Back to Journals » OncoTargets and Therapy » Volume 11
Pembrolizumab and salvage chemotherapy in EGFR T790M-positive non-small-cell lung cancer with high PD-L1 expression
Authors Tozuka T , Seike M, Minegishi Y, Kitagawa S, Kato T, Takano N , Hisakane K, Takahashi S, Kobayashi K, Kashiwada T, Sugano T, Takeuchi S, Kunugi S, Noro R, Saito Y, Kubota K, Gemma A
Received 19 March 2018
Accepted for publication 20 June 2018
Published 7 September 2018 Volume 2018:11 Pages 5601—5605
DOI https://doi.org/10.2147/OTT.S168598
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tohru Yamada
Takehiro Tozuka,1 Masahiro Seike,1 Yuji Minegishi,1 Shingo Kitagawa,1 Tomomi Kato,1 Natsuki Takano,1 Kakeru Hisakane,1 Satoshi Takahashi,1 Kenichi Kobayashi,1 Takeru Kashiwada,1 Teppei Sugano,1 Susumu Takeuchi,1 Shinobu Kunugi,2 Rintaro Noro,1 Yoshinobu Saito,1 Kaoru Kubota,1 Akihiko Gemma1
1Department of Pulmonary Medicine and Oncology, Graduate School of Medicine, Nippon Medical School, Tokyo, Japan; 2Department of Analytic Human Pathology, Graduate School of Medicine, Nippon Medical School, Tokyo, Japan
Abstract: Immuno-checkpoint inhibitors (ICI) have become an effective treatment option for non-small-cell lung cancer patients. However, ICI therapy was reported to be less effective in patients with epidermal growth factor receptor (EGFR) mutations than in those with wild-type EGFR. We report here that an non-small-cell lung cancer patient with the EGFR mutant T790M showed a programmed cell death ligand 1 (PD-L1) expression level that increased from <25% to >90% after eighth-line osimertinib therapy. He was treated with pembrolizumab as a ninth-line treatment, and attained stable disease. After the pembrolizumab therapy, he was treated with gemcitabine, which produced a good response despite being the 10th-line treatment. We should consider administering ICI and chemotherapy even to EGFR mutant patients after failure of EGFR tyrosine kinase inhibitor, especially in cases with high PD-LI expression.
Keywords: programmed cell death ligand 1, epidermal growth factor receptor mutation, lung cancer, chemotherapy post immunotherapy
Introduction
Lung cancer is the leading cause of cancer death worldwide. Non-small-cell lung cancer (NSCLC) accounts for ~85% of all lung cancer. Current treatment options are surgery, chemoradiotherapy, and chemotherapy including cytotoxic anticancer agents, antiangiogenic monoclonal antibodies, tyrosine kinase inhibitors for driver mutation-positive patients, and immuno-checkpoint inhibitors (ICI).1
In cancer progression, cancer cells induce immune suppressor cells, produce immunosuppressive cytokines, and express immune checkpoint molecules such as cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) and programmed cell death ligand 1 (PD-L1).2 The PD-L1/programmed cell-death 1 (PD-1) axis plays also an important role in immune escape mechanisms of cancer cells by regulating the activity of T cells.3 ICI targeting PD-1, such as nivolumab and pembrolizumab, have become effective treatment options in NSCLC patients.4–6 Nivolumab treatment was associated with improved overall survival (OS) compared with docetaxel in previously treated patients with advanced NSCLC in two Phase III trials.4,5 Pembrolizumab also prolonged OS compared with docetaxel in previously treated NSCLC patients with PD-L1 expression on at least 1% of tumor cells.6 However, OS was not significantly different for epidermal growth factor receptor (EGFR) mutant patients in these trials.
Expression of PD-L1 in tumor tissue has been regarded as a predictive factor for the efficacy of PD-1 inhibitors.4–6 On the other hand, some recent studies have suggested that PD-1 inhibitor therapy was less effective in patients with EGFR mutations than in those with wild-type EGFR.4–9 Several studies have shown that the frequency of high PD-L1 expression in EGFR mutant patients was low, and the efficacy of PD-1 inhibitors was poor.9 The role of PD-1 inhibitor therapy in EGFR mutant NSCLC patients is still unknown.
Osimertinib, a third-generation EGFR tyrosine kinase inhibitor (EGFR-TKI), showed good response in T790M-positive NSCLC patients. The AURA3 phase III trial showed that median progression-free survival (PFS) was significantly longer in T790M-positive NSCLC patients treated with osimertinib (10.1 months) than in patients treated with platinum-based chemotherapy (4.4 months).10 However, a mechanism of resistance to osimertinib, including the C797S secondary mutation, has already been reported. Effective treatment options after osimertinib therapy have not yet been established.
In this case, we report on an NSCLC patient with the EGFR T790M secondary mutation showing increased PD-L1 expression after treatments with cytotoxic agents and EGFR-TKI, including osimertinib. Pembrolizumab followed by gemcitabine produced a good tumor response.
Case presentation
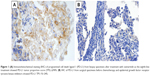
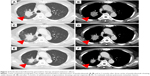
A 62-year-old male had undergone a right middle and lower lobectomy with systematic lymph node dissection for lung adenocarcinoma after initial radiofrequency ablation (pT4N2M0 stage IIIB). He had no smoking history related to harboring the EGFR exon 19 deletion. One year after the surgery, metastases were found in right hilar and mediastinal lymph nodes, right scalene muscle, and multiple bone sites. He received several chemotherapies and EGFR-TKI therapy, including carboplatin plus pemetrexed plus gefitinib, S-1, erlotinib, docetaxel, afatinib, third-generation TKI (an investigational new drug), and nab-paclitaxel (Table 1). Seven years after the surgery, he received computed tomography-guided needle biopsy (CTNB) on a pulmonary metastasis in order to evaluate the existence of secondary EGFR mutations after failure of nab-PTX as the seventh-line therapy. An EGFR exon 19 deletion and the T790M mutation on exon 20 were detected in the lung tumors by CTNB. The patient was treated with osimertinib as the eighth-line treatment. After 4 months of osimertinib treatment, the tumor had progressed as a result of acquired resistance to osimertinib, therefore, he received a re-biopsy of lung tissue by bronchoscopy in order to evaluate the histological features, EGFR mutation status, and PD-L1 expression level. We confirmed adenocarcinoma with EGFR exon 19 deletion and T790M positivity. Immunohistochemistry using 22C3 PD-L1 antibody on the lung tumor tissue revealed a high expression level of PD-L1 (tumor proportion score (TPS) 90%) (Figure 1A). The TPS of PD-L1 in surgically resected lung tumor tissue was 25% (Figure 1B). The PD-L1 expression level changed after administration of several EGFR-TKIs and chemotherapy. He was treated with pembrolizumab as the ninth-line therapy. Three cycles of pembrolizumab produced stable disease (SD) (Figure 2A–D). A left adrenal gland metastasis and a growing pulmonary metastatic lesion were evident on a computed tomography scan after 4 months. The best response to pembrolizumab was defined as SD, and the PFS was 4 months. He was treated with gemcitabine as the 10th-line chemotherapy. After 2 months, gemcitabine produced a partial response (PR) (Figure 2E and F). He remains on gemcitabine with continuous PR at the time of this case report submission.
Discussion
A recent study demonstrated two mechanisms for PD-L1 expression in lung cancer.3 First, PD-L1 expression emerges in connection with acquired resistance to a drug’s antitumor effects. Cancer cells express PD-L1 in order to prevent the activity of T cells, and they escape from antitumor immune cell attacks by producing an immunosuppressive state within the tumor microenvironment.2 PD-1 signaling in T cells could be regulated by production of cytokines such as interferon-γ, tumor necrosis factor-α, and interleukin-2. Second, the PD-L1 expression is induced by signaling from driver mutations.3 In several EGFR mutant cell lines, PD-L1 expression was regulated by EGFR signaling pathways.11 However, the effect of a PD-1 inhibitor in EGFR mutation-positive patients is poor, because these patients have a small number of CD8+ tumor-infiltrating lymphocytes.12,13 In addition, recent studies have demonstrated that the tumor mutation burden (TMB) is recognized as a predictive biomarker for ICI in addition to the PD-L1 expression level.14 A high TMB level associated with an increase in the number of neoantigens is recognized as a biomarker to predict the effect of immunotherapy.14 However, EGFR-mutated lung cancer was shown to have a low TMB.15 A recent study also suggested that PD-1 inhibitor therapy was not sufficiently effective in patients with EGFR mutations because these patients have low levels of TMB.15
In this case report, we describe an NSCLC patient with an EGFR mutation who showed an increase in the tumor PD-L1 expression from 25% to over 90% after administration of several EGFR-TKIs and cytotoxic chemotherapy. Although a potential difference between fresh and archival tissue specimens should be considered in this case, the KEYNOTE-010 trial showed that the benefit of pembrolizumab in OS was not significantly different between fresh and archival samples.5 Cytotoxic chemotherapy and EGFR-TKI therapy could affect PD-L1 expression.11,16–19 Osimertinib decreased the PD-L1 expression in EGFR-mutated NSCLC cells.18 PD-L1 expression could be regulated not only by some inflammatory cytokines such as interferon-γ but also by agents suppressing constitutive oncogenic activation, including EGFR-TKI. Various factors, such as types of anticancer agents, treatment period, and anticancer effect, may affect PD-L1 expression after the therapy.
The PD-L1 expression level was reported to be low in T790M-positive NSCLC patients.20 However, PD-1 inhibitors were expected to be effective in T790M-positive NSCLC patients with high PD-L1.20 We administered pembrolizumab in this case because of the high PD-L1 expression according to a repeated biopsy (TPS 90%), although his PD-L1 level was low (TPS 25%) before the first-line chemotherapy. The best response to pembrolizumab was defined as SD, and the PFS was 4 months.
Interestingly, gemcitabine, administered after the pembrolizumab treatment, achieved a PR despite being the 10th-line treatment. The overall response rate to gemcitabine in patients previously treated with platinum-based chemotherapy for NSCLC was reported to be 6%–19% in phase II trials.21–23 In recent studies, chemotherapy after administration of ICI produced a good response even if the efficacy of ICI was poor, especially, a gemcitabine regimen produced a good response.24,25 The overall response rate for salvage gemcitabine chemotherapy administered after ICI was 63.6%.25 Gemcitabine induced apoptosis of tumor cells and increased presentation of tumor cell antigen to immune cells.26 In addition, gemcitabine induced expression of major histocompatibility complex class I molecules on tumor cells, resulting in activation of immune cells including intratumoral CD8+ T cells.27 Moreover, cytotoxic anticancer agents decreased the immunoinhibitory cells such as regulatory T cells or myeloid-derived suppressor cells, leading to an increase of helper T cells in the tumor microenvironment.28 However, further studies should be performed to evaluate the effect of chemotherapy after ICI therapy and clarify the mechanism of the combined effect.
In this case, we demonstrated that an NSCLC patient with the EGFR T790M mutation showed high PD-L1 expression after EGFR-TKI and chemotherapy, and a favorable response to ICI followed by chemotherapy. NSCLC patients with the T790M mutation have few treatment options after failure of osimertinib therapy. Therefore, it is important to evaluate the PD-L1 expression levels in EGFR-TKI-resistant NSCLC in order to determine the next treatment strategy, including ICI therapy. Further studies should be performed to evaluate the efficacy of salvage chemotherapy administered after ICI for EGFR mutant patients with high PD-L1 expression.
Ethical approval
Written informed consent was obtained from the patient for publication of the study including the patient’s details and accompanying images.
Disclosure
The authors report no conflicts of interest in this work.
References
Gridelli C, Rossi A, Carbone DP, et al. Non-small-cell lung cancer. Nat Rev Dis Primers. 2015;1:15009. | ||
Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. | ||
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. | ||
Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. | ||
Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. | ||
Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016; 387(10027):1540–1550. | ||
Sheng Z, Zhu X, Sun Y, Zhang Y. The efficacy of anti-PD-1/PD-L1 therapy and its comparison with EGFR-TKIs for advanced non-small-cell lung cancer. Oncotarget. 2017;8(34):57826–57835. | ||
Lee CK, Man J, Lord S, et al. Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung lancer-a meta-analysis. J Thorac Oncol. 2017;12(2):403–407. | ||
Bylicki O, Paleiron N, Margery J, et al. Targeting the PD-1/PD-L1 immune checkpoint in EGFR-mutated or ALK-translocated non-small-cell lung cancer. Target Oncol. 2017;12(5):563–569. | ||
Mok TS, Wu Y-L, Ahn M-J, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376(7):629–640. | ||
Azuma K, Ota K, Kawahara A, et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol. 2014;25(10):1935–1940. | ||
Gainor JF, Shaw AT, Sequist LV, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clin Cancer Res. 2016;22(18):4585–4593. | ||
Bylicki O, Paleiron N, Margery J, et al. Targeting the PD-1/PD-L1 immune checkpoint in EGFR-mutated or ALK-translocated non-small-cell lung cancer. Target Oncol. 2017;12(5):563–569. | ||
Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. | ||
Carbone DP, Reck M, Paz-Ares L, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415–2426. | ||
Peng J, Hamanishi J, Matsumura N, et al. Chemotherapy induces programmed cell death-ligand 1 overexpression via the nuclear factor-κB to foster an immunosuppressive tumor microenvironment in ovarian cancer. Cancer Res. 2015;75(23):5034–5045. | ||
Abdelhamed S, Ogura K, Yokoyama S, Saiki I, Hayakawa Y. AKT-STAT3 pathway as a downstream target of EGFR signaling to regulate PD-L1 expression on NSCLC cells. J Cancer. 2016;7(12):1579–1586. | ||
Jiang XM, Xu YL, Huang MY, et al. Osimertinib (AZD9291) decreases programmed death ligand-1 in EGFR-mutated non-small cell lung cancer cells. Acta Pharmacol Sin. 2017;38(11):1512–1520. | ||
Han JJ, Kim DW, Koh J, et al. Change in PD-L1 expression after acquiring resistance to gefitinib in EGFR-mutant non-small-cell lung cancer. Clin Lung Cancer. 2016;17(4):263–270. | ||
Haratani K, Hayashi H, Tanaka T, et al. Tumor immune microenvironment and nivolumab efficacy in EGFR mutation-positive non-small-cell lung cancer based on T790M status after disease progression during EGFR-TKI treatment. Ann Oncol. 2017;28(7):1532–1539. | ||
Sculier JP, Lafitte JJ, Berghmans T, et al. A phase II trial testing gemcitabine as second-line chemotherapy for non small cell lung cancer. The European Lung Cancer Working Party. [email protected]. Lung Cancer. 2000;29(1):67–73. | ||
Lara PN, Gumerlock PH, Mack PC, et al. Gemcitabine in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy: a phase II California cancer consortium trial. Clin Lung Cancer. 2004;6(2):102–107. | ||
Crinò L, Mosconi AM, Scagliotti G, et al. Gemcitabine as second-line treatment for advanced non-small-cell lung cancer: A phase II trial. J Clin Oncol. 1999;17(7):2081–2085. | ||
Schvartsman G, Peng SA, Bis G, et al. Response rates to single-agent chemotherapy after exposure to immune checkpoint inhibitors in advanced non-small cell lung cancer. Lung Cancer. 2017;112:90–95. | ||
Park SE, Lee SH, Ahn JS, et al. Increased response rates to salvage chemotherapy administered after PD-1/PD-L1 inhibitors in patients with non-small cell lung cancer. J Thorac Oncol. 2018;13(1):106–111. | ||
Dwary AD, Master S, Patel A, et al. Excellent response to chemotherapy post immunotherapy. Oncotarget. 2017;8(53):91795–91802. | ||
Liu WM, Fowler DW, Smith P, Dalgleish AG. Pre-treatment with chemotherapy can enhance the antigenicity and immunogenicity of tumours by promoting adaptive immune responses. Br J Cancer. 2010;102(1):115–123. | ||
Fridlender ZG, Sun J, Singhal S, et al. Chemotherapy delivered after viral immunogene therapy augments antitumor efficacy via multiple immune-mediated mechanisms. Mol Ther. 2010;18(11):1947–1959. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.