Back to Journals » Journal of Asthma and Allergy » Volume 13
Pediatric Spectrum of Allergic Diseases and Asthma in a Tertiary Level Hospital in Botswana: an Exploratory Retrospective Cross-Sectional Study
Authors Gezmu AM , Kung SJ, Shifa JZ, Nakstad B, Brooks M, Joel D, Arscott-Mills T , Puerto EC, Šaltytė Benth J, Tefera E
Received 13 March 2020
Accepted for publication 1 June 2020
Published 1 July 2020 Volume 2020:13 Pages 213—223
DOI https://doi.org/10.2147/JAA.S253618
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Amrita Dosanjh
Alemayehu Mekonnen Gezmu,1 Shiang-Ju Kung,2 Jemal Zeberga Shifa,3 Britt Nakstad,1,4 Merrian Brooks,1,5 Dipesalema Joel,1 Tonya Arscott-Mills,1,5 Edelis Castellanos Puerto,6 Jūratė Šaltytė Benth,7,8 Endale Tefera1
1Department of Pediatrics and Adolescent Health, Faculty of Medicine, University of Botswana, Gaborone, Botswana; 2Division of Allergy and Immunology, Children’s Hospital of Philadelphia, Philadelphia, PA, USA; 3Department of Surgery, Faculty of Medicine, University of Botswana, Gaborone, Botswana; 4Institute of Clinical Medicine and Centre of Global Health, University of Oslo, Oslo, Norway; 5Center for Global Health, Children’s Hospital of Philadelphia, Philadelphia, PA, USA; 6Department of Allergy and Immunology, University Hospital Calixto Garcia, Havana, Cuba; 7Institute of Clinical Medicine, Campus Ahus, University of Oslo, Blindern, Norway; 8Health Services Research Unit, Akershus University Hospital, Lørenskog, Norway
Correspondence: Alemayehu Mekonnen Gezmu
Department of Pediatrics and Adolescent Health, Faculty of Medicine, University of Botswana, Private Bag UB00713, Gaborone, Botswana
Tel +26774063734
Fax +2673105979
Email [email protected]
Purpose: This study aims to describe the spectrum of allergic diseases of children and adolescents in a single allergy treatment centre in Botswana, over a period of 8 years.
Patients and Methods: A retrospective cross-sectional study was conducted using medical records of all patients aged 18 years or younger, seen at an allergy treatment centre in Botswana. Data were presented descriptively. Association between variables was explored by χ2-test.
Results: Four hundred and seven patients with a mean age of 5.8 years (SD 4.4) at the time of presentation included 239 (58.7%) females and 365 (87.5%) black Africans. The most common diseases were asthma (n=249, 61.2%) followed by allergic rhinitis (AR) (n=232, 57.0%) and atopic dermatitis (AD) (n=165, 40.5%). One hundred and fifteen cases (46.2%) of asthmatic patients were skin prick test positive; sensitized to grass, moulds, dust mites and animal dander, in decreasing frequency, whereas those with allergic rhinitis (AR) and allergic conjunctivitis (AC) were sensitized to trees and all allergens identified in asthmatics. Concomitant asthma was diagnosed in 171 (73.7%) with AR, 71 (68.3%) with AC, 75 (45.5%) with AD and 42 (47.7%) with food allergy. The most common triggers for asthma exacerbations include upper respiratory tract infections, weather changes, and exposure to passive cigarette smoke. Paternal allergy and allergic disease in grandparents are predisposing factors for asthma (p=0.016 and p=0.001, respectively). Paternal allergy is also predisposed to AR (p=0.007), while maternal history of allergic disease was associated with AD (p=0.019).
Conclusion: The most common chronic pediatric conditions seen in our allergic disease study were asthma, allergic rhinitis and atopic dermatitis with the most common triggers being viral upper respiratory tract infections, weather changes and exposure to cigarette smoke, all of which are modifiable risk factors. This exploratory study lays the foundation for future interventional studies that may be directed towards the spectrum of allergic diseases.
Keywords: pediatric allergy, asthma in children, allergic rhinitis, atopic dermatitis
Introduction
Allergic disorders and asthma, both allergic and non-allergic, are the non-communicable diseases with the earliest onset. Their burden is growing, especially in low and middle income countries (LMICs) where allergic rhinitis (AR) and asthma are the most prevalent chronic diseases seen in childhood.1 Globally, 300 million people at all ages are diagnosed with asthma, and there are 250,000 deaths from asthma per year worldwide.2 Asthma was formerly considered to be uncommon in children in LMIC settings, but studies indicate that the burden of childhood asthma in these areas is large and comparable to those in high income countries (HIC).3 For example, the prevalence of asthma in African children, as assessed by self-reported questionnaires in the International Study of Asthma and Allergies in Childhood (ISAAC) III study, is higher than the global average.4 Moreover, the prevalence of childhood asthma in LMICs is increasing, in contrast to HIC settings where it has stabilized or is decreasing.4 The Danish Allergy Research Center (DARC) birth cohort has prospectively followed the prevalence of atopic diseases, the pattern of sensitization, and comorbidities from birth to 14 years of age. At 14 years, the prevalence of asthma, atopic dermatitis (AD), allergic rhinoconjunctivitis (ARC) and any atopic diseases was 12.9% (9.7–16.7), 8.1% (5.5–11.3), 32.8% (28.0–37.8), and 40.3% (35.3–45.5), respectively, with no gender differences. Of these 12.1% had more than one allergic disease. The most prevalent was allergic rhinoconjunctivitis combined with asthma.5 AD is often associated with elevated serum immuno-globulin (IgE) levels and an individual or family history of type I allergies such as AR, or asthma.6,7 AD onset is most commonly between 3 and 6 months of age, with approximately 60% of patients developing the eruption in the first year of life and 90% by age 5 years. Worldwide AD affects 17% to 24% of the pediatric population.7 Food allergy is another emerging area of interest for LMICs. Much is known about the prevalence of food allergy in the developed world where food allergy affects up to 6% of children and 4% of adults in HICs,8 but there are large knowledge gaps in LMICs. Symptoms include urticarial, gastrointestinal distress, failure to thrive, anaphylaxis, and even death.9
The diagnosis of allergic disease remains a challenge in most LMICs as detailed high-quality clinical registers and diagnostic allergy testing materials are often lacking.10 Likewise, information about atopic diseases in Botswana is scanty. There are a few studies looking at this, but these are limited in scope.11,12 The prevalence of asthma is monitored by the Botswana government. However, this is based on general registries and discharge summaries from the public facilities. The numbers may be misrepresented by incorrect or missing diagnoses. Furthermore, this information does not reflect concurrent allergic diseases, allergic sensitizations or risk factors. There is no comprehensive register focusing on allergic individuals. In an attempt to delve further into allergic conditions in Botswana, we examined data that was retrospectively collected from medical records of children and adolescents, obtained at the Asthma and Allergy Clinic of the largest tertiary referral hospital in Botswana, through an 8-year period. Of note is that this was the only clinic of its kind in the entire country over this time period, reflecting referrals from both the public and private sector. Therefore, this exploratory study primarily aimed to describe demographic and clinical characteristics of the patients, and correlation between presence of allergic diseases and family history of allergy. Furthermore, we aimed to explore associations between presence of allergic diseases and family history of allergy, identify the most common allergens or combinations of allergens, assess the associations between sensitization and type of allergy, describe the variety of triggers attributed to the exacerbation of allergic diseases and demonstrate the coexistence of different allergic disorders.
Patients and Methods
Study Design, Participants and Setting
A retrospective cross-sectional study was conducted at the tertiary referral Princess Marina Hospital located in Gaborone, the capital city of Botswana. The hospital is a referral hospital that has a catchment area with 271,266 children and adolescents 18 years or younger.13 The patients were admitted to the Asthma and Allergy Clinic because they had symptoms of allergic disease, allergic asthma, or food allergy. Sensitization testing was performed to confirm the disease.
Definition of Allergic Disorder and Asthma
The diagnoses of allergic disorders and asthma were physician based, consistent with the World Allergy Organization definitions of allergic diseases and international consensus published guidelines.14–17 These were as follows.
Asthma
Asthma was diagnosed by demonstration of variable airflow obstruction with a suggestive history and examination. Symptoms included wheezing, coughing, shortness of breath or chest tightness. Pattern of symptoms was thoroughly elucidated, with particular attention to triggers such as viral infections and exercise, as well as time of day or night. Due to high prevalence of tuberculosis (TB) in Botswana, all patients were screened for pulmonary TB (by history, examination and Mantoux test if indicated). On physical examination, clubbing and other pulmonary disorders were excluded. Features of other atopic diseases were documented. Due to resource limitations, methacholine challenge test, pre/post bronchodilator spirometry and home monitoring of peak flows were not possible. Variable airflow obstruction was thus defined by an improvement 4 weeks after initiation of oral or inhaled corticosteroid therapy. For children (5 years and older), this was documented by FEV1 improvement of at least 12% (> 200 mL), or peak flow improvement by 20% or more. Children younger than 4 years of age were cognitively unable to perform spirometry or peak flow. Hence, they had to demonstrate improvement of airflow obstruction clinically, by resolution of wheezing 30–60 minutes after bronchodilator therapy and improvement of nocturnal and exertional symptoms after 4 weeks use of corticosteroid therapy. Asthma was only diagnosed in infants if there was more than one wheezing episode, with the presence of one of more of the following: parental history of asthma, atopic dermatitis or aeroallergen sensitization.
Atopic Dermatitis (AD)
Atopic dermatitis (AD) was defined by at least three of the following features: pruritis, characteristic distribution, chronic/relapsing course, and personal or family history of atopy. Distribution of skin lesions were flexural for most children, but some infants had extensor surface and/or cheek involvement. On examination, there had to be evidence of erythema, papules, excoriations, erosions, dyspigmentation or lichenification. Other skin disorders were clinically excluded.
Allergic Rhinitis (AR)
Rhinitis was diagnosed by inflammation of the nasal epithelium, presenting with two or more of the following features: rhinorrhea, nasal obstruction, sneezing or nasal pruritis. On examination, face and nasopharynx were carefully examined for Dennie-Morgan lines, shiners, nasal crease and enlarged inferior turbinates. Other pathology was excluded (such as foreign body, infection, polyps or choanal atresia). When indicated, adenoidal hypertrophy was excluded by lateral neck x-ray. AR was subsequently diagnosed if there was confirmation of aeroallergen IgE sensitization by either skin prick test or serum specific IgE test.
Allergic Conjunctivitis (AC)
AC was diagnosed if there were suggestive signs and symptoms, concurrent with IgE aeroallergen sensitization. Symptoms included eye itching and watering. On examination, eyes displayed conjunctival redness (hyperemia) and edema (chemosis), with tearing, eyelid swelling and/or cobble stoning.
Food Allergy
All patients were carefully screened for reactions to foods. Symptoms sought included one or more of the following: nausea, vomiting, abdominal pain, diarrhea, pruritis, urticaria, swelling, cough, wheeze, dizziness or syncope. If the reactions were immediate (within minutes to maximum an hour after ingestion) and reproducible (occurred each time that food was ingested), then skin prick test was performed solely to that food. Food allergy was then diagnosed if skin prick test to that food was positive. Due to resource limitations, oral food challenge was only possible in a handful of patients. The only exclusions to the above were infants (aged 1 or less) with moderate to severe eczema, unresponsive to topical corticosteroid therapy and good skin care. These infants underwent skin testing to the common food allergies, namely milk, egg, soy, wheat, and peanut. On positive testing, if removal of the foods from their diet improved skin outcome, then they were presumed to be allergic. If there was no improvement in the atopic dermatitis, then the food was reintroduced back into their diet.
Collection of Medical Records and Variables
Medical records of all pediatric patients who were diagnosed with asthma or an allergic disease seen at the Asthma and Allergy Clinic at Princess Marina Hospital (PMH) from July 1, 2010 to June 31, 2018 were included in the analysis. Medical records of patients seen at the Asthma and Allergy Clinic at PMH who did not fulfil inclusion criteria were excluded, ie, gross missing or inadequate data, patients older than 18 years and those with diagnosis other than allergic disease or asthma. In addition to information found in medical records, pediatric patients in Botswana have self-carried medical out-patient cards and a shadow file was compiled for every patient at the first visit. At all subsequent visits information was added to both their self-carried medical card, as well as the shadow file for quality and updated information. The files of all patients were stored in a locked file cabinet at the clinic. Patient files were deidentified and those who fulfilled inclusion criteria were entered into the Redcap database (http://ehealth.ub.bw/redcap). Variables were the different allergic conditions, asthma, age at time of enrollment, sex, race, place of residence, triggers of allergic symptoms, allergen sensitization, and family history of allergic disease.
Work-Up of Allergic Diseases
Standardized allergen extracts (ALK-Abello, Madrid, Spain) were used for food (cow’s milk (1:20 w/v), soya (1:20 w/v), egg white (1:20 w/v), wheat (1:20 w/v), peanut (1:20 w/v)) and aeroallergens. The latter included plane tree (Platanus acerifolia (30HEP)), oak tree (Quercus ilex (1:100 w/v)), acacia tree (Robinia pseudoacacia (1:100 w/v)), Bermuda grass (Cynodon dactylon (30HEP)), Timothy grass (Phleum pretense (30HEP)), maize pollen (Zea mays (1:100w/v)), English plantain (Plantago lanceolate (30HEP)), moulds [mix –Alternaria chaetomium, Cladosporium fulvum, Fusarium (1:20 w/v); Alternaria alternate (30HEP), Aspergillus fumigatus (1:20 w/v)], cat (10HEP), dog hair (10HEP), dust mites [Dermatophagoides mix – Dermatophagoides farinae, Dermatophagoides pteronyssinus (30HEP); Blomia tropicalis(10HEP)] and cockroach (Blatella germanica (1:100 w/v)).
A stainless steel lancet (ALK-Abelló, Madrid, Spain) was used for the skin prick, technique as described by the practice parameter.18 All tests were compared to a positive (histamine) and negative (glycerinated saline) control. A positive test was defined as a wheal size 3 mm greater than the negative control at 15 minutes. All skin prick tests (SPTs) were performed and interpreted by a single allergist (board certified with the American Board of Allergy and Immunology). SPTs were performed to food allergies in those with moderate to severe AD, or in those with a suggestive history of food allergy. Patients with rhinitis, asthma and conjunctivitis underwent SPT to indoor aeroallergens (namely, moulds, cat, dog and dust mites). Only patients older than age 3 years had additional skin testing to the other (outdoor) aeroallergens.
Statistical Methods and Data Analysis
Continuous variables were described by means and standard deviations (SD), while frequencies and percentages were used for categorical variables. χ2-test was used to assess the associations between categorical variables. Results with p-values below 0.05 were considered statistically significant. No adjustment for multiple testing was performed as the study was of an exploratory nature. The analyses were performed in SPSS, version 25 (IBM, Chicago, USA)
Results
A total of 603 children and adolescent patients were admitted for suspected allergic disease or asthma to the Asthma and Allergy Clinic at PMH during the study period of 8 years. Of these, 126 were older than 18 years of age, and an additional 29 medical records had gross loss of information, whereas 41 medical records included a main diagnosis other than allergic disease (Figure 1).
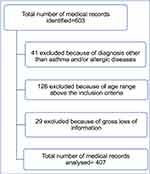 |
Figure 1 Flow diagram of selection of medical records. |
Four hundred and seven patients’ records were included in the analysis; 239 (58.7%) males with a mean age of 5.8 years (SD 4.4). The majority of patients was 1–6 years old (55.6%). Black Africans constituted 365 (87.5%). Most participants resided in urban areas, 291 (71.5%), whereas only 66 (16.2%) lived in rural areas of Botswana (Table 1).
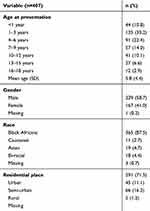 |
Table 1 Sociodemographic Descriptive Characteristics of Patients with Allergic Disease, Princess Marina Hospital, from July 1, 2010 to June 31, 2018 |
The most common diseases seen in this study were asthma (61.2%), followed by AR (57.0%) and AD (40.5%), presenting alone or coexisting with another allergic disorders (Table 2). Only 127 (31%) patients had a single allergic disease (Figure 2); 154 (37.8%) had two identified allergic illnesses and 126 (30.9%) had more than two defined allergic diseases. AC and food allergy were present in less than a third of patients (25.6% and 21.6%, respectively).
 |
Table 2 Frequency of Different Allergic Diseases at the Asthma and Allergy Clinic |
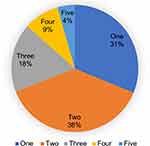 |
Figure 2 Frequency of multiple allergic diseases seen in an individual patient. |
Upper respiratory tract infection was a common trigger of allergy symptoms; 179 (44%) of patients with asthma and AR. Only a single trigger was observed in 132 (32.4%), whereas 65 (16%) children with asthma and AR had more than three triggers for symptom or disease exacerbation in children (Table 3).
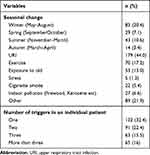 |
Table 3 Common Triggers of Disease Manifestation in Children Diagnosed with Allergic Diseases |
Due to a shortage of reagents, the skin prick test (SPT) was performed only on 252 (61.9%) of all patients and 216 (85.7%) were sensitized to one or more allergen. Among those with positive SPT, sensitization to pollens (tree, grass, weeds), moulds, dust mites, pet dander (cat and dog) and cockroach was significantly higher in those with allergic rhinoconjunctivitis than those without. In contrast, only sensitization to grass pollen, moulds, dust mites, dog dander and cockroach was significantly more pronounced among asthmatics as compared to those without asthma (Table 4). The most common food allergens detected by SPT were egg white (n=29, 13.4%), peanut (n=21, 9.7%), cow’s milk (n=7, 3.2%), wheat flour (n=7, 3.2%) and soya (n=3, 1.4%).
 |
Table 4 Allergen Sensitizations in Different Atopic Diseases as Determined by Skin Prick Allergy Test |
In this cohort, asthma in children was significantly associated to the history of allergic disease in the father (p=0.017) and grandparents (p=0.010). Paternal history of allergic disease was associated with AR (p=0.01), and patients with AD had a history of maternal allergy (p=0.014) (Table 5).
 |
Table 5 Association of Allergy Disease with First- and Second-Degree Family History |
Asthmatic children in this cohort had other allergic diagnoses, namely AR (p<0.001), AD (p<0.001) and food allergy (p=0.003). Similarly, children with AR also suffered AC (p<0.001) and AD (p<0.001) as allergic comorbidities. Furthermore, those with atopic dermatitis had food allergies too (p<0.01) (Table 6).
 |
Table 6 Associated Comorbid Allergic Disease Seen in Patients |
Discussion
This exploratory study shows the demographic and clinical characteristics of a large sample of pediatric and adolescent patients with allergic disorders and/or asthma in Botswana. We identified the presentation of allergic disease at similar ages compared with earlier reports.11,19,20 More than half of our patients admitted for allergic disease and/or asthma experienced symptoms with onset at age 1–6 years. The expression of allergic diseases varies with age, and symptoms may disappear or be replaced by other symptoms (atopic march).19,21 In infancy, the most common manifestations are atopic dermatitis, food allergy and recurrent wheezing, whereas asthma and allergic rhinoconjunctivitis are the main issues later in childhood.22 Hill et al19 reported the age distribution for different allergic diseases in a population of over one million children in the US. The incidence of atopic dermatitis (AD) during the first 5 years of life was 15.3%, with peak age at diagnosis before 5 months of age. The incidence of asthma during the first 5 years of life was 22.4%, with a peak age of first diagnosis between 12–17 months (8.7%). Our study included an array of allergic diseases, which had a similar age distribution of allergic manifestations as described by Hill et al.19
The most common allergic diseases were asthma (61.2%), AR (57.0%), AD (40.5%), AC (25.6%) and food allergy (21.6%). Sixty eight percent had more than one type of allergic disease. Patients with asthma had significantly higher comorbidity with AR, AD and food allergy. In a previous study based in Botswana, Kung et al11 reported 126 patients with allergic disease from a private practice in Gaborone. The most common clinical presentations in that study were AR (70%) and asthma (42%), followed by AD (30%), AC (21%), food allergy (20%), and angioedema (6%). Similarly, these patients often had more than one type of allergic disease. However, unlike our study, more children suffered from AR than asthma. One of the reasons for this may include the referral pattern of patients to the Asthma and Allergy Clinic at PMH. All asthmatic patients that were hospitalized as in-patients were automatically referred to the subspecialist clinic upon discharge. Uncontrolled asthmatic patients from other sections of the pediatric outpatient clinic at PMH were similarly referred to the subspecialist. Patients with sole diagnosis of AR may have been referred less frequently to the Asthma and Allergy Clinic at PMH if symptoms were not perceived to be severe. However, compared to the Kung study, our study may provide a better reflection of the spectrum of pediatric allergic diseases and asthma in southern Botswana, since PMH is the tertiary referral hospital in the public sector. All severely ill children that require specialized care from the surrounding areas are referred to this facility.
Our study showed high rates of co-existence of allergic diseases, and conditions like food allergy, atopic dermatitis, allergic rhinitis, and asthma is thought to be increasing. A number of studies examines the proportion of individuals with co-existence of AR and asthma and found rates similar to ours where almost every 3 of 4 patients showed co-morbid allergy diagnoses.11,23,24 In one of the earliest studies, Mullarkey et al25 studied 142 patients, aged 14–72 years, and 58% of them had AR and asthma. The Danish Allergy Research Center (DARC) birth cohort from 2016 followed patients prospectively with objective investigations regularly from birth to 14 years of age and 12.1% had more than one allergic disease, and most prevalent was ARC combined with asthma.5 It seems like the presence of one allergic disorder increases the risk of developing other allergic disorders in different organ systems.26,27 This is probably due to the shared predisposition and mechanism for development of these diseases including an immunological response to environmental allergens.28
Patients may typically develop a sequence of allergic diseases, ie, AD, AR and asthma, at different ages. Some may persist for several years, whereas others may resolve with increasing age.21,29 The coexistence of AD, asthma and AR may be part of the manifestation of allergic march, but the risk of developing allergic disease is complex.20,21,29 The development and progression of these diseases is strongly influenced by both genetic and environmental factors.30 Our study illustrates that co-morbid conditions are common in the southern African population at seemingly high rates.
The sensitization to various aeroallergens and the measures undertaken to control them play an important role in disease manifestations and quality of life.31 The pattern of sensitization evolves in the order of exposure throughout childhood: food, indoor allergens, outdoor allergens. Sensitization to milk and egg typically happens during the first 2–3 years of life, while sensitization to inhalant allergens usually occurs later in childhood with increasing prevalence with age,30,31 which is in accordance with our study illustrating the early onset of food-related allergy. In our study 216 of 407 (53.10%) patients were sensitized to allergens (aeroallergens and food). A large proportion of asthmatic and rhinitis patients seem to be sensitized to common allergens of indoor or outdoor origin. Sensitization to these allergens may be influenced by different factors, including genetic susceptibility, age at which the exposure begins and the degree and type of allergen exposure.32,33 Approximately two thirds of asthmatic subjects in previous studies are sensitized to at least one inhalant allergen.34 However, even though many children are sensitized to inhalant allergens by early school age, only 25% to 30% of these will eventually have asthma. It is still unclear why a subgroup of atopic children will become asthmatic, whereas others either remain asymptomatic or the atopic sensitization disappears.34 In accordance with our study, two-thirds of the patients who presented to a private allergy practice in Gaborone, Botswana, were sensitized to some type of allergen, aeroallergens (85%) more often than food allergens (19%) and 13% sensitized to both. The most common aeroallergen sensitization was to Bermuda grass (41%), with slightly lower rates to Timothy grass (33%) and maize pollen (32%).11 The present study demonstrates high asthma rates among children and adolescents and provides extended information on the relationship between asthma and allergen sensitization. In future studies we plan to follow this population prospectively and assess if sensitized patients will continue to have allergic disease manifestations.
Interestingly, 20% of our cohort showed food allergies, which is consistent with Kung’s study where 19% had food allergens and 13% of patients were sensitized to both aeroallergens and foods.11 These rates are higher than those seen in other studies, possibly due to the small sample size, and the very selected cohort. In a study done in Ghanaian school children, 11% of 1407 children reported adverse reactions to foods, and 5% of 1,431 children showed positive skin prick test (SPT) reactivity. The most common sensitizing foods were peanut and pineapple.35 Approximately 10% of 14,000 patients, all ages, referred to the only specialist allergy clinic in Harare, Zimbabwe, were diagnosed with food allergies.36 In 2008, a study done in South Africa on an unselected group of 212 Xhosa high school patients in Cape Town (age 15–24 years) showed positive SPT for food allergens in 5.4% of subjects, most commonly to egg white (3.3%), peanuts (1.9%) and milk (1.9%).37 Intriguingly, none of these students reported symptoms to these foods illustrating that the next step in our work will be to follow the correlation between sensitization proved by SPT and actual food allergy.37,38
This study identified upper respiratory tract infection, weather changes, exposure to passive cigarette smoke, and indoor pollutants as important triggers of asthma symptoms. The search for relevant genetic and environmental triggers of allergy disease has identified a variety of potential candidates.30 Prominent amongst these are factors related to respiratory infections, particularly those triggering exacerbation of asthma and allergic rhinoconjunctivitis,39 similar to those presented in this study. Allergic disease in a first-degree relative is known to be a predisposing factor for development of allergic disease in the offspring. This report demonstrates that paternal history of allergy disease is a predisposing factor for asthma and allergic rhinitis. Dold et al40 identified 3,160 families with single allergic disease in one parent where only asthma, not allergic rhinitis, was a predisposing factor for asthma in the offspring.40 A longitudinal cohort study from Germany indicated significant associations between childhood AD and maternal AD.41 These reports support our findings as asthma and AD are significantly associated with paternal and maternal history of allergic disease, respectively.
This study has limitations. Firstly, it was conducted in a single tertiary public center, and may only reflect patients with more severe disease or those opportunistically referred. In addition, there is a concurrent private health system in Botswana, that has its own network of hospitals and clinics. Although some of those patients chose to be seen at the PMH Asthma and Allergy Clinic, a systematic referral procedure does not exist. Hence, we are unable to describe disease incidence or prevalence for allergic conditions and asthma in Gaborone and Botswana. Secondly, this is a retrospective study, with all of the inherent flaws, including missing data for some patients. Unfortunately, there is no central registry of pediatric atopic diseases in the Gaborone catchment area, which may have aided in better description of disease prevalence. Thirdly, the shortage and sometimes unavailability of allergen extracts for SPT preclude the capability to do the test in all suspected patients with allergic disease. This renders the diagnosis of the allergic disease to be based on clinical suspicion in close to half of the patients included in this study. Furthermore, meeting diagnostic criteria for asthma and food allergy was a challenge in our setting. However, the diagnoses of asthma in children less than 3 years was ascertained after following up the patient for sufficient time, persistence of cough and wheeze, family history of allergy disease and, in some, evidence of allergen sensitization. The gold standard double blind placebo-controlled food challenge test was rarely done because of resource constraint. We made the diagnosis of food allergy in our patients after careful history and examination, measurement of specific IgE test and/or SPT. Despite these limitations, our data provide a large sized sample of patients referred from all over Botswana providing insights on allergy diagnoses and co-morbidities, and their relationship to allergens.
Conclusion
This study shed light on the types of common pediatric allergic diseases in a single tertiary level treatment center in Botswana. The findings of this study will help design further studies on the prevalence of allergic diseases in Botswana. The identification of common allergens and triggers of disease exacerbations will be important for planning primary and secondary prevention strategies. These strategies will improve the quality of life in children and adolescents who are suffering from these diseases. Furthermore, it can be used as a starting point for financing of research to address the need for investigation, as well as political and national planning of health care and treatment of patients with allergic diseases. However, further studies are needed to examine the role of modifiable factors and application of exposures to potentially mitigate disease exacerbations.
Ethics Approval and Informed Consent
All procedures performed and data collected in this study were adhered to the guidelines of Declaration of Helsinki and the ethical standards of institutional ethics review committees. The Institutional Review Boards (IRBs) of the University of Botswana (UBR/RES/IRB/BIO/091) and the Botswana Ministry of Health and Wellness (HPDME 13/18/1) granted approval to conduct this research. Waiver for consent was given to collect data from patients’ medical record as this study explores patient information retrospectively and some of the patients are no longer actively on follow up at the asthma and allergy clinic of PMH to provide consent. To keep patient confidentiality all identified medical records were stored in a locked filing cabinet which was accessed only by the investigators. All information collected from the medical records were coded and all patient identifiers were removed. The collected data were then entered into RedCap database (http://ehealth.ub.bw/redcap) which was password protected and only accessed by the investigators.
Acknowledgments
The authors would like to extend our heartfelt gratitude to Dr Juliett Massip for helping on statistical analysis.
Funding
None.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mastrorilli C, Posa D, Cipriani F, Caffarelli C. Asthma and allergic rhinitis in childhood: what’s new. Pediatr Allergy Immunol. 2016;27(8):795–803. doi:10.1111/pai.12681
2. Bush A, Zar HJ. WHO universal definition of severe asthma. Curr Opin Allergy Clin Immunol. 2011;11(2):115–121. doi:10.1097/ACI.0b013e32834487ae
3. Anandan C, Nurmatov U, Van Schayck OCP, Sheikh A. Is the prevalence of asthma declining? Systematic review of epidemiological studies. Allergy Eur J Allergy Clin Immunol. 2010;65(2):152–167. doi:10.1111/j.1398-9995.2009.02244.x
4. Ait-Khaled N, Odhiambo J, Pearce N, et al. Prevalence of symptoms of asthma, rhinitis and eczema in 13- to 14-year-old children in Africa: the international study of asthma and allergies in childhood phase III. Allergy Eur J Allergy Clin Immunol. 2007;62(3):247–258. doi:10.1111/j.1398-9995.2007.01325.x
5. Christiansen ES, Kjaer HF, Eller E, et al. The prevalence of atopic diseases and the patterns of sensitization in adolescence. Pediatr Allergy Immunol. 2016;27(8):847–853. doi:10.1111/pai.12650
6. Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71(1):116–132. doi:10.1016/j.jaad.2014.03.023
7. Perkin MR, Strachan DP, Williams HC, Kennedy CTC, Golding J. Natural history of atopic dermatitis and its relationship to serum total immunoglobulin E in a population-based birth cohort study. Pediatr Allergy Immunol. 2004;15(3):221–229. doi:10.1111/j.1399-3038.2004.00160.x
8. Sampson HA. Update on food allergy. J Allergy Clin Immunol. 2004;113(5):805–819. doi:10.1016/j.jaci.2004.03.014
9. Boye JI. Food allergies in developing and emerging economies: need for comprehensive data on prevalence rates. Clin Transl Allergy. 2012;2(1):1–9. doi:10.1186/2045-7022-2-25
10. Stensballe LG, Klansø L, Jensen A, Hærskjold A, Thomsen SF, Simonsen J. The validity of register data to identify children with atopic dermatitis, asthma or allergic rhinoconjunctivitis. Pediatr Allergy Immunol. 2017;28(6):535–542. doi:10.1111/pai.12743
11. Kung S-J, Steenhoff AP. Allergy in Botswana. Curr Allergy Clin Immunol. 2013;26(4):202–209.
12. Kiboneka A, Levin M, Mosalakatane T, et al. Prevalence of asthma among school children in Gaborone, Botswana. Afr Health Sci. 2016;16(3):809–816. doi:10.4314/ahs.v16i3.22
13. Statistics Botswana. 2017 Botswana demographic survey report [Internet]. 2018. Available from: www.statsbots.org.bw.
14. Papadopoulos NG, Arakawa H, Carlsen KH, et al. International consensus on (ICON) pediatric asthma. Allergy Eur J Allergy Clin Immunol. 2012;67(8):976–997. doi:10.1111/j.1398-9995.2012.02865.x
15. Nowak-Węgrzyn A, Chehade M, Groetch ME, et al. International consensus guidelines for the diagnosis and management of food protein–induced enterocolitis syndrome: executive summary—workgroup report of the adverse reactions to foods committee, American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2017;139(4):1111–1126. doi:10.1016/j.jaci.2016.12.966
16. Pawankar R, Canonica GW, Holgate ST, Lockey RF, Blaiss MS. WAO white book on allergy. World Allergy Organ. 2011;3:156–157.
17. Wise SK, Lin SY, Toskala E, et al. International consensus statement on allergy and rhinology: allergic rhinitis. In: International Forum of Allergy & Rhinology. Wiley Online Library; 2018:108–352.
18. Bernstein IL, Li JT, Bernstein DI, et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy, Asthma Immunol. 2008;100(3):S1–148.
19. Hill DA, Grundmeier RW, Ram G, Spergel JM. The epidemiologic characteristics of healthcare provider-diagnosed eczema, asthma, allergic rhinitis, and food allergy in children: a retrospective cohort study. BMC Pediatr. 2016;16(1):133. doi:10.1186/s12887-016-0673-z
20. van der Hulst AE, Klip H, Brand PLP. Risk of developing asthma in young children with atopic eczema: a systematic review. J Allergy Clin Immunol. 2007;120(3):565–569. doi:10.1016/j.jaci.2007.05.042
21. Zheng T, Yu J, Oh MH, Zhu Z. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. Allergy Asthma Immunol Res. 2011;3(2):67–73. doi:10.4168/aair.2011.3.2.67
22. Halken S, Høst A. The lessons of noninterventional and interventional prospective studies on the development of atopic disease during childhood. Allergy. 2000;55(9):793–802. doi:10.1034/j.1398-9995.2000.00117.x
23. Peters RL, Koplin JJ, Gurrin LC, et al. The prevalence of food allergy and other allergic diseases in early childhood in a population-based study: healthNuts age 4-year follow-up. J Allergy Clin Immunol. 2017;140(1):145–153.e8. doi:10.1016/j.jaci.2017.02.019
24. Beasley R. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. Lancet. 1998;351(9111):1225–1232. doi:10.1016/S0140-6736(97)07302-9
25. Mullarkey MF, Hill JS, Webb DR. Allergic and nonallergic rhinitis: their characterization with attention to the meaning of nasal eosinophilia. J Allergy Clin Immunol. 1980;65(2):122–126. doi:10.1016/0091-6749(80)90196-7
26. Viinanen A, Munhbayarlah S, Zevgee T, et al. Prevalence of asthma, allergic rhinoconjunctivitis and allergic sensitization in Mongolia. Allergy. 2005;60(11):1370–1377. doi:10.1111/j.1398-9995.2005.00877.x
27. Yuksel H, Dinc G, Sakar A, et al. Prevalence and comorbidity of allergic eczema, rhinitis, and asthma in a city in western Turkey. J Investig Allergol Clin Immunol. 2008;18(1):31.
28. Holgate ST. The epidemic of allergy and asthma. Nature. 1999;402(6760):2–4. doi:10.1038/35037000
29. Park HS, Choi GS, Cho JS, Kim -Y-Y. Epidemiology and current status of allergic rhinitis, asthma, and associated allergic diseases in Korea: ARIA Asia-Pacific workshop report. Asian Pac J Allergy Immunol. 2009;27(2–3):167.
30. Halken S. Prevention of allergic disease in childhood: clinical and epidemiological aspects of primary and secondary allergy prevention. Pediatr Allergy Immunol. 2004;15:9–32. doi:10.1111/j.1399-3038.2004.0148b.x
31. Kulig M, Bergmann R, Klettke U, Wahn V, Tacke U, Wahn U. Natural course of sensitization to food and inhalant allergens during the first 6 years of life. J Allergy Clin Immunol. 1999;103(6):1173–1179. doi:10.1016/S0091-6749(99)70195-8
32. Boulet L, Turcotte H, Laprise C, et al. Comparative degree and type of sensitization to common indoor and outdoor allergens in subjects with allergic rhinitis and/or asthma. Clin Exp Allergy. 1997;27(1):52–59. doi:10.1111/j.1365-2222.1997.tb00672.x
33. Sibanda E. Increasing trend of sensitisation to food and inhalant allergen sources in Zimbabwe. Curr Allergy Clin Immunol. 2013;26(4):214–219.
34. Illi S, von Mutius E, Lau S, et al. The pattern of atopic sensitization is associated with the development of asthma in childhood. J Allergy Clin Immunol. 2001;108(5):709–714. doi:10.1067/mai.2001.118786
35. Obeng BB, Amoah AS, Larbi IA, et al. Food allergy in Ghanaian schoolchildren: data on sensitization and reported food allergy. Int Arch Allergy Immunol. 2011;155(1):63–73. doi:10.1159/000318704
36. Sibanda EN. Inhalant allergies in Zimbabwe: a common problem. Int Arch Allergy Immunol. 2003;130(1):2–9. doi:10.1159/000068377
37. Levin ME, Le Souëf PN, Motala C. Total IgE in urban Black South African teenagers: the influence of atopy and helminth infection. Pediatr Allergy Immunol. 2008;19(5):449–454. doi:10.1111/j.1399-3038.2007.00663.x
38. Basera W, Botha M, Gray CL, et al. The South African food sensitisation and food allergy population-based study of IgE-mediated food allergy: validity, safety, and acceptability. Ann Allergy, Asthma Immunol. 2015;115(2):113–119. doi:10.1016/j.anai.2015.06.003
39. Holt PG, Sly PD. Interactions between respiratory tract infections and atopy in the aetiology of asthma. Eur Respir J. 2002;19(3):538LP– 545. doi:10.1183/09031936.02.00229302
40. Dold S, Wjst M, von Mutius E, Reitmeir P, Stiepel E. Genetic risk for asthma, allergic rhinitis, and atopic dermatitis. Arch Dis Child. 1992;67(8):1018LP– 1022. doi:10.1136/adc.67.8.1018
41. Fuertes E, Standl M, von Berg A, et al. Parental allergic disease before and after child birth poses similar risk for childhood allergies. Allergy. 2015;70(7):873–876. doi:10.1111/all.12609
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
