to make a straight scratch. After scratching, the well was gently washed twice with PBS to remove detached cells. The scratch was examined and photographed under a light microscope at 0, 24 and 48 hours.
Transwell Assay
Cell migration assays were performed using 24-well cell culture plates with 8-μm pore filter inserts (Corning). About 3×104 cells in 100μL serum-free medium were placed in upper chamber, and 600μL RPMI1640 medium supplemented with 10% FBS was added to the lower chamber. Pentamidine or vehicle was added into both upper chamber and lower chamber. After 6 hours incubation at 37°C and 5% CO2, cells on the upper surface were wiped away gently, whereas cells that migrated to the lower surface were fixed with 95% ethanol for 30 min and stained with 0.1% crystal violet for 30 min at room temperature. The cells were photographed and the migration cell numbers were counted in five random fields using ImageJ software (procedures: 1. open the target images with ImageJ 2. convert images to black and white 3. adjust threshold 4. separate the cells that are linked together with watershed 5. analyze particles 6. display results). The data are presented as mean ± standard deviation.
Real-Time Quantitative PCR
Total RNA was isolated from cells pre-treated with 20μmol/L pentamidine or vehicle for 24 hours using Trizol reagent (Invitrogen) and then reverse transcribed to cDNA using the PrimeScriptTM RT-PCR Kit (TaKaRa) according to the manufacturer’s instructions. Real-time quantitative polymerase chain reaction (qPCR) was performed using the SYBR Premix Ex Taq kit (TaKaRa) on a Light Cycler 96 instrument (Roche Life Science). Gene expression levels were calculated relative to ribosome 18s rRNA. The sequences of the primers were as follows: PTEN (FOR): 5ʹ-GCA GAA AGA CTT GAA GGC GTA-3ʹ, PTEN (REV): 5ʹ- TTG GCG GTG TCA TAA TGT CT-3ʹ, 18S (FOR): 5ʹ- TTG ACG GAA GGG CAC CAC CAG-3ʹ, 18S (REV): 5ʹ- GCA CCA CCA CCC ACG GAA TCG-3ʹ.
Western Blot and Immunoprecipitation
Cells with indicated treatments were lysed using RIPA buffer with protease cocktail inhibitor (APExBIO). The lysates were incubated on ice for 30 min with intermittent vertexing every 5 min, followed by centrifugation at 12,000g for 20 min at 4°C. Protein concentration was determined with BCA protein assay kit (Pierce). For Western blot, equivalent amounts of protein (10 µg) from each sample were separated using 8% or 12% SDS-PAGE and transferred onto a polyvinylidene difluoride (PVDF) membranes, then blocked with 5% skimmed milk. Membranes were incubated with primary antibodies overnight at 4°C. Subsequently, membranes were incubated with secondary antibodies for 1h by gentle shaking. Antibodies were dissolved in milk. Antibody binding was visualized using ECL chemiluminescence reagent (Thermo, MA, USA). For immunoprecipitation (IP), the whole cell lysates were extracted and clarified by centrifuging at 12,000 g for 20 min at 4 °C. The cell lysates were then incubated with indicated antibody for 2 h with gentle rotation followed by 30μL protein G-Sepharose overnight at 4°C. Then antibody–protein G-Sepharose-bound protein complexes were washed three times with TNE buffer. The bound immune complexes were analyzed by Western blotting.
Statistical Analysis
The data of all experiments were from three independent experiments and presented as mean±SD. Statistical difference was determined by Student’s t test between two sets of data. One-way analysis of variance (ANOVA) was applied to monitor the differences among groups. Tukey’s test was used for multiple comparisons after ANOVA. The GraphPad 5.0 software was used and a difference of P<0.05 was considered statistically significant.
Results
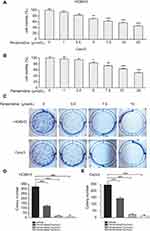
Pentamidine Represses Growth and Proliferation of OC Cells
To determine whether pentamidine has any influence on ovarian cancer cell growth and proliferation, we first examined the cell viability upon pentamidine treatment by MTS assays and found that HO8910 and Caov3 cells were significantly inhibited in a dose-dependent manner, and this suppressive effect reached a peak at the dose of 20 μmol/L (Figure 1A and B). Consistently, the results from cell colony formation experiments showed that the number of cell clones by HO8910 cells or Caov3 cells was significantly reduced in a dose-dependent manner after treatment with pentamidine for 7 days. In HO8901 cells, the number of clones decreased by 62.7%, 94.8% and 96.6%, respectively; in Caov3 cells, the number of clones decreased by 41.3%, 89.9% and 95.1%, respectively (Figure 1C–E). Taken together, these results suggested that pentamidine had a significant effect on reducing cell growth and proliferation of OC cells.
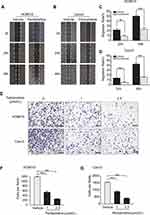
Pentamidine Suppresses the Cell Migration Ability of OC Cells
To evaluated whether pentamidine affected the migratory properties of OC cells, then wound healing assays and transwell migration assays were performed in HO8910 cells and Caov3 cells. In wound healing assays, pentamidine significantly inhibited cell movement and cell migration after 24 and 48 h of treatment (Figure 2A and B). The migration rate of HO8910 cells decreased from 53±1.48% in the control group cells to 27.33±2.55% in the pentamidine-treated cells after 48 h of treatment. The similar experiments were further performed in another OC-derived cell line, Caov3 cells. In agreement with the results generated from HO8910 cells, the data from Caov3 cells demonstrated that the migration rate reduced from 45.77±2.04% in the control group to 16.87±0.23% in the pentamidine treatment group after 48 h of treatment (Figure 2C and D). Meanwhile, the transwell migration assays without Matrigel showed cell migration was also suppressed by pentamidine in a dose-dependent manner (Figure 2E–G). Collectively, these results suggested that pentamidine inhibited ovarian cancer cell migration viability.
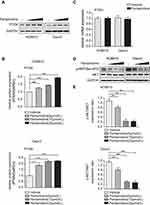
Pentamidine Upregulates PTEN Protein and Downregulates AKT Phosphorylation in OC Cells
It has been reported that phosphatase of regenerating liver-3 (PRL-3), a protein tyrosine phosphatase, down-regulates PTEN expression and the activities of PRL-3 in dephosphorylating a phosphotyrosine peptide substrate in vitro are inhibited in the presence of pentamidine.15,16 Therefore, we wondered whether pentamidine has an effect on PTEN expression in OC cells. Firstly, Western blotting was performed to detect the influence of pentamidine on the protein expression of PTEN. HO8910 cells and Caov3 cells were exposed to increasing concentration of pentamidine for 24 h and the protein levels of PTEN were assessed. The results showed that a dose-dependent increase in the levels of PTEN (Figure 3A and B and Supplementary Figure 1). Meanwhile, quantitative Real-time PCR analysis demonstrated pentamidine did not affect the mRNA levels of PTEN, implying that up-regulation of PTEN is a posttranscriptional event (Figure 3C). Next, we turn to investigate whether pentamidine-induced upregulation of PTEN enhances its suppressive effect on PI3K. Hence, we detected the phosphorylated and total level of AKT, and the data showed that the level of p-AKT decreased in a dose-dependent manner, while the level of total AKT did not change significantly in pentamidine-treated OC cells (Figure 3D and E and Supplementary Figure 2). These results indicated that pentamidine contributed to accumulated PTEN protein level, thus increasing inhibitory effect on PI3K/AKT signaling pathway.
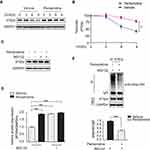
Pentamidine is Involved in Maintenance of PTEN Stability via the Ubiquitin/Proteasome Pathway in HO8910 Cell Lines
To determine whether pentamidine-induced upregulation of PTEN is mediated by the ubiquitin/proteasome pathway, cells were grown in the presence or absence of pentamidine and treated with cycloheximide (CHX), a translation inhibitor. Our data showed that upon inhibition of protein synthesis by CHX, pentamidine prolonged the half-life of PTEN in HO8910 cells, suggesting pentamidine maintained the stability of PTEN (Figure 4A and B and Supplementary Figure 3). We further treated the cells with a proteasome inhibitor MG132, and pentamidine stabilized PTEN protein to a similar level as observed with MG132 (Figure 4C and D and Supplementary Figure 4). In addition, immunoprecipitation (IP)-based ubiquitination assays were performed to provide the evidence that the influence of pentamidine on PTEN ubiquitination. PTEN was immunoprecipitated with the antibody to PTEN and immunoblotted with the antibody to ubiquitin. The results showed pentamidine markedly decreased the level of PTEN ubiquitination (Figure 4E and Supplementary Figure 5). Low migrating bands corresponding to polyubiquitinated PTEN (ladders of polyubiquitinated PTEN) were detected in HO8910 cells in absence of pentamidine but were not detectable in presence of pentamidine. Taken together, these findings indicated that pentamidine maintained PTEN stability by reducing polyubiquitination of PTEN in HO8910 cells.
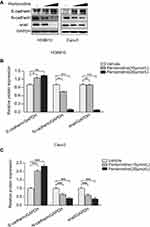
Effect of Pentamidine on the Expression Levels of EMT Marker Proteins in OC Cells
Having established that pentamidine inhibits ovarian cancer cell migration, we further wonder the molecular mechanisms underlying inhibitory activities. Previous studies have shown that PTEN suppresses the epithelial–mesenchymal transition (EMT) in cancer cell,4,17 therefore we investigated the effects of pentamidine on specific molecular associated with EMT in OC. As shown in Figure 5A–C and Supplementary Figures 6 and 7, the expression levels of mesenchymal markers N-cadherin and snail was observed to decrease in concentration-dependent manner, whereas the expression of an epithelial marker E-cadherin increased following treatment of both HO8910 and Caov3 cell lines with pentamidine. These findings clarified that the change in EMT markers suggests pentamidine suppressed EMT, leading to loss of migration.
Discussion
Pentamidine is a dicationic diarylfuran that has been studied for many decades as an antiparasitic agent.18 Recently, several studies have linked pentamidine to having anticancer effects in various cancer cells. It is reported that pentamidine can decrease HIF-1a expression by both reducing protein stability and inhibiting protein translation in DU145 prostate and MDA-MB-231 breast cancer cell lines.12 As well, pentamidine has been reported as a potent agent to inhibit prostate cancer progression by targeting mitochondria.7 In addition, in nude mice xenograft experiments, pentamidine is able to dramatically reduce the growth of WM9 human melanoma and M47 Lewis lung carcinoma tumors.12,13,15 These studies indicate the potential application of pentamidine in chemotherapy. Herein, we investigated the potential anticancer activity of pentamidine, in OC cell lines. Our study shows a dose-dependent suppression of pentamidine in the proliferation and migration of OC cells (Figures 1 and 2), suggesting that pentamidine acts as a potential antitumor agent that induced regression of ovarian cancer.
Indeed, even though pentamidine is used in humans to treat a number of parasitic diseases, it presents with 50% incidence of adverse effects including sudden death (at 4mg/Kg per day). In addition, some studies in literature that report the effect of pentamidine in reducing ovarian cancer or prostate cancer in mice, without side effects but do not speculate on the fact that hERG channel is not present in mice and therefore there will not be sudden death in mice but it may occur in human. Previous studies showed that the structural modifications of pentamidine differentially affected plasma membrane levels of hERG. Thus, if certain residues are avoided or “synthesized out”, the ability to inhibit trafficking can be abolished, such as pentamidine analogue (pentamidine-4, PA-4), which had mild effects on the hERG channel.19 In addition, it has been found that Dofetilide and its analogues could correct pentamidine-induced hERG trafficking defects.19 However, the effect of pentamidine and its analogues on hERG channel needs to be further evaluated, prior to clinical application of pentamidine.
PTEN, a tumor suppressor and lipid phosphatase, plays an important role in tumorigenesis by inhibiting the PI3K signaling pathway.20 PTEN activity, expression and localization are regulated by post-translational modifications, including phosphorylation, ubiquitination, oxidation, acetylation and SUMOylation.21,22 Ubiquitination may be the most significant post-translational modification for PTEN, which is involved in PTEN transport to different subcellular locations and regulation of its catalytic activity and stability.23 Generally, PTEN can be regulated by both polyubiquitination and monoubiquitination.24 PTEN polyubiquitination leads to its cytoplasmic retention and degradation and is therefore oncogenic in nature.25 For instance, a growth factor-activated PI3K/AKT pathway suppresses MKRN1-mediated PTEN ubiquitination and degradation to accelerate tumorigenesis in cervical cancer.26 On the other hand, deubiquitylase OTUS suppresses tumorigenesis and metastasis via de-polyubiquitylating and stabilizing PTEN in breast cancer.21 These results indicate that the tight regulation of PTEN protein stability is of great importance and more importantly, regulation in PTEN polyubiquitination levels can determine cancer outcome. In the present study, we found that the PTEN polyubiquitination level markedly decreases after pentamidine treatment compared with the control group in HO8910 cells (Figure 4E and Supplementary Figure 5), suggesting that pentamidine maybe suppress the cancer progression in OC via targeting PTEN polyubiquitination, and thus reduction in PTEN polyubiquitination level may be novel mechanism underlying pentamidine’s antitumor activity. However, further experiments are needed to elucidate the mechanism of involvement of pentamidine in regulating PTEN polyubiquitination.
The epithelial–mesenchymal transition (EMT) is the key event in distant metastasis, in which epithelial cells lose their marker proteins and acquire the mesenchymal marker proteins.17 The inactivation of PTEN may lead to the loss of apico-basal polarity and tight junctions, which are hallmarks of the EMT, which in turn promotes cell dissemination.24 PTEN has been reported to be implicated in suppressing the EMT in breast cancer.4 In the present study, we showed pentamidine maintained PTEN stability by targeting polyubiquitination of PTEN in HO8910 cells. Moreover, that the migration of ovarian cancer HO8910 and Caov3 cells was strongly inhibited by pentamidine, as shown by the wound healing assay and transwell assay (Figure 2). In addition, we showed that pentamidine modulates the levels of EMT markers, upregulates the expression of E-cadherin, and downregulates the expression of N-cadherin and snail (Figure 5 and Supplementary Figures 6 and 7), indicating that pentamidine-induced inhibition of the migratory potential of ovarian cancer cells may be associated with the upregulation of PTEN.
Multi-drug resistance (MDR) is a common cause of the failure of chemotherapy in ovarian cancer.5 Patients suffering from advanced ovarian carcinoma are, in most cases, initially responsive to chemotherapy; however, they later experience a relapse of the disease because of the eventual recurrence of the tumor and emergence of drug-resistant tumor cells.27 Cisplatin is an effective and widely used chemotherapeutic agent against ovarian cancer.28 Resistance to platinum-based chemotherapy is a major cause of treatment failure in human ovarian cancer.5 Recent years, PTEN, a tumor suppressor gene, has been reported to play an important role in the reversal of MDR and become a new potential target in sensitizing cancer cells to chemotherapy.5,29–31 A recent study showed over-expression of PTEN is able to induce apoptosis and reverse cisplatin-resistance in ovarian cancer cell line C13K.5,32 Our results showed pentamidine increases PTEN protein expression in dose-dependent manner in two OC cell lines (Figure 3A and B and Supplementary Figure 1), thus providing a possibility that pentamidine treatment may sensitize OC cancer cells to chemotherapy.
Conclusions
In summary, we demonstrated for the first time that pentamidine increases PTEN expression in human ovarian cells. The up-regulation of PTEN results in inhibiting PI3K/AKT signal pathway and EMT in OC cells, thereby exerting a potent inhibitory effect on cell proliferation and migration in OC. Collectively, these results suggest that pentamidine may serve as a promising drug candidate for the treatment of OC.
Abbreviations
OC, ovarian cancer; PTEN, phosphatase and tensin homologue deleted on chromosome ten; TEP1, TGFβ-regulated and epithelial cell-enriched phosphatase; MMAC1, mutated in multiple advanced cancers; ATCC, American Type Culture Collection; EMT, epithelial–mesenchymal transition; PRL-3, phosphatase of regenerating liver-3; PIP3, phosphatidylinositol (3,4,5)-trisphosphate; PIP2, phosphatidylinositol (4,5)-trisphosphate; AIDS, acquired immune deficiency syndrome; MDR, Multi-drug resistance; CHX, cycloheximide.
Funding
This work was supported by the Research Fund Liaoning Provincial Education Department (SYYX202007).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Stewart C, Ralyea C, Lockwood S. Ovarian cancer: an integrated review. Semin Oncol Nurs. 2019;35(2):151–156. doi:10.1016/j.soncn.2019.02.001
2. Webb PM, Jordan SJ. Epidemiology of epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol. 2017;41:3–14. doi:10.1016/j.bpobgyn.2016.08.006
3. Zhou XJ, Wu J, Shi L, et al. PTEN expression is upregulated by a RNA-binding protein RBM38 via enhancing its mRNA stability in breast cancer. J Exp Clin Cancer Res. 2017;36(1):149. doi:10.1186/s13046-017-0620-3
4. Qi Y, Liu J, Chao J, et al. PTEN suppresses epithelial-mesenchymal transition and cancer stem cell activity by downregulating Abi1. Sci Rep. 2020;10(1):12685. doi:10.1038/s41598-020-69698-1
5. Wu H, Wang K, Liu W, Hao Q. PTEN overexpression improves cisplatin-resistance of human ovarian cancer cells through upregulating KRT10 expression. Biochem Biophys Res Commun. 2014;444(2):141–146. doi:10.1016/j.bbrc.2014.01.014
6. Nero C, Ciccarone F, Pietragalla A, Scambia G. PTEN and gynecological cancers. Cancers (Basel). 2019;11(10):1458. doi:10.3390/cancers11101458
7. Liu L, Wang F, Tong Y, Li LF, Liu Y, Gao WQ. Pentamidine inhibits prostate cancer progression via selectively inducing mitochondrial DNA depletion and dysfunction. Cell Prolif. 2020;53(1):e12718. doi:10.1111/cpr.12718
8. Pearson RD, Hewlett EL. Pentamidine for the treatment of Pneumocystis carinii pneumonia and other protozoal diseases. Ann Intern Med. 1985;103(5):782–786. doi:10.7326/0003-4819-103-5-782
9. Armstrong D, Bernard E. Aerosol pentamidine. Ann Intern Med. 1988;109(11):852–854. doi:10.7326/0003-4819-109-11-852
10. Bray PG, Barrett MP, Ward SA, de Koning HP. Pentamidine uptake and resistance in pathogenic protozoa: past, present and future. Trends Parasitol. 2003;19(5):232–239. doi:10.1016/S1471-4922(03)00069-2
11. Diro E, Ritmeijer K, Boelaert M, et al. Use of pentamidine as secondary prophylaxis to prevent visceral leishmaniasis relapse in HIV infected patients, the First Twelve Months of a Prospective Cohort Study. PLoS Negl Trop Dis. 2015;9(10):e0004087. doi:10.1371/journal.pntd.0004087
12. Jung HJ, Suh SI, Suh MH, Baek WK, Park JW. Pentamidine reduces expression of hypoxia-inducible factor-1alpha in DU145 and MDA-MB-231 cancer cells. Cancer Lett. 2011;303(1):39–46. doi:10.1016/j.canlet.2011.01.008
13. Chow TY, Alaoui-Jamali MA, Yeh C, Yuen L, Griller D. The DNA double-stranded break repair protein endo-exonuclease as a therapeutic target for cancer. Mol Cancer Ther. 2004;3(8):911–919.
14. Smith J, Stewart BJ, Glaysher S, et al. The effect of pentamidine on melanoma ex vivo. Anticancer Drugs. 2010;21(2):181–185. doi:10.1097/CAD.0b013e3283340cee
15. Pathak MK, Dhawan D, Lindner DJ, Borden EC, Farver C, Yi T. Pentamidine is an inhibitor of PRL phosphatases with anticancer activity. Mol Cancer Ther. 2002;1(14):1255–1264.
16. Wang H, Quah SY, Dong JM, Manser E, Tang JP, Zeng Q. PRL-3 down-regulates PTEN expression and signals through PI3K to promote epithelial-mesenchymal transition. Cancer Res. 2007;67(7):2922–2926. doi:10.1158/0008-5472.CAN-06-3598
17. Perumal E, So Youn K, Sun S, et al. PTEN inactivation induces epithelial-mesenchymal transition and metastasis by intranuclear translocation of beta-catenin and snail/slug in non-small cell lung carcinoma cells. Lung Cancer. 2019;130:25–34. doi:10.1016/j.lungcan.2019.01.013
18. Her S, Cui L, Bristow RG, Allen C. Dual action enhancement of gold nanoparticle radiosensitization by pentamidine in triple negative breast cancer. Radiat Res. 2016;185(5):549–562. doi:10.1667/RR14315.1
19. Varkevisser R, Houtman MJ, Linder T, et al. Structure-activity relationships of pentamidine-affected ion channel trafficking and dofetilide mediated rescue. Br J Pharmacol. 2013;169(6):1322–1334. doi:10.1111/bph.12208
20. Deng L, Meng T, Chen L, Wei W, Wang P. The role of ubiquitination in tumorigenesis and targeted drug discovery. Signal Transduct Target Ther. 2020;5(1):11.
21. Yuan L, Lv Y, Li H, et al. Deubiquitylase OTUD3 regulates PTEN stability and suppresses tumorigenesis. Nat Cell Biol. 2015;17(9):1169–1181. doi:10.1038/ncb3218
22. Worby CA, Dixon JE. Pten. Annu Rev Biochem. 2014;83:641–669. doi:10.1146/annurev-biochem-082411-113907
23. Xia Q, Ali S, Liu L, et al. Role of ubiquitination in PTEN cellular homeostasis and its implications in GB drug resistance. Front Oncol. 2020;10:1569. doi:10.3389/fonc.2020.01569
24. Lee YR, Chen M, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor: new modes and prospects. Nat Rev Mol Cell Biol. 2018;19(9):547–562. doi:10.1038/s41580-018-0015-0
25. Trotman LC, Wang X, Alimonti A, et al. Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell. 2007;128(1):141–156. doi:10.1016/j.cell.2006.11.040
26. Lee MS, Jeong MH, Lee HW, et al. PI3K/AKT activation induces PTEN ubiquitination and destabilization accelerating tumourigenesis. Nat Commun. 2015;6:7769. doi:10.1038/ncomms8769
27. Selvendiran K, Tong L, Vishwanath S, et al. EF24 induces G2/M arrest and apoptosis in cisplatin-resistant human ovarian cancer cells by increasing PTEN expression. J Biol Chem. 2007;282(39):28609–28618. doi:10.1074/jbc.M703796200
28. Markman M, Howell SB, Lucas WE, Pfeifle CE, Green MR. Combination intraperitoneal chemotherapy with cisplatin, cytarabine, and doxorubicin for refractory ovarian carcinoma and other malignancies principally confined to the peritoneal cavity. J Clin Oncol. 1984;2(12):1321–1326. doi:10.1200/JCO.1984.2.12.1321
29. Cassinelli G, Zuco V, Gatti L, et al. Targeting the Akt kinase to modulate survival, invasiveness and drug resistance of cancer cells. Curr Med Chem. 2013;20(15):1923–1945. doi:10.2174/09298673113209990106
30. Hou R, Zhang J, Yin T, et al. Upregulation of PTEN by peroxynitrite contributes to cytokine-induced apoptosis in pancreatic beta-cells. Apoptosis. 2010;15(8):877–886. doi:10.1007/s10495-010-0510-z
31. Keniry M, Parsons R. The role of PTEN signaling perturbations in cancer and in targeted therapy. Oncogene. 2008;27(41):5477–5485. doi:10.1038/onc.2008.248
32. Yan X, Fraser M, Qiu Q, Tsang BK. Over-expression of PTEN sensitizes human ovarian cancer cells to cisplatin-induced apoptosis in a p53-dependent manner. Gynecol Oncol. 2006;102(2):348–355. doi:10.1016/j.ygyno.2005.12.033
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.